Abstract
Objective. Vaginal bleeding is a risk factor for preterm PROM (PPROM). A disorder of decidual hemostasis has been implicated in the genesis of PROM. Indeed, excessive thrombin generation has been demonstrated in PPROM both before and at the time of diagnosis. Decidua is a potent source of tissue factor (TF), the most powerful natural pro-coagulant. A decidual hemostatic disorder may link vaginal bleeding, PPROM and placental abruption. This study was conducted to determine the behaviour of maternal TF and its natural inhibitor, the tissue factor pathway inhibitor (TFPI) in PPROM.
Methods. This cross-sectional study included women with PPROM (n = 123) and women with normal pregnancies (n = 86). Plasma concentrations of TF and TFPI were measured by a sensitive immunoassay. Non-parametric statistics were used for analysis.
Results. (1) The median maternal plasma TF concentration was significantly higher in patients with PPROM than in women with normal pregnancies (median: 369.5 pg/mL; range: 3.27–2551 pg/mL vs. median: 291.5 pg/mL; range: 6.3–2662.2 pg/mL respectively, p = 0.001); (2) the median maternal TFPI plasma concentration was significantly lower in patients with PPROM than in women with normal pregnancies (median: 58.7 ng/mL; range: 26.3–116 ng/mL vs. median: 66.1 ng/mL; range: 14.3–86.5 ng/mL respectively, p = 0.019); (3) there was no correlation between the plasma concentration of TF and TFPI and the gestational age at sample collection; and (4) among patients with PPROM there was no association between the presence of intra-amniotic infection or inflammation and median plasma concentrations of TF and TFPI.
Conclusions. (1) Patients with PPROM have a higher median plasma concentration of TF and a lower median plasma concentration of TFPI than women with normal pregnancies. (2) These findings suggest that PPROM is associated with specific changes in the hemostatic/coagulation system.
Introduction
Preterm prelabour rupture of membranes (PROM) is associated with placental vascular lesions including a failure of physiologic transformation of the spiral arteries Citation[1], atherosis and decidual vasculopathy (i.e. fibrinoid necrosis and thrombosis of decidual vessels) Citation[1,2]. Moreover, women with preterm PROM have a higher median maternal plasma concentration of thrombin–antithrombin (TAT) complexes than women with normal pregnancies Citation[3,4]. Thus, the activation of the coagulation cascade that results in an increased thrombin generation may be one of the mechanisms of disease leading to preterm PROM.
The activation of the coagulation system in the placental and maternal compartment of patients with preterm PROM can result from the following underlying mechanisms of disease: (1) decidual hemorrhage that leads to a retro-placental clot formation Citation[5]; (2) intra-amniotic infection which can induce decidual bleeding and sub-clinical abruption Citation[6], as well as increased intra-amniotic TAT complexes Citation[7]; and (3) an increased maternal systemic inflammatory response Citation[8] that may activate the extrinsic pathway of coagulation due to the expression and release of tissue factor (TF) by activated monocytes Citation[9]. These mechanisms result in an increased thrombin generation, which has been associated with the following: (1) stimulation of decidual cell secretion of matrix metalloproteinases (MMP) (i.e. MMP-1 Citation[10] and MMP-3 Citation[11]) that can degrade the extracellular matrix of the chorioamniotic membranes; and (2) myometrial activation and uterine contractions generation that may lead to preterm labour with or without rupture of membranes and a subsequent preterm delivery Citation[12-14].
Although thrombin is generated as a consequence of activation of the coagulation cascade, TF, the most powerful natural pro-coagulant, is abundant in the uterine decidua in the normal state Citation[15,16]. Tissue factor is part of an efficient hemostatic mechanism in the uterine wall, which is activated in the course of normal pregnancy during implantation Citation[17] and after delivery Citation[18]. However, this hemostatic mechanism may also be activated due to pathological decidual bleeding in pregnancies complicated by placental abruption Citation[5],Citation[19] and intra-amniotic infection Citation[6].
The main physiological inhibitor of the TF pathway is tissue factor pathway inhibitor (TFPI), which is a glycoprotein comprised of three Kunitz domain Citation[20] that are specific inhibitors of trypsin-like proteinases Citation[21]. The mean maternal plasma concentration of total TFPI (TFPI-1 and TFPI-2) have been reported to increase during pregnancy until 20 weeks of gestation, to remain relatively constant until term Citation[22] and to decrease during labour Citation[23]. There are two types of TFPI: (1) TFPI-1 is the more prevalent form in the non-pregnant state in the maternal circulation and can also be found in the fetal blood, platelets, endothelial cells and other organs Citation[24,25]; and (2) TFPI-2, the major form of TFPI in the placenta Citation[26-31], is also known as Placental Protein 5 (PP5) Citation[32,33]. During pregnancy, the maternal plasma concentration of TFPI-2 increases gradually, reaches a plateau at 36 weeks and subsides after delivery Citation[33-38].
Pregnancy complications are associated with changes in the maternal plasma and placental concentration of TF and TFPI. Maternal plasma concentrations of TF and free TFPI are higher in women with pre-eclampsia than in patients with a normal pregnancy Citation[39-41]. Placental TF concentrations and mRNA expression are higher in patients with severe pre-eclampsia than in those with a normal pregnancy Citation[42]. In contrast, placentas of patients with gestational vascular complications (pre-eclampsia, eclampsia, fetal growth restriction, placental abruption and fetal demise) have lower placental concentrations of total TFPI and mRNA expression than in those of patients with a normal pregnancy Citation[43].
The increased thrombin generation in the maternal plasma of patients with preterm PROM Citation[3,4] reflects the activation of the coagulation cascade; however, it is not clear whether this activation is associated with changes in TF and TFPI plasma concentrations as well. Thus, the objective of this study is to determine the changes in the maternal plasma concentrations of TF and its natural inhibitor TFPI in women with preterm PROM.
Methods
Study design
This cross-sectional study included patients in the following groups: (1) patients with preterm PROM (n = 123), and (2) women with normal pregnancies (n = 86). Patients with multiple pregnancies or fetuses with congenital and/or chromosomal anomalies were excluded.
Samples and data were retrieved from the bank of biological samples and clinical databases. Many of these samples have been previously used to study the biology of inflammation, hemostasis, angiogenesis regulation and growth factor concentrations in non-pregnant women, normal pregnant women and those with pregnancy complications.
All women provided a written informed consent prior to the collection of maternal blood. The Institutional Review Boards of both Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS) approved the collection and utilisation of the samples for research purposes.
Definitions
Women were considered to have a normal pregnancy if they did not have obstetrical, medical, or surgical complication of pregnancy and delivered a term neonate of appropriate birth weight for gestational age without complications. Preterm PROM was diagnosed by a sterile speculum examination demonstrating the pooling of amniotic fluid in the vagina (with nitrazine and ferning tests when necessary) occurring <37 weeks of gestation in the absence of labour.
Amniotic fluid collection was performed by trans-abdominal amniocentesis under ultrasonographic guidance in order to determine the microbiologic state of the amniotic cavity in a subset of patients at the discretion of the treating physician. Amniotic fluid was transported to the laboratory in a capped plastic syringe and cultured for aerobic, anaerobic bacteria, as well as for genital Mycoplasmas. White blood cell (WBC) count, glucose concentration and Gram stain for microorganisms were performed in amniotic fluid shortly after collection. Intra-amniotic infection was defined by the presence of amniotic fluid cultures that were positive for microorganisms and inflammation by an amniotic fluid WBC count ≥100 cells. The results of the amniotic fluid analyses were used for clinical management. Small for gestational age (SGA) neonate was defined as birthweight below the 10th percentile Citation[44].
Placental histopathologic examinations
Placental tissue samples were taken by systematic random sampling and subsequently fixed in 10% neutral buffered formalin overnight and embedded in paraffin. Five millimetre-thick paraffin sections were stained with hematoxylin and eosin, and examined using bright-field light microscopy. Histopathologic examinations were performed by three pathologists who were blinded to the clinical information. The placental histologic lesions were classified according to a diagnostic schema proposed by Redline et al. Citation[45].
Blood samples collection
All blood samples were collected with a vacutainer into 0.109 M trisodium citrate anticoagulant solution (BD; San Jose, CA). The samples were centrifuged at 1300g for 10 min at 4°C and stored at −70°C until assayed.
Human TF immunoassays
Maternal plasma TF concentrations were determined by sensitive and specific immunoassays obtained from American Diagnostica (Greenwich, CT) which recognises TF-apo, TF and TF-FVII complexes. The assay was conducted according to the manufacturer's recommendations. The calculated coefficient of variation (CV) in our laboratory was 5.3%, and the sensitivity is 10 pg/mL.
Human TFPI immunoassay
The concentrations of TFPI in maternal plasma were determined by sensitive and specific immunoassays obtained from American Diagnostica (Greenwich, CT). The TFPI ELISA employs a murine anti-TFPI monoclonal as the capture antibody. This capturing antibody is directed against the Kunitz-1 domain of the TFPI molecule; therefore, it detects both TFPI-1 and TFPI-2 and measures the total TFPI plasma concentrations. The assay was conducted according to the manufacturer's recommendations. The calculated CV in our laboratory was 6.6% and the sensitivity is ∼10 ng/mL. The correlation between TFPI antigen concentrations and functional activity is ∼r2 = 0.785.
Statistical analysis
Plasma concentrations of TF and TFPI were not normally distributed. Thus, a Mann–Whitney U test was used for comparisons of continuous variables and a Chi-square test was used to compare categorical variables. The Spearman's rho test was used to detect a correlation between the maternal plasma TF and TFPI concentrations and the gestational age at blood collection in women with normal pregnancies. A p-value <0.05 was considered statistically significant. The statistical package employed was SPSS version 12 (SPSS, Chicago, IL).
Results
Demographic characteristics and changes in maternal plasma TF and TFPI concentrations during normal pregnancy
displays the demographic and clinical characteristics of the study groups. Patients with preterm PROM had a lower median gestational age at blood sample collection and at delivery, as well as a lower median neonatal birth weight in comparison with the control group. There was no significant correlation between the maternal plasma TF and TFPI concentrations and the gestational age at blood sample collection in patients with normal pregnancy (TF: r = −0.009, p = 0.94; TFPI: r = 0.157, p = 0.15) ().
Figure 1. The correlations between maternal plasma tissue factor (TF) (1a) and tissue factor pathway inhibitor (TFPI) (1b) concentrations and gestational age at blood collection.
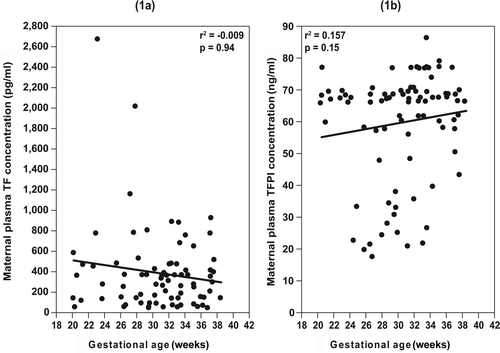
Table I. Demographic and clinical characteristics of the study population
Changes in maternal plasma TF and TFPI concentrations among patients with preterm PROM and their association with intra-amniotic infection/inflammation
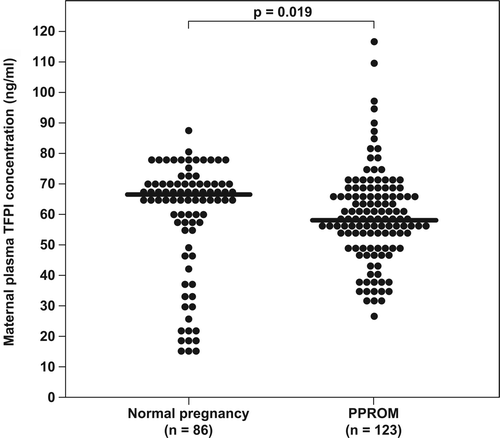
Of the 86 patients in the normal pregnancy group, 91.9% (79/86) had detectable immunoreactive TF in the plasma. Maternal plasma TF concentrations were significantly higher in women with preterm PROM than those with a normal pregnancy (median: 369.5 pg/mL; range: 3.3–2551 pg/mL vs. median: 291.5 pg/mL; range: 6.3–2662.2 pg/mL respectively, p = 0.001) (). In contrast, maternal plasma TFPI concentrations were significantly lower in women with preterm PROM than those with a normal pregnancy (median: 58.7 ng/mL; range: 26.3–116 ng/mL vs. median: 66.1 ng/mL; range: 14.3–86.5 ng/mL respectively, p = 0.019) (). Patients with preterm PROM had a significantly lower median maternal plasma TFPI/TF ratio than those with normal pregnancies (median: 142.8; range: 21.8–16422 vs. median: 221.5; range: 25.4–3355.3l respectively, p < 0.0001).
Figure 2. Comparison of maternal plasma tissue factor (TF) concentrations between women with normal pregnancy (n = 79) and patients with preterm PROM (n = 123). Tissue factor plasma concentrations were significantly higher in patients with preterm PROM than in women with a normal pregnancy (median: 369.5 pg/mL; range: 3.27–2551 pg/mL vs. median: 291.5 pg/mL; range: 6.3–2662.2 pg/mL, respectively; p = 0.001).
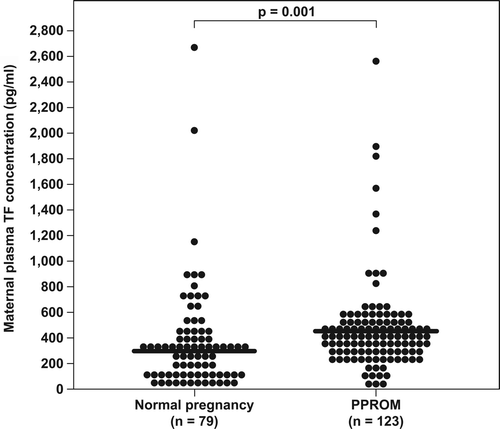
Figure 3. Comparison of maternal plasma tissue factor pathway inhibitor (TFPI) concentrations between women with normal pregnancy (n = 86) and patients with preterm PROM (n = 123). TFPI plasma concentrations were significantly lower in patients with preterm PROM than in women with a normal pregnancy (median: 58.7 ng/mL; range: 26.3–116 ng/mL vs. median: 66.1 ng/mL; range: 14.3–86.5 ng/mL, respectively; p = 0.019).
Sixty-eight patients in the preterm PROM group underwent amniocentesis, of which 54.4% (37/68) had intra-amniotic infection/inflammation. Isolated intra-amniotic inflammation was diagnosed in 16.2% (11/68) of the cases. Positive amniotic fluid cultures for microorganisms were detected in 38.2% (26/68) of patients with preterm PROM (the frequency of the specific microorganisms is presented in ).
Table II. Microorganisms isolated from the amniotic cavity in patients with preterm PROM who underwent trans-abdominal amniocentesis
displays the changes in the median maternal plasma TF and TFPI concentrations according to the presence or absence of intra-amniotic infection and/or inflammation. The patients in both subsets of the preterm PROM group (with and without intra-amniotic infection/inflammation) had a significantly higher median maternal plasma TF concentration than in women with normal pregnancies. There were no significant differences in the median maternal plasma TF and TFPI concentrations between preterm PROM patients with and without intra-amniotic infection and/or inflammation ().
Table III. Maternal plasma concentrations of tissue factor and tissue factor pathway inhibitor in patients with preterm PROM with and without intra-amniotic infection and/or inflammation in comparison to women with normal pregnancy
Table IV. Placental lesions in patients with preterm PROM
Changes in maternal plasma TF and TFPI concentrations among patients with preterm PROM according to their placental lesions and the occurrence of vaginal bleeding during pregnancy
Placental histology was available in 82.11% (101/123) of patients with preterm PROM. Of those, 58.4% (59/101) had histologic chorioamnionitis of maternal response and 48.5% (49/101) had histologic chorioamnionitis of fetal response (i.e. umbilical phlebitis, necrotising funisitis). Persistent muscularisation of the basal plate arteries was the most frequent vascular placental lesion (). The placental histologic findings were not associated with significant changes in the median maternal plasma concentrations of TF and TFPI.
Vaginal bleeding during pregnancy was documented in 21 preterm PROM patients. The median maternal plasma TFPI concentration was significantly lower in preterm PROM patients without vaginal bleeding than in women with normal pregnancies; conversely, no difference was observed in the median maternal plasma TFPI concentration between patients with preterm PROM and vaginal bleeding and those with normal pregnancies (). In addition, no significant differences were found in the median maternal plasma TF and TFPI concentrations in preterm PROM patients with and without vaginal bleeding during pregnancy [TF: median 412.6 pg/mL, range (256–2551) vs. median 369.5 pg/mL, range (3.3–1879.4), p = 0.5, respectively; and TFPI: median 56.8 ng/mL, range (31.4–74.2) vs. median 58.7 pg/mL, range (26.3–116), p = 0.6, respectively] ().
Table V. Maternal plasma concentrations of tissue factor and tissue factor pathway inhibitor in patients with preterm PROM with and without vaginal bleeding during pregnancy in comparison to women with normal pregnancy
Comments
Principal findings of the study
(1) Women with preterm PROM have significantly higher median plasma TF concentrations than those with normal pregnancies; (2) the median plasma TFPI concentration was significantly lower in patients with preterm PROM than in those with normal pregnancies; (3) there were no significant differences in maternal plasma TF or TFPI concentrations in patients with preterm PROM with or without intra-amniotic infection/inflammation; and (4) no significant correlation was observed between maternal plasma TF or TFPI concentrations and gestational age at time of blood collection.
What are the changes in TF during pregnancy?
TF is essential for the maintenance of pregnancy and about 90% of TF null mice embryo die at embryonic day 10.5 Citation[46-49] due to abnormalities in yolk sack vasculature Citation[46]. In humans, a substantial increase in decidual and myometrial TF expression and concentrations was reported during the luteal phase of the menstrual cycle Citation[15,16],Citation[50,51], as well as during early and late stages of pregnancy Citation[15,16],Citation[50-52]. Similarly, high TF concentrations have been detected in the fetal membranes (mainly the amnion) and amniotic fluid Citation[23],Citation[53-55]. In contrast, the maternal plasma TF concentrations do not change significantly during normal pregnancy in comparison to non-pregnant women Citation[56,57], though women in labour at term have significantly higher maternal plasma TF concentrations than non-pregnant women Citation[23]. Of interest, TF expression on monocytes is decreased throughout pregnancy, but returns to the normal state by the third day post-partum Citation[58,59].
Why does TF increase in patients with preterm PROM?
Our observation that women with preterm PROM have a significantly higher median maternal plasma concentration of TF is novel. Possible sources for the elevated TF in the maternal plasma could be from decidual activation that is prominent among patients with preterm PROM and from monocyte activation that is associated with the moderate systemic maternal inflammatory state reported in these patients Citation[8]. Both may lead to increased activation of coagulation cascade and higher thrombin generation that can propagate the rupture of membrane through MMP activation Citation[10,11],Citation[60].
TF and activation of coagulation cascade
The higher median maternal plasma concentration of TAT complexes Citation[3,4] along with the higher rate of placental aggregated vascular lesions (atherosis, fibrinoid necrosis of decidual vessels, decidual vessels thrombosis and fetal thrombotic vasculopathy) Citation[1,2],Citation[61] observed in patients with preterm PROM support the suggested increased activation of the coagulation cascade and thrombin generation in these patients.
It has been proposed that a high expression of TF by the decidua and myometrium during normal pregnancy is needed for tight hemostatic control that may prevent decidual bleeding during blastocyst implantation and trophoblast invasion at the early stages of pregnancy, as well as to sustain an adequate uterine hemostasis during the post-partum period Citation[17,18]. Indeed, an increased decidual expression of TF (mRNA and protein) is reported already during the luteal phase of the menstrual cycle and later during pregnancy Citation[62]. Moreover, preterm PROM is associated with an increased activation of the decidual component of the common pathway of parturition Citation[63]. Thus, in pregnancies complicated by abnormal placentation or intrauterine infection, decidual bleeding may lead to a higher expression of TF and activation of the coagulation cascade, resulting in increased thrombin generation. The latter has uterotonic properties that may generate uterine contractions that could initiate labour Citation[12-14]. Moreover, thrombin can mediate the activation of MMP-1 Citation[11], MMP-3 Citation[10] and MMP-9 Citation[60] that can digest components of the extracellular matrix, weaken the choriamniotic membranes and predispose to preterm PROM.
TF and increased maternal intravascular inflammatory response
The mechanisms described above are localised to the maternal-fetal interface. The lack of association between median maternal plasma TF concentrations and the presence of intra-amniotic infection/inflammation or vaginal bleeding in patients with preterm PROM suggest that the systemic maternal inflammatory response during preterm PROM Citation[8] may contribute to the increased median maternal plasma TF concentration in these patients regardless to the presence of infection or inflammation in the amniotic cavity or the occurrence of vaginal bleeding.
The interaction between the coagulation cascade and inflammation is well-established Citation[64-66]. Patients with chronic and acute inflammation or infection (i.e. chronic and acute pancreatitis Citation[67,68] non-crescentic glumerolonephritis Citation[69] and meningococcal sepsis Citation[70]) have higher plasma TF concentrations than healthy controls. During preterm PROM, there is a moderate maternal systemic inflammation that results in monocyte and granulocyte activation Citation[8]. Activated monocytes express TF on their membrane Citation[71-75] and shed micro-particles containing TF into the plasma Citation[71]. In addition, the lack of association between intra-amniotic infection/inflammation, as well as the placental histologic findings and median maternal plasma concentrations of TF and TFPI, suggest that the procoagulant changes observed in patients with preterm PROM may be due to a systemic rather than a local (i.e. placental, intrauterine) inflammatory process.
The procoagulant activity of immunoreactive TF in the maternal plasma (blood-born TF) is a topic of debate Citation[71,72],Citation[76-79]. In a recent in vitro study, blood-born TF had very little or no procoagulant activity Citation[71]. Moreover, whole blood and plasma clot formation, after inhibition of the contact factor (factor XIIa), was obtained only by adding exogenous, active TF. Of note, only six fentomol of exogenous TF were sufficient for clot formation Citation[71]. On the other hand, it has been proposed that blood-born TF does not initiate the coagulation cascade but rather propagates clot formation by attaching to activated platelets and further enhancing the coagulation process Citation[76-79]. This might be the reason why the administration of anti-TF antibodies has an anti-thrombotic effect without causing severe bleeding; because these antibodies inhibit blood-born TF at concentrations below those needed for inhibiting its hemostatic effect Citation[80]. Moreover, positive immunostaining of arterial thrombosis and atherosclerotic plaques for TF suggests that blood-born TF may participate in the propagation of atherosclerotic plaques and the generation of arterial thrombosis Citation[80,81]. Further, higher concentrations of blood-born TF were predictive of cardiovascular mortality of patients with acute coronary syndrome Citation[82,83]. Therefore, the higher blood-born TF concentration in patients with preterm PROM, compared with those with normal pregnancies, may result from the association between moderate maternal inflammation and an increased activation of the coagulation cascade, whether or not intra-amniotic infection or inflammation occurs.
What is TFPI?
The main physiological inhibitor of the TF pathway of coagulation is TFPI. Two types of TFPI have been described: TFPI-1 and TFPI-2. TFPI-1 directly inhibits factor Xa through the Kunitz-2 domain Citation[84], whereas the inhibition of the FVIIa/TF complex is performed by the Kunitz-1 domain in a factor Xa-dependent manner Citation[20],Citation[85,86]. TFPI-1 is found in the maternal circulation, fetal blood, platelets, endothelial cells and other organs Citation[24,25] and it is the major form of TFPI in the plasma of non-pregnant individuals Citation[87,88].
TFPI-2, first isolated from human placenta as PP5 Citation[32,33], is the major form of TFPI in the placenta Citation[26-31], and it is expressed by the syncytiotrophoblast Citation[35,36]. In the non-pregnant state, TFPI-2 is present mainly in the extracellular matrix and its plasma concentrations are very low Citation[33-35]. Recombinant TFPI-2 inhibits trypsin and factor VIIa activity (in a dose-dependant manner). High concentrations of TFPI-2 are needed for a weak inhibition of factor Xa Citation[89]. Moreover, TFPI-2 does not affect thrombin activity Citation[89]. The administration of heparin reduces the dose of TFPI-2 needed for factor VIIa inhibition, but it does not increase the inhibitory activity of TFPI-2 on factor Xa Citation[89]. In addition to its anticoagulant activity, TFPI-2 has a localised inhibitory activity on serine protease (i.e. trypsin, plasmin, plasma kalikrein) Citation[28],Citation[89-93], as well as the turnover of pro-MMP1 and pro-MMP3 to their active forms Citation[94].
Heparin and low-molecular-weight heparins increase the production and secretion of TFPI by the endothelial cells Citation[95-100], leading to an increase in the plasma concentrations of both types of TFPI Citation[95],Citation[97-112]. Heparin binds factor Xa and TFPI simultaneously, bringing them into proximity that enhances factor Xa inhibition by TFPI Citation[20],Citation[85],Citation[113,114].
What are the changes in TFPI during pregnancy?
During normal pregnancy, the maternal plasma concentration of TFPI-2 increases along gestation reaching a plateau at 36 weeks Citation[33-37] and subsiding after delivery Citation[38]. Twin pregnancies have higher median maternal plasma concentration of TFPI-2 than singleton pregnancies, but the rate of the increase in TFPI-2 during gestation is lower in twins than in singletons Citation[37]. However, the mean maternal plasma concentrations of total TFPI have been reported to increase during the first half of pregnancy until 20 weeks of gestation and then remain relatively constant until term Citation[22]. Conflicting data exists concerning the changes in maternal plasma concentrations during labour. Although some authors described an increase in TFPI plasma concentrations in early stages of labour Citation[87], others reported a decrease in the TFPI plasma concentrations during labour Citation[23].
Decreased median TFPI plasma concentrations and low TFPI/TF ratio in patients with preterm PROM: an additional mechanism for increased thrombin generation?
The procoagulable state reported in preterm PROM may be, in part, due to reduced natural anticoagulant concentrations and not only derived from the higher concentrations of procoagulant proteases (i.e. thrombin). This is supported by the novel finding of this study that patients with preterm PROM have a lower median total maternal plasma TFPI concentration than in women with normal pregnancies. Similar association between low plasma concentrations of TFPI and procoagulant state have been reported in non-pregnant and pregnant women.
In non-pregnant patients: (1) low TFPI concentrations are considered an independent risk factor for deep vein thrombosis Citation[115]; (2) among patients undergoing IVF treatments, those who developed the procoagulable state of ovarian hyper-stimulation syndrome, have lower TFPI plasma concentrations than those who did not Citation[116]; and (3) non-pregnant women with a history of recurrent pregnancy loss Citation[117] and a higher rate of thrombophilic mutation, have lower plasma TFPI concentrations than non-pregnant healthy women.
During pregnancy: (1) pregnant women with vascular complications of pregnancy (pre-eclampsia, eclampsia, placental abruption, fetal growth restriction and fetal demise) have lower placental extract total TFPI concentrations and TFPI mRNA expression than those of women with normal pregnancies Citation[43]; and (2) a different report demonstrated a lower placental immunoreactivity of TFPI-2 in patients with pre-eclampsia but not in patients with fetal growth restriction Citation[118]. Collectively, our results suggest that lower concentrations of total TFPI may contribute to the maternal procoagulant state observed in patients with preterm PROM.
The finding of a significantly lower TFPI/TF ratio in patients with preterm PROM than in women with normal pregnancy is novel and represents the procoagulant state in the maternal plasma of patients with preterm PROM. Immunoreactive TFPI is 500 to 1000 times more abundant in the maternal plasma than TF Citation[119]. Changes in this ratio have been observed in patients with disseminated intravascular coagulation (DIC) Citation[119] and thrombotic thrombocytopenic purpura (TTP) Citation[79]. Moreover, patients with DIC who had a poor outcome also had a higher TF/TFPI ratio Citation[119], whereas patients with TTP had a significant increase in TFPI/TF ratio after treatment; the authors had proposed that this reflects an improvement in the hypercoagulable state associated with TTP Citation[79]. Therefore, the ratio of TFPI/TF can serve as an additional marker for increased activation of coagulation cascade.
In conclusion, preterm PROM is associated with increased median maternal plasma TF and decreased TFPI concentrations. This may result from the moderate systemic inflammatory state associated with preterm PROM but also from activation of the coagulation cascade as reflected by the low TFPI/TF ratio. Moreover, we suggest that measurement of the ratio between TF and its inhibitor (TFPI) may be of benefit in the assessment of hypercoagulable states during pregnancy.
Acknowledgements
This research was supported by the Intramural Research Programme of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Kim Y M, Chaiworapongsa T, Gomez R, Bujold E, Yoon B H, Rotmensch S, Thaler H T, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol 2002; 187: 1137–1142
- Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol 1997; 89: 265–271
- Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim Y M, Bujold E, Edwin S, Yoon B H, Romero R. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2002; 11: 368–373
- Rosen T, Kuczynski E, O'Neill L M, Funai E F, Lockwood C J. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med 2001; 10: 297–300
- Lockwood C J, Toti P, Arcuri F, Paidas M, Buchwalder L, Krikun G, Schatz F. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin-enhanced interleukin-8 expression in term decidua. Am J Pathol 2005; 167: 1443–1449
- Gomez R, Romero R, Nien J K, Medina L, Carstens M, Kim Y M, Chaiworapongsa T, Espinoza J, Gonzalez R. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med 2005; 18: 31–37
- Gomez R, Athayde N, Pacora P, Mazor M, Yoon B H, Romero R. Increased thrombin in intrauterine inflammation. Am J Obstet Gynecol 1998; 178: S62
- Gervasi M T, Chaiworapongsa T, Naccasha N, Pacora P, Berman S, Maymon E, Kim J C, Kim Y M, Yoshimatsu J, Espinoza J, et al. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2002; 11: 171–175
- Osterud B, Bjorklid E. Sources of tissue factor. Semin Thromb Hemost 2006; 32: 11–23
- Mackenzie A P, Schatz F, Krikun G, Funai E F, Kadner S, Lockwood C J. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am J Obstet Gynecol 2004; 191: 1996–2001
- Rosen T, Schatz F, Kuczynski E, Lam H, Koo A B, Lockwood C J. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern Fetal Neonatal Med 2002; 11: 11–17
- Elovitz M A, Ascher-Landsberg J, Saunders T, Phillippe M. The mechanisms underlying the stimulatory effects of thrombin on myometrial smooth muscle. Am J Obstet Gynecol 2000; 183: 674–681
- Elovitz M A, Saunders T, Ascher-Landsberg J, Phillippe M. Effects of thrombin on myometrial contractions in vitro and in vivo. Am J Obstet Gynecol 2000; 183: 799–804
- Elovitz M A, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol 2001; 185: 1059–1063
- Lockwood C J, Krikun G, Schatz F. The decidua regulates hemostasis in human endometrium. Semin Reprod Endocrinol 1999; 17: 45–51
- Lockwood C J, Krikun G, Schatz F. Decidual cell-expressed tissue factor maintains hemostasis in human endometrium. Ann NY Acad Sci 2001; 943: 77–88
- Lockwood C J, Schatz F. A biological model for the regulation of peri-implantational hemostasis and menstruation. J Soc Gynecol Investig 1996; 3: 159–165
- Hahn L. On fibrinolysis and coagulation during parturition and menstruation. Acta Obstet Gynecol Scand Suppl 1974; 28: 7–40
- Major C A, de V M, Lewis D F, Morgan M A. Preterm premature rupture of membranes and abruptio placentae: is there an association between these pregnancy complications. Am J Obstet Gynecol 1995; 172: 672–676
- Broze G J, Jr, Warren L A, Novotny W F, Higuchi D A, Girard J J, Miletich J P. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood 1988; 71: 335–343
- Laskowski M, Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem 1980; 49: 593–626
- Sarig G, Blumenfeld Z, Leiba R, Lanir N, Brenner B. Modulation of systemic hemostatic parameters by enoxaparin during gestation in women with thrombophilia and pregnancy loss. Thromb Haemost 2005; 94: 980–985
- Uszynski M, Zekanowska E, Uszynski W, Kuczynski J. Tissue factor (TF) and tissue factor pathway inhibitor (TFPI) in amniotic fluid and blood plasma: implications for the mechanism of amniotic fluid embolism. Eur J Obstet Gynecol Reprod Biol 2001; 95: 163–166
- Tay S P, Cheong S K, Boo N Y. Circulating tissue factor, tissue factor pathway inhibitor and d-dimer in umbilical cord blood of normal term neonates and adult plasma. Blood Coagul Fibrinolysis 2003; 14: 125–129
- Edstrom C S, Calhoun D A, Christensen R D. Expression of tissue factor pathway inhibitor in human fetal and placental tissues. Early Hum Dev 2000; 59: 77–84
- Hube F, Reverdiau P, Iochmann S, Trassard S, Thibault G, Gruel Y. Demonstration of a tissue factor pathway inhibitor 2 messenger RNA synthesis by pure villous cytotrophoblast cells isolated from term human placentas. Biol Reprod 2003; 68: 1888–1894
- Iino M, Foster D C, Kisiel W. Quantification and characterization of human endothelial cell-derived tissue factor pathway inhibitor-2. Arterioscler Thromb Vasc Biol 1998; 18: 40–46
- Sprecher C A, Kisiel W, Mathewes S, Foster D C. Molecular cloning, expression, and partial characterization of a second human tissue-factor-pathway inhibitor. Proc Natl Acad Sci USA 1994; 91: 3353–3357
- Udagawa K, Miyagi Y, Hirahara F, Miyagi E, Nagashima Y, Minaguchi H, Misugi K, Yasumitsu H, Miyazaki K. Specific expression of PP5/TFPI2 mRNA by syncytiotrophoblasts in human placenta as revealed by in situ hybridization. Placenta 1998; 19: 217–223
- Udagawa K, Yasumitsu H, Esaki M, Sawada H, Nagashima Y, Aoki I, Jin M, Miyagi E, Nakazawa T, Hirahara F, et al. Subcellular localization of PP5/TFPI-2 in human placenta: a possible role of PP5/TFPI-2 as an anti-coagulant on the surface of syncytiotrophoblasts. Placenta 2002; 23: 145–153
- Kamei S, Kazama Y, Kuijper J L, Foster D C, Kisiel W. Genomic structure and promoter activity of the human tissue factor pathway inhibitor-2 gene. Biochim Biophys Acta 2001; 1517: 430–435
- Kisiel W, Sprecher C A, Foster D C. Evidence that a second human tissue factor pathway inhibitor (TFPI-2) and human placental protein 5 are equivalent. Blood 1994; 84: 4384–4385
- Butzow R, Virtanen I, Seppala M, Narvanen O, Stenman U H, Ristimaki A, Bohn H. Monoclonal antibodies reacting with placental protein 5: use in radioimmunoassay, Western blot analysis, and immunohistochemistry. J Lab Clin Med 1988; 111: 249–256
- Chand H S, Foster D C, Kisiel W. Structure, function and biology of tissue factor pathway inhibitor-2. Thromb Haemost 2005; 94: 1122–1130
- Seppala M, Wahlstrom T, Bohn H. Circulating levels and tissue localization of placental protein five (PP5) in pregnancy and trophoblastic disease: absence of PP5 expression in the malignant trophoblast. Int J Cancer 1979; 24: 6–10
- Than G N, Bohn H, Szabo D G. Advances in pregnancy – related protein research. Boca Raton, FL: CRC Press. 1993; 1–333
- Obiekwe B C, Chard T. Placental protein 5: circulating levels in twin pregnancy and some observations on the analysis of biochemical data from multiple pregnancy. Eur J Obstet Gynecol Reprod Biol 1981; 12: 135–141
- Obiekwe B C, Sooby J, Salem H T, Chard T. Placental protein 5: disappearance from the circulation after delivery. Eur J Obstet Gynecol Reprod Biol 1982; 13: 1–5
- Abdel Gader A M, Al-Mishari A A, Awadalla S A, Buyuomi N M, Khashoggi T, Al-Hakeem M. Total and free tissue factor pathway inhibitor in pregnancy hypertension. Int J Gynaecol Obstet 2006; 95: 248–253
- Bellart J, Gilabert R, Angles A, Piera V, Miralles R M, Monasterio J, Cabero L. Tissue factor levels and high ratio of fibrinopeptide A: d-dimer as a measure of endothelial procoagulant disorder in pre-eclampsia. Br J Obstet Gynaecol 1999; 106: 594–597
- Schjetlein R, Abdelnoor M, Haugen G, Husby H, Sandset P M, Wisloff F. Hemostatic variables as independent predictors for fetal growth retardation in preeclampsia. Acta Obstet Gynecol Scand 1999; 78: 191–197
- Di Paolo S, Volpe P, Grandaliano G, Stallone G, Schena A, Greco P, Resta L, Selvaggi L, Cincione R, Schena F P, et al. Increased placental expression of tissue factor is associated with abnormal uterine and umbilical Doppler waveforms in severe preeclampsia with fetal growth restriction. J Nephrol 2003; 16: 650–657
- Aharon A, Lanir N, Drugan A, Brenner B. Placental TFPI is decreased in gestational vascular complications and can be restored by maternal enoxaparin treatment. J Thromb Haemost 2005; 3: 2355–2357
- Alexander G R, Himes J H, Kaufman R B, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996; 87: 163–168
- Redline R W, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta 2005; 26(Suppl A)S114–S117
- Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van V I, Demunck H, Kasper M, Breier G, Evrard P, et al. Role of tissue factor in embryonic blood vessel development. Nature 1996; 383: 73–75
- Erlich J, Parry G C, Fearns C, Muller M, Carmeliet P, Luther T, Mackman N. Tissue factor is required for uterine hemostasis and maintenance of the placental labyrinth during gestation. Proc Natl Acad Sci USA 1999; 96: 8138–8143
- Toomey J R, Kratzer K E, Lasky N M, Stanton J J, Broze G J, Jr. Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood 1996; 88: 1583–1587
- Bugge T H, Xiao Q, Kombrinck K W, Flick M J, Holmback K, Danton M J, Colbert M C, Witte D P, Fujikawa K, Davie E W, et al. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci USA 1996; 93: 6258–6263
- Lockwood C J, Krikun G, Papp C, Toth-Pal E, Markiewicz L, Wang E Y, Kerenyi T, Zhou X, Hausknecht V, Papp Z, et al. The role of progestationally regulated stromal cell tissue factor and type-1 plasminogen activator inhibitor (PAI-1) in endometrial hemostasis and menstruation. Ann NY Acad Sci 1994; 734: 57–79
- Lockwood C J, Krikun G, Rahman M, Caze R, Buchwalder L, Schatz F. The role of decidualization in regulating endometrial hemostasis during the menstrual cycle, gestation, and in pathological states. Semin Thromb Hemost 2007; 33: 111–117
- Kuczynski J, Uszynski W, Zekanowska E, Soszka T, Uszynski M. Tissue factor (TF) and tissue factor pathway inhibitor (TFPI) in the placenta and myometrium. Eur J Obstet Gynecol Reprod Biol 2002; 105: 15–19
- Creter D. Amnioplastin: new reagent for coagulation tests. Lancet 1977; 2: 251
- Omsjo I H, Oian P, Maltau J M, Osterud B. Thromboplastin activity in amniotic fluid. Gynecol Obstet Invest 1985; 19: 1–5
- Lockwood C J, Bach R, Guha A, Zhou X D, Miller W A, Nemerson Y. Amniotic fluid contains tissue factor, a potent initiator of coagulation. Am J Obstet Gynecol 1991; 165: 1335–1341
- Holmes V A, Wallace J M. Haemostasis in normal pregnancy: a balancing act. Biochem Soc Trans 2005; 33: 428–332
- Bellart J, Gilabert R, Miralles R M, Monasterio J, Cabero L. Endothelial cell markers and fibrinopeptide A to d-dimer ratio as a measure of coagulation and fibrinolysis balance in normal pregnancy. Gynecol Obstet Invest 1998; 46: 17–21
- Oian P, Omsjo I, Maltau J M, Osterud B. Increased sensitivity to thromboplastin synthesis in blood monocytes from pre-eclamptic patients. Br J Obstet Gynaecol 1985; 92: 511–517
- Holmes V A, Wallace J M, Gilmore W S, McFaul P, Alexander H D. Tissue factor expression on monocyte subpopulations during normal pregnancy. Thromb Haemost 2002; 87: 953–958
- Stephenson C D, Lockwood C J, Ma Y, Guller S. Thrombin-dependent regulation of matrix metalloproteinase (MMP)- 9 levels in human fetal membranes. J Matern Fetal Neonatal Med 2005; 18: 17–22
- Arias F, Rodriquez L, Rayne S C, Kraus F T. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol 1993; 168: 585–591
- Schatz F, Krikun G, Caze R, Rahman M, Lockwood C J. Progestin-regulated expression of tissue factor in decidual cells: implications in endometrial hemostasis, menstruation and angiogenesis. Steroids 2003; 68: 849–860
- Romero R, Gonçalves L F, Chaiworapongsa T, Kusanovic J P, Espinoza J. Mechanisms of preterm labor and preterm premature rupture of the membranes. 2006; 1379–1393
- Esmon C T. The interactions between inflammation and coagulation. Br J Haematol 2005; 131: 417–430
- Levi M, van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med 2005; 15: 254–259
- Levi M, van der Poll T, ten Cate H. Tissue factor in infection and severe inflammation. Semin Thromb Hemost 2006; 32: 33–39
- Haas S L, Jesnowski R, Steiner M, Hummel F, Ringel J, Burstein C, Nizze H, Liebe S, Lohr J M. Expression of tissue factor in pancreatic adenocarcinoma is associated with activation of coagulation. World J Gastroenterol 2006; 12: 4843–4849
- Sawa H, Ueda T, Takeyama Y, Yasuda T, Matsumura N, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Elevation of plasma tissue factor levels in patients with severe acute pancreatitis. J Gastroenterol 2006; 41: 575–581
- Naumnik B, Borawski J, Chyczewski L, Pawlak K, Mysliwiec M. Tissue factor and its inhibitor in human non-crescentic glomerulonephritis – immunostaining vs. plasma and urinary levels. Nephrol Dial Transplant 2006; 21: 3450–3457
- Roldan V, Marin F, Blann A. Soluble E-selectin, interleukin-6 and tissue factor in two cases of meningococcal septicaemia. Blood Coagul Fibrinolysis 2004; 15: 179–182
- Butenas S, Bouchard B A, Brummel-Ziedins K E, Parhami-Seren B, Mann K G. Tissue factor activity in whole blood. Blood 2005; 105: 2764–2770
- Osterud B. Cellular interactions in tissue factor expression by blood monocytes. Blood Coagul Fibrinolysis 1995; 6(Suppl 1)S20–S25
- Rivers R P, Hathaway W E, Weston W L. The endotoxin-induced coagulant activity of human monocytes. Br J Haematol 1975; 30: 311–316
- Bach R R, Moldow C F. Mechanism of tissue factor activation on HL-60 cells. Blood 1997; 89: 3270–3276
- Egorina E M, Sovershaev M A, Bjorkoy G, Gruber F X, Olsen J O, Parhami-Seren B, Mann K G, Osterud B. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol 2005; 25: 1493–1498
- Balasubramanian V, Grabowski E, Bini A, Nemerson Y. Platelets, circulating tissue factor, and fibrin colocalize in ex vivo thrombi: real-time fluorescence images of thrombus formation and propagation under defined flow conditions. Blood 2002; 100: 2787–2792
- Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie B C, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood 2004; 104: 3190–3197
- Balasubramanian V, Vele O, Nemerson Y. Local shear conditions and platelet aggregates regulate the incorporation and activity of circulating tissue factor in ex vivo thrombi. Thromb Haemost 2002; 88: 822–826
- Kobayashi M, Wada H, Wakita Y, Shimura M, Nakase T, Hiyoyama K, Nagaya S, Minami N, Nakano T, Shiku H. Decreased plasma tissue factor pathway inhibitor levels in patients with thrombotic thrombocytopenic purpura. Thromb Haemost 1995; 73: 10–14
- Rauch U, Nemerson Y. Circulating tissue factor and thrombosis. Curr Opin Hematol 2000; 7: 273–277
- Westmuckett A D, Lupu C, Goulding D A, Das S, Kakkar V V, Lupu F. In situ analysis of tissue factor-dependent thrombin generation in human atherosclerotic vessels. Thromb Haemost 2000; 84: 904–911
- Campo G, Valgimigli M, Ferraresi P, Malagutti P, Baroni M, Arcozzi C, Gemmati D, Percoco G, Parrinello G, Ferrari R, et al. Tissue factor and coagulation factor VII levels during acute myocardial infarction: association with genotype and adverse events. Arterioscler Thromb Vasc Biol 2006; 26: 2800–2806
- Morange P E, Blankenberg S, Alessi M C, Bickel C, Rupprecht H J, Schnabel R, Lubos E, Munzel T, Peetz D, Nicaud V, et al. Prognostic value of plasma tissue factor and tissue factor pathway inhibitor for cardiovascular death in patients with coronary artery disease: the AtheroGene study. J Thromb Haemost 2007; 5: 475–482
- Girard T J, Warren L A, Novotny W F, Likert K M, Brown S G, Miletich J P, Broze G J, Jr. Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature 1989; 338: 518–520
- Broze G J, Jr, Girard T J, Novotny W F. Regulation of coagulation by a multivalent Kunitz-type inhibitor. Biochemistry 1990; 29: 7539–7546
- Kaiser B, Hoppensteadt D A, Jeske W, Wun T C, Fareed J. Inhibitory effects of TFPI on thrombin and factor Xa generation in vitro– modulatory action of glycosaminoglycans. Thromb Res 1994; 75: 609–616
- Novotny W F, Brown S G, Miletich J P, Rader D J, Broze G J, Jr. Plasma antigen levels of the lipoprotein-associated coagulation inhibitor in patient samples. Blood 1991; 78: 387–393
- Butzow R, Alfthan H, Stenman U H, Suikkari A M, Bohn H, Seppala M. Immunofluorometric demonstration and quantification of placental protein 5 in the absence of pregnancy. Clin Chem 1988; 34: 1591–1593
- Petersen L C, Sprecher C A, Foster D C, Blumberg H, Hamamoto T, Kisiel W. Inhibitory properties of a novel human Kunitz-type protease inhibitor homologous to tissue factor pathway inhibitor. Biochemistry 1996; 35: 266–272
- Rao C N, Peavey C L, Liu Y Y, Lapiere J C, Woodley D T. Partial characterization of matrix-associated serine protease inhibitors from human skin cells. J Invest Dermatol 1995; 104: 379–383
- Rao C N, Liu Y Y, Peavey C L, Woodley D T. Novel extracellular matrix-associated serine proteinase inhibitors from human skin fibroblasts. Arch Biochem Biophys 1995; 317: 311–314
- Rao C N, Gomez D E, Woodley D T, Thorgeirsson U P. Partial characterization of novel serine proteinase inhibitors from human umbilical vein endothelial cells. Arch Biochem Biophys 1995; 319: 55–62
- Rao C N, Reddy P, Liu Y, O'Toole E, Reeder D, Foster D C, Kisiel W, Woodley D T. Extracellular matrix-associated serine protease inhibitors (Mr 33,000, 31,000, and 27,000) are single-gene products with differential glycosylation: cDNA cloning of the 33-kDa inhibitor reveals its identity to tissue factor pathway inhibitor-2. Arch Biochem Biophys 1996; 335: 82–92
- Rao C N, Mohanam S, Puppala A, Rao J S. Regulation of ProMMP-1 and ProMMP-3 activation by tissue factor pathway inhibitor-2/matrix-associated serine protease inhibitor. Biochem Biophys Res Commun 1999; 255: 94–98
- Li Y, Rodriquez M, Spencer F A, Becker R C. Comparative effects of unfractionated heparin and low molecular weight heparin on vascular endothelial cell tissue factor pathway inhibitor release: a model for assessing intrinsic thromboresistance. J Thromb Thrombolysis 2002; 14: 123–129
- Hansen J B, Svensson B, Olsen R, Ezban M, Osterud B, Paulssen R H. Heparin induces synthesis and secretion of tissue factor pathway inhibitor from endothelial cells in vitro. Thromb Haemost 2000; 83: 937–943
- Menabawey M, Silman R, Rice A, Chard T. Dramatic increase of placental protein 5 levels following injection of small doses of heparin. Br J Obstet Gynaecol 1985; 92: 207–210
- Meisser A, Bischof P, Bohn H. Placental protein 5 (PP5) inhibits thrombin-induced coagulation of fibrinogen. Arch Gynecol 1985; 236: 197–201
- Nisbet A D, Brenner R D, Horne C H, Bohn H. Placental protein 5 (PP5) in pregnancy and malignant disease: the influence of heparin binding. Clin Chim Acta 1982; 119: 21–29
- Jones G R, Davey M W, Sinosich M, Grudzinskas J G. Specific interaction between placental protein 5 and heparin. Clin Chim Acta 1981; 110: 65–70
- Jeske W, Fareed J. Pharmacodynamic considerations in the selection of dosage of tinzaparin for various indications: experimental studies in primates. Semin Thromb Hemost 2004; 30(Suppl 1)41–47
- Kemme M J, Burggraaf J, Schoemaker R C, Kluft C, Cohen A F. Quantification of heparin-induced TFPI release: a maximum release at low heparin dose. Br J Clin Pharmacol 2002; 54: 627–634
- Kaiser B, Hoppensteadt D A, Fareed J. Tissue factor pathway inhibitor: an update of potential implications in the treatment of cardiovascular disorders. Expert Opin Investig Drugs 2001; 10: 1925–1935
- Sandset P M, Bendz B, Hansen J B. Physiological function of tissue factor pathway inhibitor and interaction with heparins. Haemostasis 2000; 30(Suppl 2)48–56
- Kaiser B, Glusa E, Hoppensteadt D A, Breddin H K, Amiral J, Fareed J. A supersulfated low-molecular-weight heparin (IK-SSH) increases plasma levels of free and total tissue factor pathway inhibitor after intravenous and subcutaneous administration in humans. Blood Coagul Fibrinolysis 1998; 9: 517–523
- Hansen J B, Sandset P M, Huseby K R, Huseby N E, Bendz B, Ostergaard P, Nordoy A. Differential effect of unfractionated heparin and low molecular weight heparin on intravascular tissue factor pathway inhibitor: evidence for a difference in antithrombotic action. Br J Haematol 1998; 101: 638–646
- Jeske W, Hoppensteadt D, Klauser R, Kammereit A, Eckenberger P, Haas S, Wyld P, Fareed J. Effect of repeated Aprosulate and Enoxaparin administration on tissue factor pathway inhibitor antigen levels. Blood Coagul Fibrinolysis 1995; 6: 119–124
- Jesty J, Wun T C, Lorenz A. Kinetics of the inhibition of factor Xa and the tissue factor-factor VIIa complex by the tissue factor pathway inhibitor in the presence and absence of heparin. Biochemistry 1994; 33: 12686–12694
- Huang Z F, Wun T C, Broze G J, Jr. Kinetics of factor Xa inhibition by tissue factor pathway inhibitor. J Biol Chem 1993; 268: 26950–26955
- Ostergaard P, Nordfang O, Petersen L C, Valentin S, Kristensen H. Is tissue factor pathway inhibitor involved in the antithrombotic effect of heparins?. Biochemical considerations. Haemostasis 1993; 23(Suppl 1)107–111
- Wun T C. Lipoprotein-associated coagulation inhibitor (LACI) is a cofactor for heparin: synergistic anticoagulant action between LACI and sulfated polysaccharides. Blood 1992; 79: 430–438
- Lindahl A K, Abildgaard U, Larsen M L, Aamodt L M, Nordfang O, Beck T C. Extrinsic pathway inhibitor (EPI) and the post-heparin anticoagulant effect in tissue thromboplastin induced coagulation. Thromb Res Suppl 1991; 14: 39–48
- Wesselschmidt R, Likert K, Huang Z, MacPhail L, Broze G J, Jr. Structural requirements for tissue factor pathway inhibitor interactions with factor Xa and heparin. Blood Coagul Fibrinolysis 1993; 4: 661–669
- Broze G J, Jr. Tissue factor pathway inhibitor and the revised theory of coagulation. Annu Rev Med 1995; 46: 103–112
- Dahm A, Van H V, Bendz B, Rosendaal F, Bertina R M, Sandset P M. Low levels of tissue factor pathway inhibitor (TFPI) increase the risk of venous thrombosis. Blood 2003; 101: 4387–4392
- Rogolino A, Coccia M E, Fedi S, Gori A M, Cellai A P, Scarselli G F, Prisco D, Abbate R. Hypercoagulability, high tissue factor and low tissue factor pathway inhibitor levels in severe ovarian hyperstimulation syndrome: possible association with clinical outcome. Blood Coagul Fibrinolysis 2003; 14: 277–282
- Gardiner C, Cohen H, Austin S K, Machin S J, Mackie I J. Pregnancy loss, tissue factor pathway inhibitor deficiency and resistance to activated protein C. J Thromb Haemost 2006; 4: 2724–2726
- Ogawa M, Yanoma S, Nagashima Y, Okamoto N, Ishikawa H, Haruki A, Miyagi E, Takahashi T, Hirahara F, Miyagi Y. Paradoxical discrepancy between the serum level and the placental intensity of PP5/TFPI-2 in preeclampsia and/or intrauterine growth restriction: possible interaction and correlation with glypican-3 hold the key. Placenta 2007; 28: 224–232
- Shimura M, Wada H, Wakita Y, Nakase T, Hiyoyama K, Nagaya S, Mori Y, Shiku H. Plasma tissue factor and tissue factor pathway inhibitor levels in patients with disseminated intravascular coagulation. Am J Hematol 1997; 55: 169–174