Abstract
Objective. Hemoglobin and its catabolic products have been associated with amniotic fluid (AF) discoloration and intra-amniotic infection/inflammation (IAI). However, the origin of AF hemoglobin (maternal or fetal) has not been determined. The aims of this study were to determine if fetal hemoglobin can be detected in AF obtained from normal pregnancies, and whether there is an association between AF fetal hemoglobin concentrations and gestational age, spontaneous labor (term and preterm), preterm prelabor rupture of membranes (PPROM) and IAI.
Study design. This cross-sectional study included pregnant women in the following groups: (1) mid-trimester (n = 60); (2) term not in labor (n = 21); (3) term in labor (n = 47); (4) spontaneous preterm labor with intact membranes (PTL) without IAI who delivered at term (n = 89); (5) PTL without IAI who delivered preterm (n = 74); (6) PTL with IAI (n = 78); (7) PPROM with (n = 48) and (8) without IAI (n = 48). AF fetal hemoglobin concentrations were determined by ELISA. Non-parametric statistics were used for analyses.
Results. (1) Fetal hemoglobin was detected in 80.4% of all AF samples; (2) women at term not in labor had a higher median AF fetal hemoglobin concentration than those at mid-trimester (p = 0.008); (3) labor at term was not associated with a significant difference in the median AF fetal hemoglobin concentration; (4) the median AF fetal hemoglobin concentration was not significantly different among the three PTL groups or between the PPROM groups; (5) women with PTL and IAI had a lower AF fetal hemoglobin percentage of the total hemoglobin than those without IAI who delivered preterm (p = 0.03) or at term (p < 0.001); (6) The median AF fetal hemoglobin concentration was higher in pregnancies complicated with PTL or PPROM than in women at term (p < 0.001 for all comparison).
Conclusions. (1) The concentration of immunoreactive AF fetal hemoglobin increases with gestational age; (2) the median AF fetal hemoglobin concentration is higher in pregnancies complicated with PTL or PPROM than in term pregnancies; (3) among women with PTL or PPROM, the AF fetal hemoglobin concentrations were not associated with IAI; (4) however, women with PTL and IAI had a lower percentage of AF fetal hemoglobin of the total hemoglobin than those without IAI, suggesting different mechanisms of disease.
Introduction
Hemoglobin and its catabolic products have been associated with discolored amniotic fluid (AF) Citation[1],Citation[2], which is considered as a risk factor for intra-amniotic infection and/or inflammation (IAI) Citation[3]. A putative explanation is that even small amounts of intra-amniotic bleeding can serve as a medium for bacterial growth Citation[4],Citation[5], and may increase the risk for intra-amniotic infection and subsequent preterm parturition. Indeed, we recently reported that IAI is associated with elevated median AF total hemoglobin concentration in patients with spontaneous preterm labor and intact membranes (PTL), as well as in those with preterm prelabor rupture of membranes (preterm PROM) Citation[6]. However, it is unclear if the origin of the detected hemoglobin is maternal or fetal.
Gomez et al. Citation[7] proposed that a sub-clinical intra-uterine infection can cause deciduitis and decidual bleeding, leading to the clinical manifestation of vaginal bleeding and subsequently to preterm PROM and early preterm delivery. Thus, we hypothesised that the main origin of AF hemoglobin detected in pregnancies complicated with IAI, as well as in those with spontaneous labor at term is of maternal origin Citation[6]. Indeed, less than 1% of the hemoglobin in the normal adult corresponds to the fraction of hemoglobin F (α2γ2), also known as fetal hemoglobin Citation[8],Citation[9], whereas in the fetal circulation the majority of hemoglobin is hemoglobin F Citation[9]. Therefore, determination of fetal hemoglobin concentrations in AF can assist in determining the origin of the total hemoglobin in the amniotic cavity Citation[6].
The aims of this study were to determine: (1) if fetal hemoglobin can be detected in normal pregnancies; and (2) whether there is an association between the fetal hemoglobin concentrations and advancing gestational age, spontaneous labor (term and preterm), preterm PROM and the presence of IAI.
Materials and methods
Study design and population
This cross-sectional study was conducted by searching our clinical database and bank of biological specimens, and included 465 pregnant women in the following groups: (1) women in the mid-trimester of pregnancy (14–18 weeks) who underwent amniocentesis for genetic indications and delivered a healthy neonate at term (n = 60); (2) normal pregnant women at term not in labor (n = 21) and (3) at term in spontaneous labor (n = 47); (4) women with an episode of PTL without IAI who delivered at term (n = 89); (5) PTL without IAI who delivered preterm (n = 74); (6) PTL with IAI (n = 78); (7) women with preterm PROM without IAI (n = 48); and (8) preterm PROM with IAI (n = 48).
All women provided written informed consent prior to the collection of AF. The collection of AF and its utilisation for research purposes was approved by the Institutional Review Boards of the participating institutes and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, (NICHD/NIH/DHHS). Many of these samples have been used previously to study the biology of inflammation, hemostasis, angiogenesis regulation and growth factors, as well as for measurement of the total hemoglobin concentration, in normal pregnant women and those with pregnancy complications.
Clinical definitions
Pregnant women were considered to have a normal pregnancy if they did not have obstetrical complications, and delivered a term (≥37 weeks) neonate with appropriate for gestational age birthweight Citation[10],Citation[11] without complications. Spontaneous PTL was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 min associated with cervical changes before 37 completed weeks of gestation that required hospitalisation. Preterm PROM was diagnosed by sterile speculum examination confirming pooling of AF in the vagina in association with nitrazine and ferning tests when necessary, before 37 weeks of gestation and in the absence of labor. Women at term in labor consisted of women who were suspected to have PTL because of uncertain dates and had an amniocentesis for the assessment of fetal lung maturity and microbial invasion of the amniotic cavity. If analysis of AF was consistent with maturity, tocolysis was not used. However, if they delivered a baby larger than 2500 grams without complications of prematurity, they were considered to represent patients in spontaneous labor at term. Intra-amniotic infection was defined as a positive AF culture for micro-organisms. Intra-amniotic inflammation was diagnosed by an AF interleukin (IL)-6 concentration >2.6 ng/mL Citation[12].
AF sample collection
Ultrasound guided transabdominal amniocenteses were performed for evaluation of microbial status of the amniotic cavity and/or assessment of fetal lung maturity. AF white blood cell (WBC) count, glucose concentration and Gram-stain were performed shortly after collection, and AF was cultured for aerobic/anaerobic bacteria and genital mycoplasmas (Ureaplasma urealyticum and Mycoplasma hominis). The results of these tests were used for subsequent clinical management. AF not required for clinical assessment was centrifuged for 10 min at 4°C and the supernatant was aliquoted and stored at −70°C until analysis. Mid-trimester samples were not evaluated for infection, but intra-amniotic inflammation was assessed by measuring IL-6 concentration, and all mid-trimester samples included in this study had an AF IL-6 concentration <2.6 ng/mL Citation[12].
Determination of fetal hemoglobin concentration in AF
AF concentration of human fetal hemoglobin was determined by sensitive enzyme-linked immunoassays (Bethyl Laboratories, Montgomery, TX USA). The fetal hemoglobin immunoassay was validated for human AF in our laboratory prior to the conduction of this study, including spike and recovery experiments which produced parallel curves indicating that AF constituents did not interfere with antigen-antibody binding in this assay. Immunoassays were carried out according to manufacturer recommendations. AF samples were incubated in duplicate wells of the micro titer plates pre-coated with an antibody specific for the fetal hemoglobin. During this incubation, the fetal hemoglobin present in the standards or AF samples was bound by the immobilised antibodies in the respective assay plates. After repeated washing and aspiration to remove all unbound substances, an enzyme-linked polyclonal antibody specific for the analyte was added to the wells of the assay plates. Unbound enzyme conjugate was removed by repeated washing and a substrate solution was added to the wells of the assay plates and colour developed in proportion to the amount of the fetal hemoglobin bound in the initial step. Colour development was stopped with the addition of an acid solution and the intensity of colour was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA USA). The concentrations of fetal hemoglobin in AF samples were determined by interpolation from individual standard curves. The calculated inter- and intra-assay coefficients of variation for fetal hemoglobin immunoassays in our laboratory were 2.5% and 2%, respectively. The sensitivity for the fetal hemoglobin assay was calculated to be 3.48 ng/mL.
Statistical analysis
Shapiro-Wilk and Kolmogorov-Smirnov tests were used to test for normal distribution of the data. Because the AF fetal hemoglobin concentrations were not normally distributed, non-parametric tests were used for analyses. Correlation between continuous variables was assessed by the Spearman's rank test. Comparisons between proportions were performed with the Chi-square test. Kruskal-Wallis with post-hoc tests (Mann–Whitney U) were used for continuous variables. Analysis of covariance (ANCOVA) was performed to investigate the association between the sub-groups of PTL and preterm PROM and the AF fetal hemoglobin concentration, gestational age at amniocentesis and sample storage time. A p-value of <0.05 was considered statistically significant. Statistical analysis was performed with SPSS package version 14 (SPSS, Chicago, IL, USA).
Results
Demographic and clinical characteristics
displays the median gestational age at amniocentesis among the study groups and the percentage of samples with measurable fetal hemoglobin, which was detected in 80.4% (374/465) of all AF samples, but in only 31.7% (19/60) of mid-trimester samples. and display the demographic and clinical characteristics of patients with spontaneous PTL and women with preterm PROM, respectively. The median gestational age at amniocentesis differ significantly among the sub-groups of PTL (Kruskal-Wallis, p = 0.002; ). Patients with PTL and IAI had a lower median gestational age at amniocentesis than those who delivered preterm without IAI (p = 0.001) and than those who delivered at term (p = 0.005). Similarly, among patients with preterm PROM, those with IAI had a lower median gestational age at amniocentesis than those without IAI (p = 0.001, ). Among patients with PTL, those who delivered preterm (with or without IAI) were more likely to have a history of vaginal bleeding during pregnancy that those who delivered at term (p = 0.04; ).
Table I. Gestational age at amniocentesis and the proportion of samples that had detectable amniotic fluid fetal hemoglobin concentration in each group.
Table II. Clinical and demographic characteristics of women presenting with spontaneous preterm labor.
Table III. Clinical and demographic characteristics of women presenting with preterm PROM.
AF fetal hemoglobin concentrations in mid-trimester and term patients
Women at term not in labor had a higher median AF fetal hemoglobin concentration than patients at mid-trimester [term not in labor: 19.5 ng/mL, interquartile range (IQR): 0–49 vs. mid-trimester: 0.0 ng/mL, IQR: 0–19.9, p < 0.001; ]. Among patients at term, the median AF fetal hemoglobin concentration did not differ significantly between women not in labor and those in labor (19.5 ng/mL, IQR: 0–49 vs. 19.1 ng/mL, IQR: 0–36.2, respectively; p = 0.4).
Figure 1. AF concentration of fetal hemoglobin in normal pregnancies in the mid-trimester and at term, in labor and not in labor. The median AF fetal hemoglobin concentration was significantly higher in women at term not in labor than in women in the mid-trimester [19.5 ng/mL, IQR: 0–49 vs. 0.0 ng/mL, IQR: 0–19.9; p < 0.001]. Women at term, whether in labor or not in labor, had a similar median AF fetal hemoglobin concentration (term in labor; 19.1 ng/mL, IQR: 0–36.2, p = 0.4).
![Figure 1. AF concentration of fetal hemoglobin in normal pregnancies in the mid-trimester and at term, in labor and not in labor. The median AF fetal hemoglobin concentration was significantly higher in women at term not in labor than in women in the mid-trimester [19.5 ng/mL, IQR: 0–49 vs. 0.0 ng/mL, IQR: 0–19.9; p < 0.001]. Women at term, whether in labor or not in labor, had a similar median AF fetal hemoglobin concentration (term in labor; 19.1 ng/mL, IQR: 0–36.2, p = 0.4).](/cms/asset/e447bd26-e77a-4fa6-b676-bc78525e2b94/ijmf_a_357996_f0001_b.gif)
Changes in AF fetal hemoglobin concentrations in patients with spontaneous PTL or preterm PROM
Among women with PTL, the median AF fetal hemoglobin concentration did not differ significantly between the three subgroups (PTL without IAI who delivered at term: 134.2 ng/mL, IQR: 56.3–255.7 vs. PTL without IAI who delivered preterm: 109.6 ng/mL, IQR: 42.0–247.1 vs. PTL with IAI: 114.6 ng/mL, IQR: 33.2–283.9; Kruskal-Wallis, p = 0.7; ). Similarly, among women with preterm PROM, no significant differences were found among patients without IAI and those with IAI (without IAI: 109.7 ng/mL, IQR: 51.4–293.1 and with IAI: 148.4 ng/mL, IQR: 82.8–811.1; p = 0.07, ). However, the median AF fetal hemoglobin concentration was significantly higher in pregnancies complicated by either PTL or preterm PROM (with or without IAI) than in pregnant women at term, either in labor or not in labor (p < 0.001 for all comparisons).
Figure 2. AF concentration of fetal hemoglobin among women with spontaneous PTL and intact membranes. The median AF concentration of fetal hemoglobin was not significantly different in patients with IAI or without IAI who delivered preterm or at term [with IAI: 114.6 ng/mL, IQR: 33.2–283.9, without IAI delivered preterm: 109.6 ng/mL, IQR: 42–247.1, delivered at term: 134.2 ng/mL, IQR: 56.3–255.7, p = 0.7].
![Figure 2. AF concentration of fetal hemoglobin among women with spontaneous PTL and intact membranes. The median AF concentration of fetal hemoglobin was not significantly different in patients with IAI or without IAI who delivered preterm or at term [with IAI: 114.6 ng/mL, IQR: 33.2–283.9, without IAI delivered preterm: 109.6 ng/mL, IQR: 42–247.1, delivered at term: 134.2 ng/mL, IQR: 56.3–255.7, p = 0.7].](/cms/asset/056ce959-6198-430b-b66d-f1eb32a13056/ijmf_a_357996_f0002_b.gif)
Figure 3. AF concentration of fetal hemoglobin in women with preterm prelabor rupture of the membranes (preterm PROM). The median AF concentration of fetal hemoglobin was not significantly different among patients with preterm PROM, with and without IAI [preterm PROM with IAI: 148.4 ng/mL, IQR: 82.8–811.1 vs. without IAI: 109.7 ng/mL, IQR: 51.4–293.1; p = 0.07].
![Figure 3. AF concentration of fetal hemoglobin in women with preterm prelabor rupture of the membranes (preterm PROM). The median AF concentration of fetal hemoglobin was not significantly different among patients with preterm PROM, with and without IAI [preterm PROM with IAI: 148.4 ng/mL, IQR: 82.8–811.1 vs. without IAI: 109.7 ng/mL, IQR: 51.4–293.1; p = 0.07].](/cms/asset/cb15800a-4469-4282-9df1-8ae0619959f0/ijmf_a_357996_f0003_b.gif)
Fetal hemoglobin percentage of the total hemoglobin
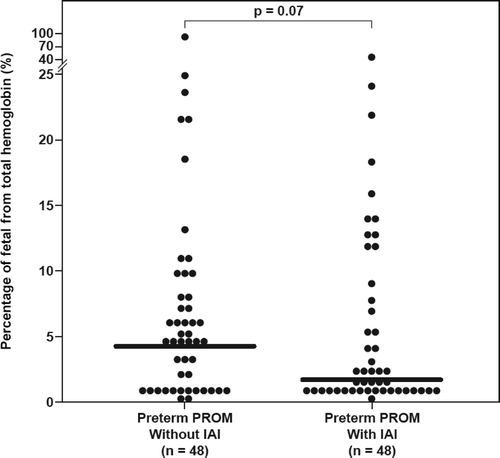
Recently, we reported the AF concentrations of total hemoglobin in these patients Citation[6]. To study the origin of the hemoglobin detected Citation[6], the percentage of fetal hemoglobin of the total hemoglobin was calculated in each sample, and the following results were found: (1) the median AF fetal hemoglobin percentage of the total hemoglobin concentration was lower in women at term in labor than those not in labor (0.2%, IQR: 0–1.1 vs. 2.2%, IQR: 0–7.1; p = 0.03); (2) the median AF fetal hemoglobin percentage of the total hemoglobin differed significantly among the three groups of patients with spontaneous PTL (Kruskal-Wallis, p = 0.001). Women with PTL and IAI had a lower median AF fetal hemoglobin percentage of the total hemoglobin than those without IAI who delivered preterm (2.8%, IQR: 1.2–7.4 vs. 5.6%, IQR: 1.8–13.5; p = 0.03) and than those with PTL who delivered at term (7.3%, IQR: 3–15.4; p < 0.001) (). There was no significant difference in the median AF fetal hemoglobin percentage of the total hemoglobin in patients with PTL without IAI who delivered preterm or at term (p = 0.3); (3) among women with preterm PROM, the median AF fetal percentage of the total hemoglobin did not differ significantly between patients with and without IAI (1.8%, IQR: 0.5–8.4 vs. 4.3%, IQR: 0.7–9, respectively; p = 0.07, ).
Figure 4. The fetal hemoglobin percentage of the total hemoglobin detected in AF among women with spontaneous PTL and intact membranes. The median AF fetal hemoglobin percentage of the total hemoglobin was lower in women with IAI than those without IAI who delivered preterm (2.78%, IQR: 1.18–7.44 vs. 5.61%, IQR: 1.76–13.52; p = 0.03) or at term (7.33%, IQR: 3–15.4; p < 0.001). There was no significant difference in the median AF fetal hemoglobin percentage of the total hemoglobin in those without IAI who delivered preterm or at term (p = 0.3).
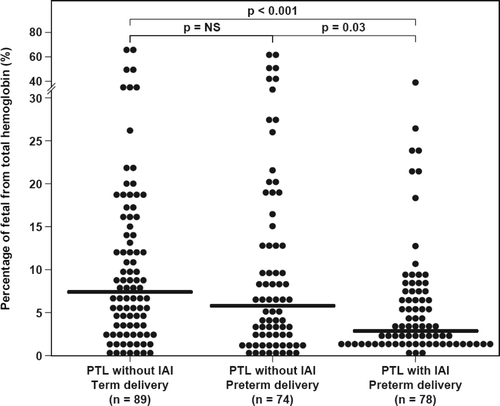
Figure 5. The fetal hemoglobin percentage of the total hemoglobin detected in AF among women with preterm prelabor rupture of the membranes (PPROM). The median AF fetal percentage of the total hemoglobin did not differ significantly in women with and without IAI (1.76%, IQR: 0.46–8.37 vs. 4.31%, IQR: 0.72–8.96, respectively; p = 0.07).
Discussion
Principal findings of the study
(1) Fetal hemoglobin was detected in 80.4% of all AF samples, but in only 31.7% of mid-trimester samples; (2) the median AF fetal hemoglobin concentration was higher in pregnancies complicated by PTL or preterm PROM than in term pregnancies; (3) there were no significant differences in the median AF fetal hemoglobin concentrations among patients with and without IAI (regardless of the membrane status); (4) women with PTL and IAI had a lower AF fetal hemoglobin percentage of the total hemoglobin when compared to those without IAI; and (5) similarly, women at term in labor had a lower median fetal hemoglobin percentage than those at term not in labor.
Potential origins of the detectable fetal hemoglobin in the AF
Normal adult hemoglobin consists of approximately 96–98% of hemoglobin A (α2β2), less than 2% of hemoglobin A2 (α2δ2) and less than 1% of fetal hemoglobin (hemoglobin F, α2γ2) Citation[13]. However, up to 2% of hemoglobin F in the adult blood is considered normal Citation[8],Citation[13], whereas in the fetus the majority is fetal hemoglobin Citation[9]. At birth, 60–80% of the neonatal hemoglobin is of the fetal type Citation[8],Citation[14], and after birth, most of the fetal hemoglobin is replaced by the adult type, being less than 10% by 4 months of age Citation[14].
In the study presented herein, we identified fetal hemoglobin in 80.4% of the AF samples using an immunoassay specific for fetal hemoglobin. Because hemoglobin F is present in both maternal and fetal circulations, the origin of AF fetal hemoglobin could not be ascertained.
Sources for the detected hemoglobin in AF include contamination during amniocentesis Citation[15] (maternal or fetal); however, maternal or placental injury at the time of amniocentesis is relatively rare, ranging from 0.3% to 10.8%, whereas fetal injury rates range from 0.6% to 2%Citation[16]. Although we cannot completely rule out the possibility of contamination during amniocentesis, the detection of fetal hemoglobin in most of our AF samples makes the possibility unlikely.
Another source for hemoglobin in AF can be intra-amniotic bleeding prior to amniocentesis that originated either from the maternal Citation[17-19] or fetal Citation[20] circulation. Maternal intra-amniotic bleeding has been reported in cases of placental abruption Citation[21],Citation[22], circumvallate placenta Citation[17], as well as PTL Citation[17],Citation[18] with clinical Citation[23] or histological chorioamnionitis Citation[18]. Intra-amniotic bleeding of fetal origin has been associated with a sacrococcygeal teratoma Citation[20] and fetal transfusion Citation[24]. Of note, all the case reports above mentioned were acute events with a noticeable amount of intra-amniotic maternal or fetal bleeding, leading to maternal or fetal compromise. A more subtle or chronic intra-amniotic bleed (maternal/fetal) may remain sub-clinical Citation[15].
Hyperechogenic fetal bowel can be associated with an asymptomatic intra-amniotic bleeding Citation[24-26]. Indeed, an association between fetal small bowel hyperechogenicity and the presence of heme pigments in AF retrieved during the second trimester was described Citation[24-26]; and heme pigments were also detected in 5% of the fetuses without echogenic bowel Citation[26]. Interestingly, echogenic bowel was diagnosed in 25% (7/28) of fetuses within the first 12 h after an episode of intra-amniotic fetal bleeding (diagnosed by follow-up ultrasound after fetal transfusion), but in only three fetuses it persisted for 4 weeks Citation[24]. These studies indicate that intra-amniotic bleeding could be sub-clinical.
Fetal hemoglobin concentration in AF is not associated with IAI
In the study presented herein, women with spontaneous PTL or preterm PROM, with or without IAI, had a significantly higher median AF fetal hemoglobin concentration than normal pregnant women at term. However, among patients with PTL or preterm PROM, there were no significant differences in the median AF fetal hemoglobin concentration between those with or without IAI. This is in contrast to the increased AF total hemoglobin concentrations in pregnancies complicated with IAI Citation[6]. This observation suggests that the main source of fetal hemoglobin in cases of PTL with IAI is of maternal origin, and the detected fetal hemoglobin represents the normal percentage of hemoglobin F in the maternal blood. Indeed, Gomez et al. Citation[7] proposed that a sub-clinical intrauterine infection can cause deciduitis and decidual bleeding leading to the clinical manifestation of vaginal bleeding and subsequent adverse pregnancy outcomes. Moreover, among patients with PTL, we found a lower median fetal hemoglobin percentage of the total hemoglobin in women with IAI compared to those without IAI and a similar trend, although not statistically significant, was found among patients with preterm PROM. Collectively, these findings support the hypothesis that maternal origin is the major contributor to the detected hemoglobin in patients with IAI.
Fetal hemoglobin in PTL or preterm PROM
PTL and preterm PROM are syndromic in nature, resulting from IAI as well as other underlying mechanisms Citation[27-29]. Among patients with spontaneous PTL, the median AF fetal hemoglobin percentage of the total hemoglobin was significantly higher in women with PTL who delivered preterm or at term without IAI than those with IAI. Furthermore, the median percentage of fetal hemoglobin of the total hemoglobin detected in AF of women with PTL without IAI who delivered preterm (5.6%) or at term (7.3%), and in women with preterm PROM without IAI (4.3%) was higher than the normal percentage in normal adult blood Citation[8]. Taken together, these findings suggest that in a subset of patients, a sub-clinical intra-amniotic bleed of fetal origin can be associated with spontaneous PTL or preterm PROM. Our findings suggest that conditions other than IAI can lead to intra-amniotic bleeding. Indeed, Hankins et al. Citation[30] detected total and fetal hemoglobin in brown, green and clear second trimester AF samples, with higher concentrations in the brown AF, and the fetal hemoglobin was determined to be 20–100% of the total hemoglobin Citation[30]. However, different insults may result in intra-amniotic fetal bleeding that can potentially originate from the immature skin, gastrointestinal tract, urinary or respiratory systems, and lead to the initiation of the common pathway of parturition, or, teleologically, fetal intra-amniotic bleeding may be a ‘stress signal’ that the fetus uses to initiate labor.
Fetal hemoglobin and term pregnancy
Among women with a normal pregnancy at term, spontaneous labor was not associated with a significant change in the median AF fetal hemoglobin concentration. However, the fetal hemoglobin percentage of the total hemoglobin was significantly lower among women in labor than that of those not in labor. This suggests that the finding of a significantly higher total hemoglobin concentration found in women at term in labor compared to those not in labor Citation[6] is predominantly of maternal origin. Possible explanations for the maternal intra-amniotic bleeding during spontaneous term labor are: (1) uterine contractions or active labor may cause vessel damage and minute intra-amniotic bleeding; and (2) the counteracting changes in the hemostatic system (bleeding/thrombosis) associated with labor Citation[31-34] may result in some degree of intra-amniotic bleeding.
Conclusions
The concentration of fetal hemoglobin in AF increases with gestational age. Among women with PTL or preterm PROM, the AF fetal hemoglobin concentrations were not associated with IAI. However, the percentage of fetal hemoglobin of the total hemoglobin in AF was lower in pregnancies complicated by PTL with IAI than those without IAI, suggesting a different source of bleeding and, thus, a different mechanism of disease. In PTL with IAI the hemoglobin detected in AF can be attributed to originate mainly from the maternal circulations, whereas in PTL without IAI the fetus contributes at least for some of the detected hemoglobin.
Acknowledgement
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Alger L S, Kisner H J, Nagey D A. The presence of a meconium-like substance in second-trimester amniotic fluid. Am J Obstet Gynecol 1984; 150: 380–385
- Zorn E M, Hanson F W, Greve L C, Phelps-Sandall B, Tennant F R. Analysis of the significance of discolored amniotic fluid detected at midtrimester amniocentesis. Am J Obstet Gynecol 1986; 154: 1234–1240
- Romero R, Hanaoka S, Mazor M, Athanassiadis A P, Callahan R, Hsu Y C, Avila C, Nores J, Jimenez C. Meconium-stained amniotic fluid: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1991; 164: 859–862
- Weinberg E D. Iron withholding: a defense against infection and neoplasia. Physiol Rev 1984; 64: 65–102
- Kontoghiorghes G J, Weinberg E D. Iron: mammalian defense systems, mechanisms of disease, and chelation therapy approaches. Blood Rev 1995; 9: 33–45
- Vaisbuch E, Romero R, Erez O, Kusanovic J P, Gotsch F, Than N G, Mazaki-Tovi S, Mittal P, Edwin S, Hassan S. Total hemoglobin concentration in amniotic fluid is increased in intraamniotic infection/inflammation. Am J Obstet Gynecol 2008; 199: 426e1–7
- Gomez R, Romero R, Nien J K, Medina L, Carstens M, Kim Y M, Chaiworapongsa T, Espinoza J, Gonzalez R. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med 2005; 18: 31–37
- Laros R K. The hemoglobinopathies. Blood Disorders in Pregnancy, R K Laros. Lea & Febiger, Philadelphia 1986; 37–61
- Telen M J, Kaufman R E. The mature erythrocyte. Wintrobe's Clinical Hematology, Vol. 1, J P Greer, J Foerster, J N Lukens, G M Rodgers, F Paraskevas, B Glader. Lippincott Williams & Wilkins, Philadelphia 2004; 217–247
- Alexander G R, Kogan M, Martin J, Papiernik E. What are the fetal growth patterns of singletons, twins, and triplets in the United States. Clin Obstet Gynecol 1998; 41: 114–125
- Gonzalez R P, Gomez R M, Castro R S, Nien J K, Merino P O, Etchegaray A B, Carstens M R, Medina L H, Viviani P G, Rojas I T. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev Med Chil 2004; 132: 1155–1165
- Yoon B H, Romero R, Moon J B, Shim S S, Kim M, Kim G, Jun J K. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001; 185: 1130–1136
- Perkins S L. Normal blood and bone marrow values in humans. Wintrobe's Clinical Hematology, Vol. 2, J P Greer, J Foerster, J N Lukens, G M Rodgers, F Paraskevas, B Glader. Lippincott Williams & Wilkins, Philadelphia 2004; 2698
- Bard H. The postnatal decline of hemoglobin F synthesis in normal full-term infants. J Clin Invest 1975; 55: 395–398
- Giorlandino C, Gambuzza G, D'Alessio P, Santoro M L, Gentili P, Vizzone A. Blood contamination of amniotic fluid after amniocentesis in relation to placental location. Prenat Diagn 1996; 16: 180–182
- Galle P C, Meis P J. Complications of amniocentesis: a review. J Reprod Med 1982; 27: 149–155
- Cutillo D P, Swayne L C, Schwartz J R, Dise C A, Faux R G. Intra-amniotic hemorrhage secondary to placenta circumvallate. J Ultrasound Med 1989; 8: 399–401
- Kurata H, Sekizuka N, Kato R, Yoshizawa H, Tanaka K. Intra-amniotic maternal hemorrhage in preterm labor: a case report. J Perinat Med 1995; 23: 229–232
- Sijanovic S, Selthofer R, Abicic-Zuljevic K, Milojkovic M, Topolovec Z, Sijanovic I, Kulas T. A case of intra-amniotic maternal hemorrhage in term pregnancy. Fetal Diagn Ther 2007; 22: 299–301
- Yamaguchi Y, Tsukimori K, Hojo S, Nakanami N, Nozaki M, Masumoto K, Taguchi T, Wake N. Spontaneous rupture of sacrococcygeal teratoma associated with acute fetal anemia. Ultrasound Obstet Gynecol 2006; 28: 720–722
- Naftolin F, Khudr G, Benirschke K, Hutchinson D L. The syndrome of chronic abruptio placentae, hydrorrhea, and circumvallate placenta. Am J Obstet Gynecol 1973; 116: 347–350
- Hill L M, Breckle R. Fetal outcome after intraamniotic hemorrhage with placental abruption. A report of three cases. J Reprod Med 1986; 31: 1065–1070
- Witter F R, Sanders R C. Maternal hemorrhage into the amniotic sac producing an apparent umbilical cord mass on sonogram. Am J Obstet Gynecol 1986; 155: 649–651
- Petrikovsky B, Smith-Levitin M, Holsten N. Intra-amniotic bleeding and fetal echogenic bowel. Obstet Gynecol 1999; 93: 684–686
- Sepulveda W, Hollingsworth J, Bower S, Vaughan J I, Fisk N M. Fetal hyperechogenic bowel following intra-amniotic bleeding. Obstet Gynecol 1994; 83: 947–950
- Sepulveda W, Reid R, Nicolaidis P, Prendiville O, Chapman R S, Fisk N M. Second-trimester echogenic bowel and intraamniotic bleeding: association between fetal bowel echogenicity and amniotic fluid spectrophotometry at 410 nm. Am J Obstet Gynecol 1996; 174: 839–842
- Romero R. The child is the father of the man. Prenat Neonat Med 1996; 1: 8–11
- Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. Preterm Birth, H Critchely, P Bennett, S Thornton. RCOG Press, London 2004; 28–60
- Romero R, Espinoza J, Kusanovic J P, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG 2006; 113(Suppl 3)17–42
- Hankins G D, Rowe J, Quirk J G, Jr, Trubey R, Strickland D M. Significance of brown and/or green amniotic fluid at the time of second trimester genetic amniocentesis. Obstet Gynecol 1984; 64: 353–358
- Yoshimura T, Ito M, Nakamura T, Okamura H. The influence of labor on thrombotic and fibrinolytic systems. Eur J Obstet Gynecol Reprod Biol 1992; 44: 195–199
- Andersson T, Lorentzen B, Hogdahl H, Clausen T, Mowinckel M C, Abildgaard U. Thrombin-inhibitor complexes in the blood during and after delivery. Thromb Res 1996; 82: 109–117
- Watanabe T, Minakami H, Sakata Y, Matsubara S, Tamura N, Obara H, Wada T, Onagawa T, Sato I. Effect of labor on maternal dehydration, starvation, coagulation, and fibrinolysis. J Perinat Med 2001; 29: 528–534
- Kusanovic J P, Espinoza J, Romero R, Hoppensteadt D, Nien J K, Kim C J, Erez O, Soto E, Fareed J, Edwin S. Plasma protein Z concentrations in pregnant women with idiopathic intrauterine bleeding and in women with spontaneous preterm labor. J Matern Fetal Neonatal Med 2007; 20: 453–463