Abstract
Objective: To investigate changes in maternal ECG ST index, blood pressure (BP), cardiac left ventricular (LV) ejection function and vascular tone/stiffness in large and small arteries occurring during elective cesarean section (CS) in spinal anesthesia.
Material and methods: Twenty-six women were monitored with photoplethysmographic digital pulse wave (PW) analysis (DPA) before and after spinal anesthesia, after delivery of the baby, after 5 IU oxytocin bolus IV, and 5 min later. Statistics with Wilcoxon matched-pairs signed-rank and Friedman tests at a p < 0.05 were performed.
Results: Spinal anesthesia resulted in significantly decreased BP, increased ST index and LV ejection time, and small-artery vasodilation. Delivery of the baby resulted in global vasoconstriction and increases in systolic BP and heart rate (HR). Oxytocin lowered BP, HR and ST index, increased LV ejection power and caused both large- and small-artery vasodilation. ST index and BP recovered after 5 min, but low HR and low vascular tone persisted.
Conclusions: Spinal anesthesia and oxytocin caused arterial vasodilation and cardiac affection. Oxytocin caused a decrease in HR despite a fall in BP, indicating a direct negative chronotropic effect. Delivery of the baby caused momentous cardiovascular changes, possibly due to maternal emotions and auto-transfusion of blood from the uterus.
Introduction
Oxytocin is routinely administered at cesarean section (CS) to contract the uterus and prevent hemorrhage. However, many women then experience discomfort, nausea and chest pain. These symptoms have been attributed to the significant circulatory dose-dependent effects of oxytocin [Citation1] including ECG ST-depression, increase in heart rate (HR), stroke volume and cardiac output (CO), and decrease in systemic vascular resistance and arterial blood pressure (BP) [Citation2–8]. Detailed studies of the immediate hemodynamic response show an increase in HR and decreases in systemic vascular resistance and BP within 30–40 s after a 5 IU oxytocin bolus, with a concomitant increase of CO, followed by a rebound decrease in HR and a slow restitution of the BP [Citation5,Citation9].
Pharmacological vascular effects can be studied by analyzing pulse wave (PW) curve contour characteristics, determined by propagation of the forward percussion PW along the vascular tree and the reflection of the tidal PW from distal arteries. PW characteristics can be determined by digital PW analysis (DPA), which is a rapid, noninvasive and operator-independent photoplethysmographic (PPG) method. The DPA has been validated against invasive aortic measurement and correlates well with radial pulse applanation tonometry [Citation10,Citation11]. The DPA method can assess cardiac ejection time and distinguish between vascular tone/stiffness in large and small arteries [Citation11].
The primary objective of the study was to investigate the effects of oxytocin during elective CS on cardiac left ventricular (LV) ejection function and systemic arterial stiffness. We hypothesized that oxytocin decreases arterial vascular tone, but there is no knowledge yet whether oxytocin affects both large and small arteries.
Spinal anesthesia is frequently associated with maternal hypotension despite precautions with plasma volume expansion and vasopressor substances [Citation12]. The secondary objectives of the study were to investigate the cardiovascular effects of spinal anesthesia and delivery of the baby; due to adjunctive effects of fluid co-load and vasopressors, and to a lack of previous studies with the DPA method, we could not settle any hypotheses for these aims.
Material and methods
Study design
The study was prospective, with no interventions added to the routine management, carried out at the Skåne University Hospital in Lund, Sweden. Women who met the inclusion criteria were recruited consecutively and gave their informed consent to be monitored by a Meridian DPA during elective CS in spinal anesthesia. The study recordings were all performed by one of the authors (S.R.). The study was approved by the Regional Research Ethics Committee in Lund (Dnr 2012/649).
The inclusion criteria were healthy women at ≥34 gestational weeks scheduled for elective CS in spinal anesthesia with singleton pregnancy and informed consent. The exclusion criteria were hypertension, preeclampsia, abnormal pregnancy with expected surgical problems, coagulopathy, cardiovascular disease, American Society of Anesthesiologists physical status classification system (ASA-class) III or more, disease of upper extremities impeding measurements, or women unwilling to participate.
The pre-defined drop-out factors were blood loss greater than 1000 mL within the time frame of DPA measurements, initial dose of oxytocin other than 5 IU (8.35 μg), insufficient anesthesia, conversion to general anesthesia, administration of other vasoactive or uterotonic drugs than in the protocol, other deviations from the study protocol, technical errors, or patient unwilling to participate further.
Study protocol
All recordings were performed during maternal quiescence in the supine position, with the operation table tilted approximately 15 degrees to the left. Two liters per minute of oxygen was delivered through the nasal route throughout the procedure. All women were connected to a Philips Intellivue MP70 (Philips Healthcare, Stockholm, Sweden) surveillance device and continuously monitored with an oxygen saturation probe, an automatic BP cuff and a 3-lead ECG. From this was derived the ST index, a summation of the absolute values from ECG leads V2, V5 and aVF [Citation13]. For the DPA measurements, the PPG probe (Meridian DPA, Meridian Co., Ltd. Korea, and Salcor AB, Uppsala, Sweden), connected to a laptop (HP 625, Hewlett Packard, Solna, Sweden), was placed on the right second or third finger.
The baseline measurement (T0) was made after 5 min of rest before spinal anesthesia. The next recording (T1) was made 15 min after spinal anesthesia, i.e. just before the start of surgery. Measurement T2 was made immediately after delivery of the baby, but before oxytocin administration and further surgery. Immediately after the T2 recording was finished and the umbilical cord was clamped, a 5 IU (8.35 μg) bolus of oxytocin (Syntocinon, Swedish Orphan AB, Stockholm, Sweden) was given IV during 60 s. When the bolus was finished, a stopwatch was started and 60 s later the next DPA recording was started (T3). The DPA recordings were then continued with measurements 5 min after the bolus was finished (T4).
The BP was measured intermittently every 2 min as well as immediately after at each T recording point. The measurements were performed in the contralateral arm to avoid interference with the DPA measurements. Recordings of ST index, HR and systolic and diastolic BPs (SBP, DBP) were noted manually in a case report form at each T point. The volumes of blood loss and IV fluid given, vasopressor treatment, as well as any other specific treatment were also noted in the case report form at each specific T point.
Spinal anesthesia was administered with the patient sitting. The standard dose was bupivacaine hyperbaric solution 5 mg/mL (Marcain Tung, AstraZeneca, Södertälje, Sweden) 2 mL (10 mg) mixed with 1 mL sufentanil 5 μg/mL (Sufenta, Janssen-Cilag, Sollentuna, Sweden). Short women (<160 cm) received 9 mg of bupivacaine (n = 3) and tall women (>179 cm) received 12 mg (n = 1). After approximately 15 min preoperative preparation time, spinal anesthesia depth and spread was tested with pinprick and cold, and then surgery was allowed to start.
The protocol for plasma volume expansion implicated co-loading with Ringer-acetat (Fresenius Kabi, Uppsala, Sweden), approximately 20 mL/kg in the first 20 min, starting after the baseline measurement (T0), followed by 5–10 mL/kg during the rest of the procedure. In case the blood loss was >500 mL, or if clinical signs of hypovolemia occurred (low BP, tachycardia, poor capillary perfusion), 500 mL of Venofundin (B. Braun Medical, Danderyd, Sweden) could be given. Greater blood loss than 1000 mL was an exclusion criterion.
The protocol for vasoactive drugs implicated the use of phenylephrine 50–100 μg IV if mean arterial pressure (MAP) fell below 20% of baseline, or below 60 mmHg, or if clinical signs of low BP occurred, such as nausea or pallor. Atropine or ephedrine was administered in case of bradycardia. This standard protocol was used also after the delivery of the baby.
Digital photoplethysmography
The physiological background to the DPA method has been described previously [Citation10,Citation14]. The Meridian DPATM reports 17 different parameters, but for this study we selected parameters with the best repeatability and best correlation to gold standard applanation tonometry: pulse height (PH), aging index (AI), ejection time compensated (ETc), cardiac ejection elasticity index (EEI), dicrotic index (DI), dicrotic dilation index (DDI), and the ratios b/a and d/a [Citation11]. Descriptions of the parameters are shown in .
Table 1. Description of the digital pulse wave analysis parameters used in the study (for detailed description, see [Citation11]).
The DPA method cannot distinguish between decreased arterial wall elasticity due to structural remodeling of the arterial wall (aging, vascular disease), low compliance due to vascular volume expansion, or vasoconstriction; in the literature and in this paper, the terms “vascular tone” and “stiffness” are used interchangeably.
Statistical analyses
Some of the DPA variables are HR dependent [Citation11] and the statistical analyses were accordingly performed with both crude and HR-adjusted DPA values. If simple linear regression analyses between HR and a DPA variable at T0 yielded a statistically significant correlation (p < 0.05), and the intervention (spinal anesthesia, delivery of baby, oxytocin administration) resulted in a significant change in HR, the DPA variable in question was adjusted to a HR of 75 bpm, denoted DPA@75, with the equation DPA@75 = DPA+C (75-HR). C denotes the slope constant.
The cardiovascular effects of spinal anesthesia were analyzed with recordings from point T0 to T1, the effects of the start of surgery and the delivery of the baby between T1 and T2, and the effects of oxytocin were analyzed with recordings T2–T3–T4. The longitudinal changes in single T–T steps were analyzed with the Wilcoxon matched-pairs signed-rank test with a two-sided p values < 0.05 considered significant. To evaluate the risk of type I errors the Friedman non-parametric one-way ANOVA for repeated measurements T2–T3–T4, and Holm-Bonferroni adjustments of the p values achieved at the Wilcoxon tests, were also calculated: in the three T2–T3, T3–T4 and T2–T4 comparisons the Holm–Bonferroni significance level is <0.05/3 equal to <0.0167 for the Wilcoxon test with the lowest p values, < 0.05/2 equal to < 0.025 for the second lowest, and < 0.05/1 equal to < 0.05 for the third.
Results
Among the 26 recruited women, three women were excluded from DPA analyses from T3 and onwards because they were given a bolus of 10 IU (16.70 μg) oxytocin instead of 5 IU. In five women the measurements T0–T1 (spinal anesthesia) could not be analyzed due to technical recording errors at T0, but they were included in the measurements T1–T4. Six women had missing ETc, EEI, or DDI values at T2 and/or T3.
At T0 significant correlations were only found between HR and EEI (p = 0.037, R2 =0.22) and DDI (p = 0.048, R2 =0.20).
Effects of spinal anesthesia (T0–T1)
From measurement point T0 to point T1 the HR was not affected and hence no HR-adjustments of DPA parameters were made. Spinal anesthesia resulted in significant decreases in SBP and DBP and an increase in ECG ST index (, ). The DPA parameters PH, DI and DDI showed peripheral/small-artery vasodilation; ETc indicated increased LV ejection time suggesting decreased CO and/or large-artery vasoconstriction, while EEI (large-artery stiffness, LV ejection capacity), b/a (large-artery stiffness, LV ejection capacity), d/a (small-artery stiffness) and AI (global vascular stiffness) were unchanged.
Figure 1. Box plots showing sequential changes in maternal mean arterial blood pressure (MAP), heart rate, ECG ST index, ejection elasticity index, dicrotic index and pulse height from before spinal anesthesia (point T0), after spinal anesthesia but before surgery (T1), after delivery of the baby but before oxytocin injection (T2), 1 min after IV oxytocin (T3), and 5 min after oxytocin (T4). Arrows denote significant changes (Wilcoxon matched-pairs signed-rank test, p < 0.05; within brackets p < 0.10 but ≥0.05) and direction of change. Figures denote number of women in paired comparisons.
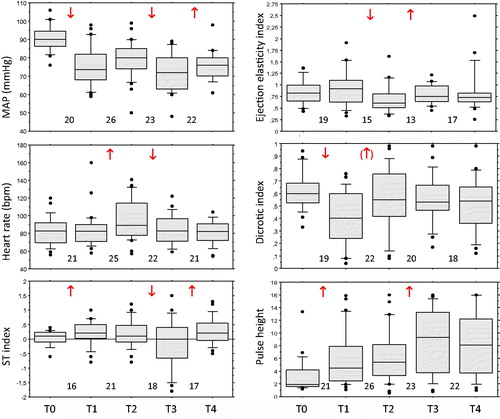
Table 2. Hemodynamic effects of spinal anesthesia and delivery of the baby at cesarean section.
Effects of surgery and delivery of the baby (T1–T2)
The HR increased significantly from T1 to T2 and the DPA parameters EEI and DDI were accordingly adjusted to EEI@75 and DDI@75, respectively. After the start of surgery and delivery of the baby (point T2), the SBP increased significantly but the DBP and MAP as well as the ST index remained unchanged (, ). A large-artery vasoconstriction and/or decreased LV ejection power were indicated by significant changes of b/a and EEI@75, a marginally significant small-artery vasoconstriction by DI (p = 0.062), and a global arterial vasoconstriction by AI. No significant changes were found for PH, ETc and d/a.
Effects of oxytocin (T2–T4)
The hemodynamic effects of oxytocin are shown in and . The HR decreased significantly at T2–T3, and EEI and DDI were accordingly HR-adjusted. From T2 to T3, the oxytocin injection resulted in significant decreases in DBP and MAP as well as in ST index, but the SBP remained unchanged (, ). A large-artery vasodilation and/or increased LV ejection power were indicated by a significant change of EEI@75, and a small-artery vasodilation by PH. No significant changes were found for ETc, DI, DDI@75, b/a, d/a and AI.
Table 3. Hemodynamic effects of oxytocin administration (T2 to T4) during cesarean section.
Restitution to T2 values of the DBP, MAP and ST index had occurred at point T4, 5 min after the oxytocin bolus. The initial T2–T3 changes in HR and PH were still significant at T4. In addition, from T3 to T4 changes in d/a and AI indicated small-artery and global vasodilation. Throughout T2–T3–T4, oxytocin had no significant effects on SBP, ETc, DI, DDI@75 and b/a.
Discussion
The procedures with spinal anesthesia, intravenous fluids, vasoactive drugs and delivery of the baby and placenta make it problematic to interpret the hemodynamic effects of oxytocin at CS. In addition, relief of aorto-caval compression when emptying the uterus, bleeding and maternal emotions may interfere [Citation15]. At the start of the serial post-oxytocin recordings, significant circulatory changes had already occurred. Spinal anesthesia, and the concomitant procedures, resulted in a vasodilation of small arteries and peripheral hyperemia, accompanied by a fall in both SBP and DBP and an increase in the ECG ST index. Even so, the DPA parameter ETc increased, indicating a prolongation of the LV ejection time [Citation11], i.e. large-artery vasoconstriction and/or a decrease in CO. The ETc elevation could be an effect of phenylephrine, a vasoconstricting alpha-1-adrenergic receptor agonist with well-known side effects of decreased HR and CO [Citation16].
Start of surgery and delivery of the baby resulted in increases of HR and SBP and a global vasoconstriction. A further deepening of the spinal anesthesia during this time interval is not unlikely, but would have a further vasodilatory effect. We found no previous studies addressing the hemodynamic effects of the cesarean delivery procedure per se, but it seems clear that surgery and delivery of the baby had profound effects on the maternal circulation. During surgery and delivery of the baby the mother is exposed to both positive and negative mental stress and, in addition, the circulatory effects could be due to a catecholamine surge or auto-transfusion of blood from the empty and shrunk uterus.
Negative stress triggers increases in oxygen consumption, respiration, BP, CO and peripheral vascular resistance, whereas relaxation responses are mostly the opposite [Citation17]. Sinha et al. [Citation18] found that in healthy young males happiness induces increases in HR and SBP, decreases in LV ejection time, stroke volume and peripheral vascular resistance, whereas DBP and CO remain unaffected. In accordance, watching a comedy induces a rise in BP and vasodilation [Citation19].
Regarding auto-transfusion of blood, the effects of acute blood volume expansion have been investigated in experiments on healthy animals and humans. Jandhyala and Hom [Citation20] showed in dogs that blood transfusion significantly increased BP and central venous pressure and reduced HR. The decrease in HR was explained by a reflex compensation to the elevated BP. Increases in systemic BP and central venous pressure have been shown in several animal and human studies, with linear relations between the magnitudes of volume expansion and increase in pressure [Citation21–23]. Most of the transfused blood is pooled in the low-pressure vasculature, acting as a distensible reservoir [Citation21–23].
To study the isolated effect of blood volume expansion on vascular smooth muscles, Jandhyala and Hom [Citation20] denervated the vasculature in a hind limb of dogs. By volume expansion, the vascular resistance increased. However, in other vascular beds a volume expansion may result in a decrease in vascular resistance, as demonstrated in the pulmonary vasculature in dogs [Citation22]. Thus, the findings in our study could point to a combined hemodynamic effect of maternal emotions and blood volume expansion: the increase in HR being a result of positive emotions, the increase in SBP being a result of positive emotions and auto-transfusion of blood, and the increase in vascular tone being a result of auto-transfusion.
A 5 IU IV oxytocin bolus given during 60 s resulted within 1–5 min in a vasodilation of both large and small arteries, accompanied by a fall in HR, DBP and ST index. Since the normal response to a decline in BP due to vasodilation would be an increase in HR, the findings indicate a direct negative chronotropic effect of oxytocin. Although none of the women in the present series experienced chest pain or discomfort, the findings point to a transient cardiac ischemia caused by oxytocin. The coronary arteries are perfused mainly during diastole, but since oxytocin tended to slow down the HR rather than to increase it, and the LV ejection time was not significantly affected, a shortening of diastole was not etiological of ischemia. An alternative mechanism to cardiac ischemia caused by oxytocin is coronary vasoconstriction, which has been demonstrated in dogs [Citation24].
Five minutes after the oxytocin administration, a global vasodilation and fall in HR persisted, but the DBP and ST index had returned to levels recorded before the oxytocin administration. Hence, the vasodilation and negative chronotropic effects were not just transient.
Previous studies on IV oxytocin bolus effects during CS have shown a decrease in peripheral vascular resistance and a positive chronotropic effect resulting in an increase in CO [Citation5,Citation7]. In contrast, we found a negative chronotropic effect lasting for at least 6 min and a possible positive inotropic effect as indicated by a rapid increase in EEI (increased LV ejection power). Also, animal studies have shown a negative chronotropic effect [Citation2,Citation25], but the inotropic effect is not clear, since studies have shown a decrease in LV contraction force [Citation2] as well as an increase [Citation25]. Cardiac synthesis of oxytocin and oxytocin receptors have been found in rats and dogs [Citation2,Citation26], suggesting not only a systemic vascular effect of oxytocin but also direct cardiac effects by autocrine and/or paracrine pathways.
The divergent results can possibly be explained by the fact that oxytocin has a biphasic vascular effect, as demonstrated by Thomas et al. [Citation5] and Moertl et al. [Citation9]: within the first post-oxytocin minute the HR increases and the SBP decreases, after which the HR decreases and the SBP increases with a slight rebound bradycardia occurring with a nadir at 3–4 min post oxytocin. Thomas et al. [Citation5] injected a 5 IU IV bolus as quick as possible and Moertl et al. [Citation9] during 10 s, which might maximize the cardiovascular effects.
The maternal hemodynamic effects apparently depend on the oxytocin injection time: when given as a statim bolus of 5 IU oxytocin the peak effects on BP and HR occur within 30–60 s [Citation1,Citation5,Citation9], but when the same dose is given as an infusion over 5 min the effects are blunted with no biphasic effect curve [Citation5]. It is also clear that the hemodynamic effects depend on the oxytocin dose: Sartain et al. [Citation15] found less hemodynamic effects of a 2 IU oxytocin bolus compared with a 5 IU bolus when injected over 5–10 s, and Jonsson et al. [Citation8] made the same experience when comparing 5 and 10 IU doses injected during one minute, with peak hemodynamic effects after 2 min.
The different doses of oxytocin used and the different injections times explain the inconsistency in the literature concerning the half-life as well as the peak effect of IV oxytocin. The pharmacokinetics of oxytocin in pregnant baboons has been explained by Kowalski et al. [Citation27] using a two-compartment model, with a redistribution phase half-life of 1.1–1.7 min and an elimination phase half-life of 8.0–9.6 min. To add to the complexity, the two-compartment model seems to be valid only with high doses (>0.5 μg/kg), but at lower doses the pharmacokinetics is described with a one-compartment model [Citation28].
Given these pharmacokinetic data, and adding the results from the studies by Thomas et al. [Citation5] and Jonsson et al. [Citation8] and considering patient safety, we gave the oxytocin bolus during 60 s, assuming a delayed and blunted peak effect. The DPA recording at time point T3 began 60 s after the last drop of oxytocin and lasted for a good minute; thus, it is possible that our T3 measurement covered parts of both the initial and the rebound phases.
It is well known that the chest discomfort experienced by some women during a CS is related to the dose and speed of oxytocin injection [Citation5,Citation15]. The adverse hemodynamic effects of oxytocin are added to the already present extensive adverse effects of spinal anesthesia, with global vasodilation, fall in BP and cardiac affection, as demonstrated in the present study (). In the perspective of our findings, we believe it is wise to administer even a small bolus like 5 IU over a longer time than the minute used in this study, particularly in women showing circulatory instability. Furthermore, efforts should be made to enhance the spinal anesthesia procedure in order to reduce the adverse circulatory effects. Spinal anesthesia with concomitant procedures carries a risk also for the fetus [Citation29].
Apart from a decline in HR after oxytocin, our results generally support the findings in previous studies. However, our study is the first to use the DPA technology and to show that oxytocin causes vasodilation in large as well as in small and peripheral arteries. Since the DPA is noninvasive, simple to use and the recording time is only about one minute, it is well suited for pharmacological research and for screening. A disadvantage is that the method is sensitive to body movements and cold fingers [Citation14,Citation30,Citation31]. Other methods for PW analysis, like applanation tonometry and oscillometry, are too slow to catch the rapid hemodynamic responses to vasoactive drugs like oxytocin.
Weaknesses and strengths
The complex interaction of hemodynamic events makes it difficult to selectively analyze the effects of the individual procedures during a CS. A weakness is that the study series was relatively small, comprising less than 20 paired observations for some of the statistical analyzes. Small series is a common problem in clinical experimental research, though our sample sizes were in only two paired comparisons below the recommended threshold for using the Wilcoxon matched-pairs signed-rank test [Citation32]. This is a non-parametric statistical tests, which then is more robust than its parametric equivalent, the paired t-test. Furthermore, to evaluate the risk of type I errors we also tested with the Friedman non-parametric one-way ANOVA for repeated measurements and performed Holm–Bonferroni adjustments of p values. The strengths of the study are the novelty of digital photoplethysmography for PW analysis, a hitherto not explored method to study arterial stiffness in obstetrics, and the longitudinal analyses of circulatory events occurring during the different steps of the CS procedure.
Summary
Both spinal anesthesia and oxytocin 5 IU IV bolus gave rise to profound maternal circulatory effects, mainly arterial vasodilation and cardiac affection with ST index changes. Contrary to previous studies, oxytocin resulted in a decrease in HR, suggesting a direct negative chronotropic effect. The DPA parameters implied that oxytocin within minutes results in vasodilation in both large and small arteries and increased LV ejection power. Cesarean surgery and delivery of the baby resulted in a global increase in vascular tone and increases in SBP and HR, suggesting momentous circulatory effects by these procedures. We believe these seemingly contradictory changes can be a combined effect of maternal emotions and auto-transfusion of blood from the empty and reduced uterus.
Declaration of interest
The authors declare no conflicts of interest. This study was supported by grants from the Lund University Medical Faculty (ALF) and Region Skåne.
References
- Hendricks CH, Brenner WE. Cardiovascular effects of oxytocic drugs used post partum. Am J Obstet Gynecol 1970;108:751–60.
- Mukaddam-Daher S, Yin YL, Roy J, et al. Negative inotropic and chronotropic effects of oxytocin. Hypertension 2001;38:292–6.
- Pinder AJ, Dresner M, Calow C, et al. Haemodynamic changes caused by oxytocin during caesarean section under spinal anaesthesia. Int J Obstet Anesth 2002;11:156–9.
- Carvalho JCA, Balki M, Kingdom J, et al. Oxytocin requirements at elective cesarean delivery: a dose-finding study. Obstet Gynecol 2004;104:1005–10.
- Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing caesarean section. Br J Anaesth 2007;98:116–9.
- Svanström MC, Biber B, Hanes M, et al. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during caesarean section. Br J Anaesth 2008;100:683–9.
- Langesaeter E, Rosseland LA, Stubhaug A. Haemodynamic effects of repeated doses of oxytocin during caesarean delivery in healthy parturients. Br J Anaesth 2009;103:260–2.
- Jonsson M, Hanson U, Lidell C, et al. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. BJOG 2010;117:76–83.
- Moertl M, Friedrich S, Kraschl J, et al. Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. BJOG 2011;118:1349–56.
- Millasseau SC, Ritter JM, Takazawa K, et al. Contour analysis of the photoplethysmographic pulse measured at the finger. J Hypertens 2006;24:1449–56.
- von Wowern E, Ostling G, Nilsson PM, et al. Digital photoplethysmography for assessment of arterial stiffness: repeatability and comparison with applanation tonometry. PLoS One 2015;10:e0135659. doi:10.1371/journal.pone.0135659.
- Cyna AM, Andrew M, Emmett RS, et al. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev 2006Oct 18;CD002251.
- Philips IntelliVue MP2 Patient Monitor. Available from: http://www.ems.philips.com/assets/documents/PM_-_IntelliVue_MP2_Patient_Monitor.pdf [last accessed 29 Apr 2016].
- Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev 2012;8:14–25.
- Sartain JB, Barry JJ, Howat PW, et al. Intravenous oxytocin bolus of 2 units is superior to 5 units during elective Caesarean section. Br J Anaesth 2008;101:822–6.
- Stewart A, Fernando R, McDonald S, et al. The dose-dependent effects of phenylephrine for elective cesarean delivery under spinal anesthesia. Anesth Analg 2010;111:1230–7.
- Dusek JA, Benson H. Mind-body medicine: a model of the comparative clinical impact of the acute stress and relaxation responses. Minn Med 2009;92:47–50.
- Sinha R, Lovallo WR, Parsons OA. Cardiovascular differentiation of emotions. Psychosom Med 1992;54:422–35.
- Miller M, Fry WF. The effect of mirthful laughter on the human cardiovascular system. Med Hypotheses 2009;73:636–9.
- Jandhyala BS, Hom GJ. Effects of acute blood volume expansion on vascular resistance and reactivity in anaesthetized dogs. Clin Sci 1983;65:9–17.
- Gauer OH, Henry JP, Sieker HO. Changes in central venous pressure after moderate hemorrhage and transfusion in man. Circ Res 1956;4:79–84.
- Henry JP, Gauer OH, Sieker HO. The effect of moderate changes in blood volume on left and right atrial pressures. Circ Res 1956;4:91–4.
- Echt M, Düweling J, Gauer OH, Lange L. Effective compliance of the total vascular bed and the intrathoracic compartment derived from changes in central venous pressure induced by volume changes in man. Circ Res 1974;34:61–8.
- Fortner CL, Manley ES Jr, Woodbury RA. Effects of synthetic oxytocin with and without preservatives upon coronary blood flow in the dog. J Pharmacol Exp Ther 1969;165:258–66.
- Coulson CC, Thorp JM Jr, Mayer DC, et al. Central hemodynamic effects of oxytocin and interaction with magnesium and pregnancy in the isolated perfused rat heart. Am J Obstet Gynecol 1997;177:91–3.
- Jankowski M, Hajjar F, Kawas S, et al. Rat heart: a site of oxytocin production and action. Proc Natl Acad Sci USA. 1998;95:14558–63.
- Kowalski WB, Diveky L, Mehendale R, et al. Effect of pregnancy on the metabolic clearance rate and the volume of distribution of oxytocin in the baboon. Am J Physiol 1998;274:E791–5.
- Morin V, Del Castillo JR, Authier S, et al. Evidence for non-linear pharmacokinetics of oxytocin in anesthetizetized rat. J Pharm Pharm Sci 2008;11:12–24.
- Reynolds F, Seed PT. Anaesthesia for Caesarean section and neonatal acid-base status: a meta-analysis. Anaesthesia 2005;60:636–53.
- Takazawa K, Tanaka N, Fujita M. Assessment of vasoactive agents and vascular aging by the second derivative of photoplethysmogram waveform. Hypertension 1998;32:365–70.
- Chowienczyk PJ, Kelly RP, MacCallum H, et al. Photoplethysmographic assessment of pulse wave reflection: blunted response to endothelium-dependent beta2-adrenergic vasodilation in type II diabetes mellitus. J Am Coll Cardiol 1999;34:2007–14.
- Mundry R, Fischer J. Use of statistical programs for nonparametric tests of small samples often leads to incorrect p values: examples from animal behaviour. Anim Behav 1998;56:256–9.
