Abstract
Objective. Low maternal plasma protein Z (PZ) concentrations were reported in patients with pre-eclampsia (PE), a small for gestational age (SGA) neonate, and a fetal demise (FD). Anti-protein Z antibodies (APZ-AB) have been proposed as a possible underlying mechanism leading to low plasma PZ concentrations. The objective of this study was to determine the maternal plasma concentration of APZ-AB in women with a normal pregnancy, and patients with PE, an SGA neonate or a FD.
Study design. A cross-sectional study included women in the following groups: (1) non-pregnant women (n = 45); and pregnant women with: (2) normal pregnancies (n = 70); (3) PE (n = 123); (4) SGA neonates (n = 51); and (5) a FD (n = 51). Plasma concentrations of anti-protein Z IgM and IgG antibodies were measured by ELISA. Elevated APZ-AB was defined as >75th, 90th and 95th percentile of the normal pregnancy group. Non-parametric statistics were used for analyses.
Results. (1) Patients with an SGA neonate had a higher median maternal plasma IgG APZ-AB concentration than women with normal pregnancies (p < 0.001), and patients with PE (p < 0.001) or with a FD (p = 0.001). (2) The proportion of patients with a maternal plasma IgM APZ-AB concentration >90th percentile was higher in the SGA group than in the PE group (p = 0.01). (3) Patients with PE maternal plasma IgM APZ-AB concentration >90th percentile had a higher rate of villous thrombosis (p = 0.03) and persistent muscularisation of basal plate arteries (p = 0.01) than those with IgM APZ-AB concentration <90th percentile; and (5) Patients with FD and maternal plasma IgM APZ-AB concentration >90th percentile had a higher rate of umbilical phlebitis and arteritis than those with IgM APZ-AB concentration <90th percentile (p = 0.003).
Conclusions. (1) Patients with SGA neonates have a higher median plasma concentration of IgG APZ-AB than normal pregnant women, or patients with PE or FD; and (2) maternal plasma IgM APZ-AB concentration >90th percentile was associated with vascular placental lesions in patients with PE, but not in those with an SGA neonate, suggesting that in a subset of patients, these antibodies can be associated with abnormal placentation and pregnancy complications.
Introduction
Protein Z (PZ), a vitamin-K-dependent plasma glycoprotein, has an anticoagulant effect which is derived from its function as a co-factor for PZ dependent protease inhibitor (ZPI). The latter inhibits activated factor X (FXa) by interaction with its catalytic residue Citation[1] and reduces thrombin generation Citation[2]. In the absence of PZ, the activity of ZPI is reduced by more than a thousand fold Citation[3-5]. Thus, PZ deficiency is associated with a procoagulant state Citation[2]. During pregnancy, maternal plasma PZ concentration increases with advancing gestation and gradually subsides post-partum Citation[6]. The major increase in the plasma PZ concentrations is during the second trimester Citation[6], it has been proposed that this reflects a possible protective mechanism of PZ by inhibiting the activation of FX during normal pregnancy Citation[6]. Low plasma concentration of PZ has been associated with pregnancy complications Citation[7-9] including: early fetal losses Citation[10], fetal demise Citation[8],Citation[11], pre-eclampsia Citation[8] and a small for gestational age (SGA) neonate Citation[11].
Anti-protein Z antibodies (APZ-AB) are present in the plasma of pregnant and non-pregnant women Citation[12],Citation[13]. Among patients with normal pregnancies, maternal plasma concentrations of APZ-AB increase during pregnancy. The plasma concentrations of (APZ) IgM antibodies are significantly higher during pregnancy than in the non-pregnant state, throughout gestation Citation[12], and there is a correlation between maternal plasma anti-protein Z IgM antibodies and PZ concentrations in the second and third trimesters Citation[12]. In contrast, maternal plasma (APZ) IgG concentrations are higher than in non-pregnant women only in the third trimester Citation[12].
Non-pregnant women with a history of adverse pregnancy outcomes (i.e. recurrent pregnancy loss <8 weeks gestation with and without PZ deficiency, unexplained fetal death after the 10th week of gestation, and a history of severe PE) have higher concentrations of APZ-AB (IgG and IgM) than those with a history of normal pregnancies Citation[13]. Moreover, the risk for abnormal pregnancy outcome was positively correlated with maternal plasma APZ-AB concentration Citation[13]. In a randomised controlled trial Citation[14], when comparing the efficacy of low molecular weight heparin (LMWH) vs. low dose aspirin in women with thrombophilic mutation and a history of an unexplained fetal demise, women with PZ deficiency and a positive APZ-AB titer had an increased rate of fetal death regardless of the treatment. Moreover, PZ deficiency and a positive titer of APZ-AB were independent risk factors for a fetal demise Citation[14].
The objectives of this study were to determine: (1) the maternal plasma APZ-AB concentration in non-pregnant and normal pregnant women; (2) The changes in the maternal plasma APZ-AB concentration in pregnancies complicated by PE, an SGA neonate, and a fetal demise when compared with normal pregnant women; and (3) the association between elevated maternal plasma APZ-AB and placental lesions.
Materials and methods
This cross-sectional study included patients in the following groups: (1) non pregnant women (n = 45); (2) patients with a normal pregnancy (n = 70); (3) PE (n = 123); (4) SGA neonates (n = 51); and (5) fetal demise (n = 51). Patients with multiple pregnancies or with fetal congenital and chromosomal anomalies were excluded.
Samples and data were retrieved from our bank of biological samples and clinical databases. Many of these samples have been previously employed to study the biology of inflammation, hemostasis, angiogenesis regulation and growth factor concentrations in non-pregnant women, normal pregnant women, and those with pregnancy complications. All women provided a written informed consent prior to the collection of maternal blood. The Institutional Review Boards of both Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS) approved the collection and utilisation of samples for research purposes.
Clinical definitions
Women with normal pregnancies met the following criteria: (1) no medical, obstetrical or surgical complications at the time of the study; (2) gestational age between 20 and 41 weeks; and (3) delivery of a term infant, appropriate for gestational age, without complications. Preeclampsia was defined as hypertension (systolic blood pressure of ≥140 mmHg or diastolic blood pressure of ≥90 mmHg on at least two occasions, 4 h to 1 week apart) associated with proteinuria (≥300 mg in a 24-h urine collection or at least one dipstick measurement of ≥2+) Citation[15]. Fetal demise was defined as a fetal death occurring during the second half of pregnancy. SGA was defined as a birthweight below the 10th percentile Citation[16]. Placental histologic findings were classified according to a diagnostic schema proposed by Redline et al. Citation[17].
Blood samples
All blood samples were collected with a vacutainer into 0.109 M trisodium citrate anticoagulant solution (BD; San Jose, CA). The samples were centrifuged at 1300g for 10 min at 4°C and stored at −70°C until assay.
Measurements of anti-protein Z IgG and IgM isotypes
Immunoassays to quantify anti-protein Z IgG and IgM isotypes were obtained from HYPHEN BioMed (Neuville-sur-Oise, France). Briefly, diluted citrated plasma samples were incubated in duplicate wells of the micro titer plates pre-coated with highly purified human PZ. During this incubation, APZ-AB (IgG or IgM) present in the standards or samples bound to immobilised human PZ, forming antigen antibody complexes. Repeated washing and aspiration removed all other unbound materials from the assay plate. Further incubations with a peroxidase conjugated goat anti-human IgG that react specially with the IgG isotype were conducted. Following the washing step, to detect bound antibodies of the IgG isotype. Similarly, to detect bound antibodies of the IgM isotype, further incubations with a peroxidase conjugated goat anti-human IgM that reacts specifically with the IgM isotype were performed. Following a washing step to remove excess and unbound materials, a substrate solution (tetramethylbenzidine (TMB) in the presence of hydrogen peroxide) was added to the wells of the micro titer plate, and color developed in proportion to the amount of IgG or IgM bound in the initial step of the individual assays. The color development was stopped with the addition of an acid solution (0.45 M sulphuric acid) and the intensity of color was read using a programmable micro titer plate spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA). The concentrations of anti-protein Z IgG or IgM in samples were determined by interpolation from individual standard curves composed of purified human anti-protein Z IgG or IgM (calibrators). The calculated inter- and intra-assay CVs for anti-protein Z IgG isotype immunoassay in our laboratory were 6.03% and 5.41%, respectively. The sensitivity for the anti-protein Z IgG isotype immunoassay was 1.11 AU/ml. The calculated inter- and intra-assay CVs for anti-protein Z IgM isotype immunoassay were 7.04% and 2.18%, respectively, and the sensitivity for the anti-protein Z IgM isotype immunoassay was 2.02 AU/ml. The cutoff for elevated plasma APZ-AB concentrations was set at the, 75th, 90th or 95th percentiles of the normal population next the association of this cutoff with adverse pregnancy outcomes and placental pathology was examined.
Statistical analysis
The Shapiro–Wilk and the Kolmogorov–Smirnov tests were used to test the distribution of the data. Since APZ-AB (IgM and IgG) plasma concentrations were not normally distributed, a Mann–Whitney U test was employed for comparisons of continuous variables. Chi-square and Fisher exact tests were used to compare categorical variables. Spearman correlation was employed to detect an association between the concentrations between APZ-AB (IgG and IgM) and gestational age at sample collection in women with a normal pregnancy. Multiple logistic regression analysis was performed to investigate the association between anti-protein Z IgM antibodies and the delivery of an SGA neonate. A p-value < 0.05 was considered statistically significant. Analysis was performed with SPSS package, version 12 (SPSS, Chicago, IL).
Results
Demographic and clinical characteristics of the study population are displayed in . The median gestational age at blood collection was significantly lower in the normal pregnancy and the fetal demise groups than in the preeclampsia and the SGA groups. The median gestational age at delivery was lower among patients with preeclampsia, SGA and a fetal demise than in normal pregnant women. The 75th, 90th and 95th percentiles of maternal plasma APZ-AB concentrations of the normal pregnancy group are presented in .
Table I. Demographic and clinical characteristics of the study population.
Table II. Maternal plasma concentration of anti-protein Z antibodies.
Comparison of APZ-AB between non-pregnant and pregnant patients
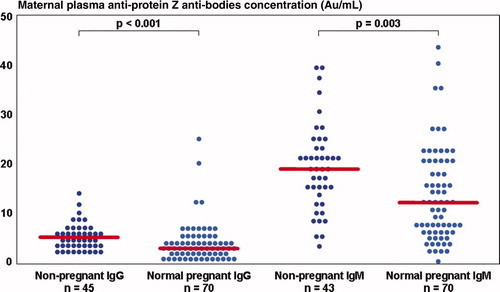
Normal pregnant women had a lower median plasma concentration of APZ-AB than non-pregnant patients; (IgM: normal pregnancy – median 13.3 AU/ml, interquartile range (IQR) 7.8–21.1 vs. non pregnant – 19.2 AU/ml, IQR 13.6–23.8, p = 0.003; IgG: normal pregnancy: median 3.2 AU/ml, IQR 2.1–5.3 vs. non-pregnant: median 5.2 AU/ml, IQR 3.9–6.9, p < 0.001) ().
APZ-AB concentrations during normal pregnancy
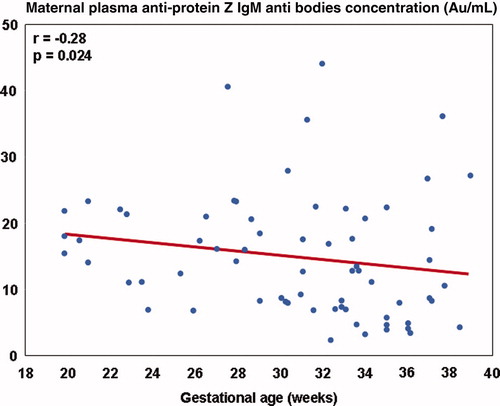
In the normal pregnancy group, there was a negative correlation between anti-protein Z IgM plasma concentrations and gestational age at blood collection (r = −0.28, p = 0.024) (). Anti-protein Z IgG plasma concentrations did not correlated with gestational age at blood collection (r = 0.009, p = 0.94). There was no correlation between anti-protein Z IgM and IgG plasma concentrations and parity (r = −0.008, p = 0.95; r = −0.22, p = 0.07, respectively).
Maternal plasma APZ-AB concentrations in patients with pregnancy complications
Patients in the SGA group had a significantly higher median maternal plasma anti-protein Z IgG antibodies concentration than patients with a normal pregnancy (SGA: median 5.5 AU/ml, IQR 3.5–7.6 vs. normal pregnancy: median 3.2 AU/ml, IQR 2.1–5.3, p < 0.001) (). The median maternal plasma anti-protein Z IgM antibodies concentration did not differ significantly among the groups (p = 0.52).
Figure 3. Maternal plasma concentrations of anti-protein Z antibodies in normal and complicated pregnancies.
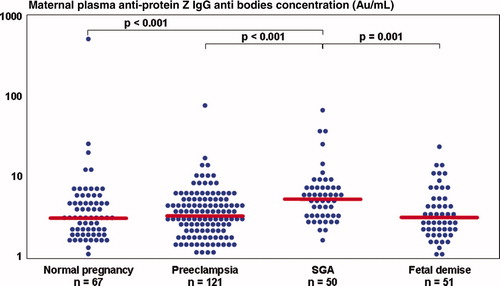
The median maternal plasma APZ-AB (IgG and IgM) concentrations of patients presenting with preeclampsia (IgM: median 12.0 AU/ml, IQR 8.8–16.5; IgG: median 3.4 AU/ml, IQR 2.3–5.2) or a fetal demise (IgM: median 11.1 AU/ml, IQR 6.8–18.1; IgG: median 3.3 AU/ml, IQR 2.2–5.5) did not differ significantly from that of women with normal pregnancies. The median maternal plasma concentration of anti-protein Z IgG antibodies was higher in the SGA group than in patients with preeclampsia (p < 0.001) or a fetal demise (p = 0.001) (). The median maternal plasma anti-protein Z IgM antibodies concentration did not differ significantly between the three groups (preeclampsia, SGA, fetal demise).
The proportion of patients in the SGA group who had anti-protein Z IgG antibodies concentration >75th percentile was higher than that observed in both the normal pregnancy and preeclampsia groups (). The proportion of patients in the SGA group who had anti-protein Z IgM antibodies concentration >75th, 90th and 95th percentiles was higher than that observed in the preeclampsia group ().
Table III. Comparison of IgG anti-protein Z antibodies percentiles between the study groups.
Table IV. Comparison of IgM anti-protein Z antibodies percentiles between the study groups.
In a multiple logistic regression model controlling for gestational age at sample collection, the maternal plasma anti-protein Z IgM antibodies were independently associated with the delivery of an SGA neonate (OR 1.03, 95%CI 1.007–1.06).
The association between elevated APZ-AB and placental lesions
Placental histology was available in 82.9% (102/123) of patients with preeclampsia, 86.3% (44/51) of those with an SGA neonate, and 76.5% (39/51) of patients with a fetal demise.
Among women with preeclampsia, the proportion of villous infarcts and persistent muscularization of basal plate arteries was higher in patients with elevated anti-protein Z IgM antibodies >90th percentile than in those with anti-protein Z IgM antibodies <90th percentile (villous thrombosis: anti-protein Z IgM antibodies >90th percentile 60% (3/5) vs. anti-protein Z IgM antibodies <90th percentile 13.4% (13/97), p = 0.03; persistent muscularisation of basal plate arteries: anti-protein Z IgM antibodies >90th percentile 40% (2/5) vs. anti-protein Z IgM antibodies <90th percentile 2.1% (2/97), p = 0.01.
Among patients with a fetal demise, the proportion of funisitis (umbilical phlebitis and arteritis) was higher in patients with elevated IgM antibodies >90th percentile of the normal pregnancy group than those with anti-protein Z IgM antibodies <90th percentile (anti-protein Z IgM antibodies >90th percentile 33.3% (2/6) vs. anti-protein Z IgM antibodies <90th percentile 0% (0/33), p = 0.003). Of interest, women with SGA neonates had no association between placental lesion and the rate of elevated APZ-AB.
Comments
Principal findings
(1) Non-pregnant women had higher median plasma APZ-AB (IgG and IgM) concentrations than women with a normal pregnancy. (2) Patients with SGA neonates had a higher median maternal plasma anti-protein Z IgG antibodies concentration women with normal pregnancies; as well as patients with preeclampsia or a fetal demise. (3) There was a higher rate of patients in the SGA group with maternal plasma anti-protein Z IgM antibodies concentration above the 90th percentile than in the preeclampsia group. (4) Among patients with preeclampsia, the proportion of patients with villous infarcts or persistent muscularisation of basal plate arteries was higher in those with maternal plasma anti-protein Z IgM antibodies concentration >90th percentile than in those with maternal plasma anti-protein Z IgM antibodies concentration <90th percentile. (5) Among patients with a fetal demise, the proportion of inflammation of the umbilical vessels was higher in patients with maternal plasma anti-protein Z IgM antibodies concentration above the 75th percentiles.
What are Anti-protein Z antibodies?
The finding of low PZ plasma concentration in patients with anti-phospholipid syndrome Citation[18],Citation[19] led to the hypothesis that antibodies against PZ may cause a rapid clearance of this glycoprotein Citation[18]. A functional study demonstrated that anti-phospholipid antibodies of patients with anti-phospholipid syndrome impair the inhibition of FXa by the PZ/ZPI complex in the presence of β2 glycoprotein-1 Citation[20]. The authors proposed that the β2 glycoprotein-1-antiphospholipid-IgGs complexes impair FXa inhibition by competing with the PZ-ZPI-FXa complex for the same phospholipid binding sites Citation[20]. Of note, the authors could not demonstrate antibodies directed against PZ Citation[20] and APZ-AB were classified as a subclass of antiphospholipid antibodies Citation[21]. Gris et al. Citation[13] were the first to report antibodies directed against protein Z among non-pregnant women; and this observation was subsequently supported by others Citation[12],Citation[22],Citation[23]. In their study, Gris et al. Citation[13] did not find a correlation between maternal plasma concentration of anti-cardiolipin (IgG and IgM) and anti-β2 glycoprotein-1 (IgG and IgM) and APZ-AB Citation[13]. A recent study Citation[23] tested the clinical significance of APZ-AB in patients with lupus anticoagulant. The proportion of elevated anti-protein Z IgG antibodies (>the 75th percentile) was higher among patients with elevated anticardiolipin antibodies concentrations than in those with anticardiolipin antibodies concentrations in the normal range. However, there was no association between anti-β2 glycoprotein and APZ-AB Citation[23]. The proportion of elevated anti-protein Z IgM antibodies was higher among patients with lupus anticoagulant than in the control group Citation[23]. Yet, there was no association between APZ-AB and previous thrombosis or history of recurrent pregnancy loss in these patients Citation[23]. Thus, the current view is that APZ-AB may constitute a different class of antibodies than anti-phospholipid antibodies, and it is not clear whether these antibodies interact on the phospholipids bilayer of the vascular endothelium.
Are Anti-protein Z an additional type of natural autoantibodies?
The detection of APZ-AB in non-pregnant patients and the lower concentrations during normal pregnancy suggest that they may be regarded as natural antibodies. This class of antibodies, which are present in the serum without a known antigenic stimulation Citation[24-26], are a component of the normal humoral arm of the immune system in human. It has been proposed that natural antibodies participate in enhancement of: (1) opsonisation of foreign antigens Citation[27]; (2) independent B-cells and T-cells respond to foreign antigens Citation[28],Citation[29]; (3) clearance of catabolic products and soluble immune complexes Citation[30-34]; and (4) increased protection against infection Citation[35-37]. Autoantibodies are a subgroup of natural antibodies that react with self-antigens Citation[24-26],Citation[38]. During normal pregnancy, the total IgG concentrations in the maternal serum of healthy women decreases significantly in comparison to the non-pregnant state Citation[39]. In contrast, the maternal serum concentrations of autoantibodies (i.e. against phospholipids, histone, histone subfractions and polynucleotides) do not change significantly Citation[39]. Other autoantibodies including antinuclear Citation[40],Citation[41], anticardiolipin Citation[40],Citation[42], antiphospholipids Citation[43] and others Citation[44] have been detected in women with normal pregnancies.
The presence of autoantibodies to members of the coagulation/ anti-coagulation system has been previously described. Antibodies to factor VIII were detected in non-hemophilic patients Citation[45-50], and antibodies to prothrombin Citation[51-55], factor VII Citation[56] and protein S Citation[57-60] have been reported in patients with antiphospholipid syndrome. However, antiphospholipid antibodies can be found in 3–10% of the normal population Citation[61-63]. These observations and our finding of APZ-AB in non-pregnant healthy women raise the following question: when does the pathologic transformation of the autoantibodies occur? Lieby et al. Citation[64] tried to provide an answer by studying the pathogenic effect of five randomly selected monoclonal antiphospholipid antibodies originated from a patient with antiphospholipid syndrome. When the different five monoclonal antibodies were injected to pregnant mice, only one caused a significantly higher fetal resorption in comparison to human IgG Citation[64]. The authors suggested that the affinity maturation process of natural autoantibodies can transform them into pathologic autoantibodies Citation[64]. Currently, the measurement of antiphospholipid concentration gives only the total ‘quantity’ (reactivity) of the autoantibodies rather than the ‘quality’ (which of the different monoclonal antiphospholipid antibodies is potentially harmful) Citation[64]. This may also be the case with APZ-AB; women with APZ-AB plasma concentrations >90th percentile can have either a normal pregnancy or develop pregnancy complications such as recurrent abortions, delivering an SGA neonate, and a fetal demise.
What are the changes in anti-protein Z antibodies in complicated pregnancies?
The finding that patients with SGA neonates had a higher median plasma concentration of anti-protein Z IgG antibodies in comparison to patients with normal pregnancies is novel. A previous study Citation[13] demonstrated higher APZ-AB (IgG and IgM) concentrations in non-pregnant women with a history of unexplained primary recurrent embryo losses and women with unexplained fetal loss Citation[13]. It has been proposed Citation[65],Citation[66] that recurrent abortion, preeclampsia, and SGA are different spectrum of the same disease and the latter two are associated with a fetal demise. However, the maternal compartment does not always reflect the changes occurring in the fetal–placental compartment in women who delivered an SGA neonate Citation[8],Citation[67],Citation[68], similar to the changes in the maternal coagulation and anticoagulation factors reported in patients with SGA neonates. In contrast to the increased median maternal plasma thrombin-anti-thormbin III complexes concentrations Citation[67], the median maternal plasma tissue factor concentration was lower Citation[68], and the concentrations of PZ Citation[8] and tissue factor pathway inhibitor Citation[68] did not differ from those of women with normal pregnancies. Thus, the higher median maternal plasma anti-protein Z IgG antibodies along with the higher proportion of elevated anti-protein Z IgG antibodies suggest a role for these antibodies in the underlying mechanism leading to an SGA neonate.
The association between elevated maternal plasma anti-protein Z IgM antibodies concentrations and specific placental lesions in patients with PE or a fetal death is novel. Interestingly, in the PE group elevated anti-protein Z IgM antibodies were associated with vascular placental lesions (e.g. failure of transformation of basal plate arteries and villous infarcts). On the other hand, in the fetal demise group, elevated median maternal plasma concentration of these antibodies was associated with inflammatory lesions of the umbilical cord (umbilical phlebitis/chronic vasculitis or umbilical arteritis). Of note, among women with a history of severe preeclampsia and women with PZ deficiency who had unexplained primary recurrent embryo losses, only the concentrations of IgM APZ-AB were higher than that of normal pregnant women Citation[13]. Moreover, a dose effect between the plasma concentrations of APZ-AB and previous pathologic pregnancy was reported Citation[13].
Although there is no correlation between PZ and APZ-AB plasma concentrations, the combination of PZ deficiency and high titer of PZ antibodies has been reported to be associated with recurrent primary pregnancy losses Citation[13] and a fetal demise Citation[14]. This study Citation[14] included women with thrombophilia and history of previous fetal demise that were randomly assigned to treatment with LMWH or low dose aspirin. In a sub-analysis of the pregnancy outcomes according to the presence of high titer of APZ-AB (75th–97th percentile) and PZ deficiency (<1 mg/l) Citation[14]. The combination of high titer of APZ-AB and PZ deficiency was associated with a poor response to either treatments and increased risk for recurrent fetal death in these patients Citation[14]. Hence, the presence of maternal thrombophilia along with the combination of PZ deficiency and high titer of APZ-AB may lead to a less favorable pregnancy outcome even under anticoagulant treatment.
What is the mechanism of action of APZ-AB in complicated pregnancies?
The mechanisms in which high plasma concentrations of APZ-AB contribute to the development of a SGA neonate are not clear. Two possible explanation for the association between high maternal plasma concentrations of APZ-AB and pregnancy complications have been proposed: (1) enhanced immune-complex formation that is associated with cellular or complement activation similar to that observed with anti-β2-glycoprotein Citation[13], leading to recurrent first trimester losses Citation[13] and (2) inhibition of PZ by APZ-AB Citation[13] which coat the PZ molecule and inhibition of its activity Citation[69]. This mechanism has been proposed to cause a preference for hypercoagulation in the maternal side of the placenta Citation[21], leading to a fetal demise and preeclampsia Citation[13]. Evidences in support of the association of both mechanisms with the delivery of an SGA neonate include increased placental inflammatory processes such as villitis of unknown origin Citation[70],Citation[71], maternal side thrombosis and placental vascular lesions Citation[72-76]. However, in a previous study by our group, women with an SGA neonate did not have a significantly lower median maternal plasma PZ concentrations or a higher rate of PZ deficiency than women with normal pregnancies Citation[8], suggesting that the mechanism of immune complexes formation may be the possible explanation for the finding of a higher median maternal plasma concentration of APZ-AB in pregnant patients who delivered an SGA neonate.
In conclusion, the presence of APZ-AB can be physiologic in both non-pregnant and pregnant women. However, in a subgroup of patients, higher concentrations of APZ-AB are associated with pregnancy complications such as recurrent abortions, SGA neonate and fetal death.
Acknowledgements
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Rezaie A R, Manithody C, Yang L. Identification of factor Xa residues critical for interaction with protein Z-dependent protease inhibitor: both active site and exosite interactions are required for inhibition. J Biol Chem 2005; 280: 32722–32728
- Yin Z F, Huang Z F, Cui J, Fiehler R, Lasky N, Ginsburg D, Broze G J, Jr, et al. Prothrombotic phenotype of protein Z deficiency. Proc Natl Acad Sci USA 2000; 97: 6734–6738
- Han X, Fiehler R, Broze G J, Jr. Isolation of a protein Z-dependent plasma protease inhibitor. Proc Natl Acad Sci USA 1998; 95: 9250–9255
- Han X, Huang Z F, Fiehler R, Broze G J, Jr. The protein Z-dependent protease inhibitor is a serpin. Biochemistry 1999; 38: 11073–11078
- Han X, Fiehler R, Broze G J, Jr. Characterization of the protein Z-dependent protease inhibitor. Blood 2000; 96: 3049–3055
- Quack Loetscher K C, Stiller R, Roos M, Zimmermann R. Protein Z in normal pregnancy. Thromb Haemost 2005; 93: 706–709
- Paidas M J, Ku D H, Lee M J, Manish S, Thurston A, Lockwood C J, Arkel Y S. Protein Z. Protein S levels are lower in patients with thrombophilia and subsequent pregnancy complications. J Thromb Haemost 2005; 3: 497–501
- Erez O, Hoppensteadt D, Romero R, Espinoza J, Goncalves L, Nien J K, Kusanovic J P, Fareed J, Gotsch F, Pineles B, et al. Pre-eclampsia is associated with low concentrations of protein Z. J Matern Fetal Neonatal Med 2007; 20: 661–667
- Kusanovic J P, Espinoza J, Romero R, Hoppensteadt D, Nien J K, Kim C J, Kim C J, Erez O, Soto E, Fareed J, Edwin S, et al. Plasma protein Z concentrations in pregnant women with idiopathic intrauterine bleeding and in women with spontaneous preterm labor. J Matern Fetal Neonatal Med 2007; 20: 453–463
- Gris J C, Quere I, Dechaud H, Mercier E, Pincon C, Hoffet M, Vasse M, Mares P. High frequency of protein Z deficiency in patients with unexplained early fetal loss. Blood 2002; 99: 2606–2608
- Bretelle F, Arnoux D, Shojai R, D'Ercole C, Sampol J, Dignat F, Camoin-Jau L. Protein Z in patients with pregnancy complications. Am J Obstet Gynecol 2005; 193: 1698–1702
- Paidas M J, Ku D H, Arkel Y S, Elizabeth T, Christine F, Ben H, Ku Evelyn, Lockwood C J. Normal pregnancy is associated with the development of protein S and protein Z antibodies, independent of PS and PZ levels. Am J Obstet Gynecol 2004; 6(Supp.1)s140
- Gris J C, Amadio C, Mercier E, Lavigne-Lissalde G, Dechaud H, Hoffet M, Quere I, Amiral J, Dauzat M, Mares P. Anti-protein Z antibodies in women with pathologic pregnancies. Blood 2003; 101: 4850–4852
- Gris J C, Mercier E, Quere I, Lavigne-Lissalde G, Cochery-Nouvellon E, Hoffet M, Ripart-Neveu S, Tailland M L, Dauzat M, Mares P. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 2004; 103: 3695–3699
- ACOG Practice Bulletin. Diagnosis and management of pre-eclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol 2002; 99: 159–167
- Alexander G R, Himes J H, Kaufman R B, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996; 87: 163–168
- Redline R W, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta 2005; 26(Suppl A)S114–S117
- Steffano B, Forastiero R, Martinuzzo M, Kordich L. Low plasma protein Z levels in patients with antiphospholipid antibodies. Blood Coagul Fibrinolysis 2001; 12: 411–412
- McColl M D, Deans A, Maclean P, Tait R C, Greer I A, Walker I D. Plasma protein Z deficiency is common in women with antiphospholipid antibodies. Br J Haematol 2003; 120: 913–914
- Forastiero R R, Martinuzzo M E, Lu L, Broze G J. Autoimmune antiphospholipid antibodies impair the inhibition of activated factor X by protein Z/protein Z-dependent protease inhibitor. J Thromb Haemost 2003; 1: 1764–1770
- Shoenfeld Y, Blank M. Autoantibodies associated with reproductive failure. Lupus 2004; 13: 643–648
- Pardos-Gea J, Ordi-Ros J, Serrano S, Balada E, Nicolau I, Vilardell M. Protein Z levels and anti-protein Z antibodies in patients with arterial and venous thrombosis. Thromb Res 2007; 121: 727–734
- Sailer T, Vormittag R, Koder S, Quehenberger P, Kaider A, Pabinger I. Clinical significance of anti-protein Z antibodies in patients with lupus anticoagulant. Thromb Res 2008; 122(2)153–160
- Avrameas S. Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton’. Immunol. Today 1991; 12: 154–159
- Coutinho A, Kazatchkine M D, Avrameas S. Natural autoantibodies. Curr Opin Immunol 1995; 7: 812–818
- Boyden S V. Natural antibodies and the immune response. Adv Immunol 1966; 5: 1–28
- Navin T R, Krug E C, Pearson R D. Effect of immunoglobulin M from normal human serum on Leishmania donovani promastigote agglutination, complement-mediated killing, and phagocytosis by human monocytes. Infect Immun 1989; 57: 1343–1346
- Elson C J, Naysmith J D, Taylor R B. B-cell tolerance and autoimmunity. Int Rev Exp Pathol 1979; 19: 137–203
- Michael J G. Natural antibodies. Curr Top Microbiol Immunol 1969; 48: 43–62
- Kay M M, Goodman S R, Sorensen K, Whitfield C F, Wong P, Zaki L, Rudloff V. Senescent cell antigen is immunologically related to band 3. Proc Natl Acad Sci USA 1983; 80: 1631–1635
- Galili U, Clark M R, Shohet S B. Excessive binding of natural anti-α-galactosyl immunoglobin G to sickle erythrocytes may contribute to extravascular cell destruction. J Clin Invest 1986; 77: 27–33
- Hintner H, Romani N, Stanzl U, Grubauer G, Fritsch P, Lawley T J. Phagocytosis of keratin filament aggregates following opsonization with IgG-anti-keratin filament autoantibodies. J Invest Dermatol 1987; 88: 176–182
- Lutz H U, Bussolino F, Flepp R, Fasler S, Stammler P, Kazatchkine M D, Arese P. Naturally occurring anti-band-3 antibodies and complement together mediate phagocytosis of oxidatively stressed human erythrocytes. Proc Natl Acad Sci USA 1987; 84: 7368–7372
- Grabar P. Hypothesis. Auto-antibodies and immunological theories: an analytical review. Clin Immunol Immunopathol 1975; 4: 453–466
- Mold C, Rodic-Polic B, Du Clos T W. Protection from Streptococcus pneumoniae infection by C-reactive protein and natural antibody requires complement but not Fcγ receptors. J Immunol 2002; 168: 6375–6381
- Shaw P X, Horkko S, Chang M K, Curtiss L K, Palinski W, Silverman G J, Witztum J L. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest 2000; 105: 1731–1740
- Quan C P, Berneman A, Pires R, Avrameas S, Bouvet J P. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect Immun 1997; 65: 3997–4004
- Lacroix-Desmazes S, Kaveri S V, Mouthon L, Ayouba A, Malanchere E, Coutinho A, Kazatchkine M D. Self-reactive antibodies (natural autoantibodies) in healthy individuals. J Immunol Methods 1998; 216: 117–137
- el-Roeiy A, Myers S A, Gleicher N. The prevalence of autoantibodies and lupus anticoagulant in healthy pregnant women. Obstet Gynecol 1990; 75: 390–396
- Matthiesen L S, Berg G, Ernerudh J, Skogh T. A prospective study on the occurrence of autoantibodies in low-risk pregnancies. Eur J Obstet Gynecol Reprod Biol 1999; 83: 21–26
- Farnam J, Lavastida M T, Grant J A, Reddi R C, Daniels J C. Antinuclear antibodies in the serum of normal pregnant women: a prospective study. J Allergy Clin Immunol 1984; 73: 596–599
- Lockwood C J, Romero R, Feinberg R F, Clyne L P, Coster B, Hobbins J C. The prevalence and biologic significance of lupus anticoagulant and anticardiolipin antibodies in a general obstetric population. Am J Obstet Gynecol 1989; 161: 369–373
- Pattison N S, Chamley L W, McKay E J, Liggins G C, Butler W S. Antiphospholipid antibodies in pregnancy: prevalence and clinical associations. Br J Obstet Gynaecol 1993; 100: 909–913
- Patton P E, Coulam C B, Bergstralh E. The prevalence of autoantibodies in pregnant and nonpregnant women. Am J Obstet Gynecol 1987; 157: 1345–1350
- Wootla B, Dasgupta S, Dimitrov J D, Bayry J, Levesque H, Borg J Y, Borel-Derlon A, Rao D N, Friboulet A, Kaveri S V, et al. Factor VIII hydrolysis mediated by anti-factor VIII autoantibodies in acquired hemophilia. J Immunol 2008; 180: 7714–7720
- Allain J P, Croissant M P, Lerolle D, Houbouyan L, Zuzel M, Frommel D. In vivo interactions of autoantibodies to factor VIII with the factor VIII complex. Thromb Haemost 1982; 48: 142–145
- Croissant M P, Zuzel M, Allain J P. Heterogeneity of autoantibodies to factor VIII: differences in specificity for apparently distinct antigenic determinants of factor VIII coagulant protein. Blood 1983; 62: 133–140
- Sultan Y, Rossi F, Kazatchkine M D. Recovery from anti-VIII:C (antihemophilic factor) autoimmune disease is dependent on generation of antiidiotypes against anti-VIII: C autoantibodies. Proc Natl Acad Sci USA 1987; 84: 828–831
- Hart H C, Kraaijenhagen R J, Kerckhaert J A, Verdel G, Freen M, van de W A. A patient with a spontaneous factor VIII: C autoantibody: successful treatment with cyclosporine. Transplant Proc 1988; 20: 323–328
- Algiman M, Dietrich G, Nydegger U E, Boieldieu D, Sultan Y, Kazatchkine M D. Natural antibodies to factor VIII (anti-hemophilic factor) in healthy individuals. Proc Natl Acad Sci USA 1992; 89: 3795–3799
- Dahlback B, Nilsson I M, Frohm B. Inhibition of platelet prothrombinase activity by a lupus anticoagulant. Blood 1983; 62: 218–225
- Fleck R A, Rapaport S I, Rao L V. Anti-prothrombin antibodies and the lupus anticoagulant. Blood 1988; 72: 512–519
- Horbach D A, Derksen R H, de Groot P G. The presence of anti-β 2-glycoprotein I and anti-prothrombin antibodies and their correlations with venous and arterial thrombosis. Ann Med Interne (Paris) 1996; 147(Suppl 1)42–43
- Swadzba J, De Clerck L S, Stevens W J, Bridts C H, van Cotthem K A, Musial J, Jankowski M, Szczeklik A. Anticardiolipin, anti-β(2)-glycoprotein I, antiprothrombin antibodies, and lupus anticoagulant in patients with systemic lupus erythematosus with a history of thrombosis. J Rheumatol 1997; 24: 1710–1715
- Forastiero R R, Martinuzzo M E, Cerrato G S, Kordich L C, Carreras L O. Relationship of anti β2-glycoprotein I and anti prothrombin antibodies to thrombosis and pregnancy loss in patients with antiphospholipid antibodies. Thromb Haemost 1997; 78: 1008–1014
- Bidot C J, Jy W, Horstman L L, Huisheng H, Jimenez J J, Yaniz M, Ahn Y S. Factor VII/VIIa: a new antigen in the anti-phospholipid antibody syndrome. Br J Haematol 2003; 120: 618–626
- Ruiz-Arguelles G J, Ruiz-Arguelles A, Perez-Romano B, Arcon-Segovia D. Protein S deficiency associated to anti-protein S antibodies in a patient with mixed connective-tissue disease and its reversal by danazol. Acta Haematol 1993; 89: 206–208
- Sorice M, Arcieri P, Griggi T, Circella A, Misasi R, Lenti L, Di Nucci G D, Mariani G. Inhibition of protein S by autoantibodies in patients with acquired protein S deficiency. Thromb Haemost 1996; 75: 555–559
- Erkan D, Zhang H W, Shriky R C, Merrill J T. Dual antibody reactivity to β2-glycoprotein I and protein S: increased association with thrombotic events in the antiphospholipid syndrome. Lupus 2002; 11: 215–220
- Bertolaccini M L, Sanna G, Ralhan S, Gennari L C, Merrill J T, Khamashta M A, Hughes G R. Antibodies directed to protein S in patients with systemic lupus erythematosus: prevalence and clinical significance. Thromb Haemost 2003; 90: 636–641
- Mateo J, Oliver A, Borrell M, Sala N, Fontcuberta J. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism – results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997; 77: 444–451
- Jones J V, Eastwood B J, Jones E, James H, Mansour M. Antiphospholipid antibodies in a healthy population: methods for estimating the distribution. J Rheumatol 1995; 22: 55–61
- Rand J H, Senzel L. Antiphospholipid antibodies and the antiphospholipid syndrome. Hemostasis and thrombosis basic principles and clinical practice, R W Colman, A E Clowes, S Z Goldhaber, V J Marder, J N George. Lippincott Williames & Wilkins, Philadelphia 2006; 1621–1636
- Lieby P, Poindron V, Roussi S, Klein C, Knapp A M, Garaud J C, Cerutti M, Martin T, Pasquali J L. Pathogenic antiphospholipid antibody: an antigen-selected needle in a haystack. Blood 2004; 104: 1711–1715
- von Dadelszen P, Watson R W, Noorwali F, Marshall J C, Parodo J, Farine D, Lye S J, Ritchie J W, Rotstein O D. Maternal neutrophil apoptosis in normal pregnancy, pre-eclampsia, and normotensive intrauterine growth restriction. Am J Obstet Gynecol 1999; 181: 408–414
- Burton G J, Jauniaux E. Placental oxidative stress: from miscarriage to pre-eclampsia. J Soc Gynecol Investig 2004; 11: 342–352
- Chaiworapongsa T, Yoshimatsu J, Espinoza J, Kim Y M, Berman S, Edwin S, Yoon B H, Romero R. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med 2002; 11: 362–367
- Erez O, Romero R, Hoppensteadt D, Than N G, Fareed J, Mazaki-Tovi S, Espinoza J, Chaiworapongsa T, Kim S S, Yoon B H, et al. Tissue factor and its natural inhibitor in pre-eclampsia and SGA. Reprod Sci A 2007; 182
- Dorner T, Hoppe B, Salama A, Pruss A, Kiesewetter H. Antibodies against protein Z and fetal loss: current perspectives. Clin Exp Med 2005; 5: 50–54
- Nordenvall M, Sandstedt B. Placental villitis and intrauterine growth retardation in a Swedish population. APMIS 1990; 98: 19–24
- Becroft D M, Thompson J M, Mitchell E A. Placental villitis of unknown origin: epidemiologic associations. Am J Obstet Gynecol 2005; 192: 264–271
- Katzman P J, Genest D R. Maternal floor infarction and massive perivillous fibrin deposition: histological definitions, association with intrauterine fetal growth restriction, and risk of recurrence. Pediatr Dev Pathol 2002; 5: 159–164
- Laurini R, Laurin J, Marsal K. Placental histology and fetal blood flow in intrauterine growth retardation. Acta Obstet Gynecol Scand 1994; 73: 529–534
- Althabe O, Labarrere C, Telenta M. Maternal vascular lesions in placentae of small-for-gestational-age infants. Placenta 1985; 6: 265–276
- Altshuler G, Russell P, Ermocilla R. The placental pathology of small-for-gestational age infants. Am J Obstet Gynecol 1975; 121: 351–359
- Becroft D M, Thompson J M, Mitchell E A. Placental infarcts, intervillous fibrin plaques, and intervillous thrombi: incidences, co-occurrences, and epidemiological associations. Pediatr Dev Pathol 2004; 7: 26–34