Abstract
Objective: Hypertensive disorders of pregnancy (HDP) affect foetal outcome. Labetalol is frequently used to lower maternal blood pressure and prolong pregnancy. Conflicting evidence exists for specific neonatal side effects described after maternal labetalol treatment. Our aim was to investigate neonatal effects of foetal exposure to labetalol on cerebral oxygenation and extraction.
Methods: In a prospective observational study, clinical characteristics, vital parameters and cerebral oxygen delivery and extraction were collected during the first 24 h of life in labetalol-exposed preterm neonates and compared with two control groups.
Results: Twenty-two infants with a mean gestational age of 28.9 weeks, born from labetalol-treated mothers with HDP were included and matched with 22 infants with non-labetalol-treated mothers with HDP and 22 infants without maternal HDP. No significant differences between groups were found neither in heart rate, blood pressure and inotropic support, nor in mean regional cerebral oxygen saturation and fractional tissue oxygen extraction.
Conclusion: Foetal labetalol exposure associated effects on preterm heart rate, blood pressure, cerebral oxygenation and extraction are not demonstrated. Maternal disease severity seems to play a more important role in neonatal cerebral haemodynamics. Maternal labetalol treatment has no clinically important short term side effects in the preterm neonate.
Introduction
Hypertension is a common disorder encountered in about 6–8% of pregnancies. Gestational hypertension is defined as a blood pressure ≥140/90 mmHg after 20 weeks of gestation. When accompanied by proteinuria (≥300 mg/24 h), it fulfills the criteria for diagnosis of preeclampsia, which can deteriorate to eclampsia, a life threatening event for mother and child. Diagnosis and treatment thresholds of hypertensive disorders in pregnancy (HDP) differ between societies but the pathology causes increased perinatal morbidity and mortality when compared with uneventful gestations [Citation1].
The treatment goals of HDP are preventing maternal complications and improving foetal growth and maturity by permitting prolongation of pregnancy. Both oral and intravenous labetalol are used worldwide as first- or second-line treatment to normalize acute and chronic elevated maternal blood pressure in HDP due to its highly effective antihypertensive properties and a better profile than hydralazine and other β-blockers [Citation2]. Labetalol is a selective α-1 and non-selective β-receptor antagonist inducing peripheral vasodilation but preventing reflex tachycardia and maintaining cardiac output [Citation3]. Due to its lipophilic properties labetalol passes easily the placental barrier. Half-life in hypertensive pregnant mothers in the third trimester is 4.3–6.9 h [Citation4], but can increase up to 24 h in an exposed preterm baby [Citation5]. Most associations of maternal labetalol treatment with adverse infant outcomes are derived from retrospective cohort studies. A recent nationwide population-based cohort study found a β-blocker-associated increase in small for gestational age infants, preterm birth and perinatal mortality – as described by others – but confounding by maternal disease could not be ruled out given the study design [Citation6]. Detailed data on neonatal tolerance and side effects (bradycardia, hypoglycemia, hypotension) are described [Citation7–10] but definitions differ between studies and most severe toxicities attributed to labetalol are published as case reports [Citation11–13].
Scarce detailed information on labetalol-exposed neonatal systemic and cerebral haemodynamics in HDP is available [Citation14]. Moreover foetal cerebral circulation is subject to changes due to brain sparing in severe placental insufficiency as seen in HDP. This response in hypoxic pregnancies with a redistribution of foetal cardiac output towards essential vascular beds such as the brain vasculature, may have an impact on cerebral oxygenation as cerebral blood flow increases. We aimed to investigate labetalol-induced effects on neonatal haemodynamics and cerebral oxygenation in the first 24 h after birth. We hypothesized that maternal exposure to labetalol in preterm offspring would lead to slower heart rate, lower blood pressure, increased need for inotropic support and signs of cerebral hypoperfusion reflected in decreased values of cerebral oxygenation.
Methods
Patients
An observational case-control study was performed in neonates of mothers treated with labetalol for HDP with a gestational age (GA) < 32 weeks admitted in 2010 to the NICU of the Wilhelmina Children’s Hospital in Utrecht (HDPlab), a tertiary referral center in the Netherlands. Infants participated in a longitudinal study in which cerebral oxygenation and extraction were monitored using near infrared spectroscopy (NIRS) for the first 72 h of life. Exclusion criteria were neonates with known or strongly suspected congenital abnormalities. HDPlab group infants were individually matched to infants in two control groups in the 2009–2010 cohort. The HDPnonlab and noHDP group consisted of neonates of mothers with HDP without labetalol treatment and neonates of mothers without HDP, respectively. Beside sex, matching was performed by GA and birthweight with median (range) 6 (1–13) days and 212.5 (80–425) grams, respectively. Definitions used for HDP were the recommendations made by the International Society for the Study of Hypertension in Pregnancy [Citation1]. First and second line treatment for early (GA < 35 weeks) HDP in our hospital consists of the centrally acting α2-receptor antagonist methyldopa and oral labetalol, respectively. If diastolic blood pressure is repeatedly >110 mmHg IV treatment is started with labetalol or dihydralazine sulphate. Magnesium sulphate (MgSO4) is given in preeclampsia and corticosteroids are given to promote foetal maturity if indicated. Informed consent was obtained in all cases. The Medical Ethical committee of the University Medical Center Utrecht approved the study.
Clinical data
Obstetrical data were collected from the hospital record. Prenatal maternal exposure and maximum dose and route of labetalol were noted as was exposure to MgSO4, dihydralazine sulphate and methyldopa. Neonatal data were collected prospectively. Initiation of blood pressure support was decided by the attending neonatologist following written guidelines. Treatment was started if mean arterial blood pressure (MABP) in mmHg was lower than GA in weeks. A blood pressure support scoring system, depending on the intensity of the treatment, was used to assess the intensity of support as earlier described by Krediet et al. [Citation15]. Mechanical ventilation was defined as endotracheal intubation and ventilation during the first 24 h with exclusion of the INSURE-procedure (INtubate-SURfactant-Extubate). Surfactant administration in patients with respiratory distress disease was noted. Using ultrasound, prenatal foetal brain sparing was defined as a pulsatility index (PI) less than percentile 3 in the medial cerebral artery with PI of umbilical artery more than percentile 95 with or without end-diastolic block during pregnancy [Citation16] and intraventricular haemorrhage (IVH) was diagnosed according to the classification of Papile [Citation17] on neonatal admission.
Monitoring cerebral tissue oxygenation
NIRS-derived regional cerebral oxygen saturation (rScO2) measured with the INVOS 5100C monitor and adult SomaSensor (Covidien, Mansfield, MA) was used as a reliable estimator for changes in tissue cerebral oxygenation [Citation18]. To investigate the balance between oxygen delivery and oxygen consumption, a relative cerebral fractional tissue oxygen extraction (cFTOE) measurement can be formulated as a ratio with arterial oxygen saturation (SaO2): cFTOE = (SaO2−rScO2)/SaO2. An increase in cFTOE reflects an increase of oxygen extraction by brain tissue suggesting a higher consumption than delivery of oxygen. Conversely, a decrease of cFTOE suggests less utilization of oxygen by brain tissue in comparison with the supply [Citation19].
Study design
Invasively measured MABP, heart rate (HR), SaO2 and rScO2 were monitored from admission up to 24 h after birth, collected simultaneously and stored on a personal computer (software: Poly 5, Inspektor Research Systems, Amsterdam, NL). Data were analysed offline with 1 Hz sample frequency (SignalBase, Utrecht, NL). Signals were resampled to median samples using windows of one minute (a set of one minute data points around a destination sample) after artefact removal. Each resulting sample was calculated as the median value of all data points in the window. A sample was included if values in the window were present for more than 50% of time. From resampled data, mean values of MABP (mmHg), SaO2 (%), rScO2 (%), cFTOE (1/1) were calculated in six-hourly time frames, started from birth until 24 h afterwards.
Statistical analysis
For statistical analysis, SPSS 20.0 (SPSS Inc., IL) was used. Data were summarized as mean values ± SD or median values and ranges when appropriate. Differences between groups were analysed with the Kruskal–Wallis test, one-way ANOVA, independent t-test, the Pearson Chi-Square or Fisher’s Exact test and between and within groups by linear mixed model analysis, if appropriate. Adjustments for multiple comparisons were made by the Bonferroni post hoc test. The degree of relation between variables was tested using Spearman’s correlation coefficient. A p value of <0.05 was considered statistically significant.
Results
Study population
The clinical characteristics of the three groups: HDPlab, HDPnonlab and noHDP are shown in .
Table 1. Clinical characteristics of offsprings of mothers with HDP with and without labetalol-treatment (HDPlab and HDPnonlab) and offsprings of mothers without HDP (noHDP).
No significant differences were found in Apgar score, inotropic support, need for mechanical ventilation, surfactant administration, severe early onset sepsis and occurrence of large IVH (≥grade 3) between the three groups. There was a significant difference in the occurrence of brain sparing between the three groups (p = 0.003), being highest in HDPnonlab group. No significant differences were found between the HDPlab and the HDPnonlab group concerning exposure to MgSO4, methyldopa and dihydralazine sulphate. The median [range] dose of labetalol was 480 [100–2400] mg/24 h. Sixteen and six mothers received oral (400 [100–1200] mg/24 h) and IV (740 [300–2400] mg/24 h) labetalol treatment, respectively.
Patterns of heart rate, blood pressure and cerebral oxygen delivery and extraction
Heart rate was not significantly different between groups or within groups during the four periods () and always between normal clinical limits (mean ± sd 147 ± 14.6 beats per minute (bpm)). Heart rate and labetalol dose were not significantly correlated ().
Figure 1. Clustered boxplots of mean heart rate (a: HR, bpm), mean arterial blood pressure (b: MABP, mmHg), mean cerebral oxygen saturation (c: rScO2, %) and mean cerebral fractional tissue oxygen extraction (d: cFTOE, 1/1) during the first 24 h of life in offsprings of hypertensive mothers exposed to labetalol (HDPlab), offsprings of hypertensive mothers not exposed to labetalol (HDP-lab) and offsprings of non-hypertensive mothers (noHDP). Mean heart rate did not differ significantly between and within groups. Mean MABP increased over time in all groups but only reached significance in the noHDP group. No significant differences were measured between groups for mean rScO2 and mean cFTOE at any time point. In the noHDP group rScO2 increased while cFTOE decreased significantly over time (#p < 0.001, *p < 0.05).
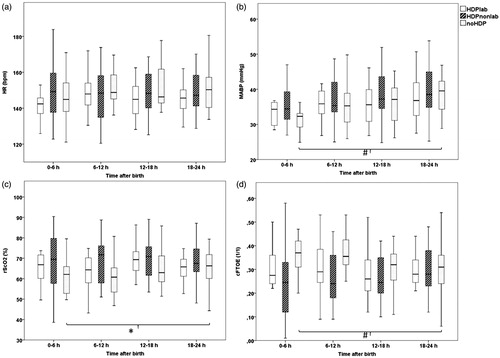
Table 2. Relation between maternal dose of labetalol and haemodynamic parameters of labetalol-exposed infants in the first 24 h after birth.
An expected physiological increase in mean MABP was seen during the first day in all groups, only reaching significance in the noHDP group (mean ± sd HDPlab 35 ± 6.3 to 37 ± 6.6 mmHg, p = 0.08; HDPnonlab 36 ± 5.5 to 40 ± 6.9 mmHg, p = 0.05; noHDP 31 ± 3.6 to 39 ± 6.5 mmHg, p < 0.001). MABP was not different between groups on the different time points and significant correlation between MABP and labetalol dose was not found (, ). As already shown in , no difference between groups in inotropic support was seen on all time points.
To assess cerebral oxygenation and extraction, patterns of rScO2 and cFTOE were compared between groups and no significant differences at any time point were found (). In the noHDP group rScO2 increased while cFTOE decreased significantly over time (from 61 ± 9.1 to 65 ± 11%, p < 0.05 and 0.36 ± 0.09 to 0.32 ± 0.11, p < 0.001, respectively). This finding was lacking in both HDP groups. There was no significant correlation between labetalol dose and rScO2 or cFTOE at any time point ().
Discussion
In this study we compared vital haemodynamic parameters and cerebral oxygenation and extraction of 22 preterm neonates prenatally exposed to labetalol with two control groups during the first 24 h of life. Within the described dose range of maternal labetalol, no differences compared to controls concerning neonatal blood pressure, heart rate, blood pressure support nor differences concerning regional cerebral oxygen saturation and oxygen extraction were found. Labetalol dosage had no effect neither on cerebral oxygenation, heart rate nor on blood pressure of the preterm infant during the first 24 h after birth. To our best knowledge this is the first paper describing the exclusive effect of maternal labetalol on NIRS measured cerebral oxygenation.
Contradictory reports concerning neonatal blood pressure and/or heart rate after foetal labetalol exposure are published. Macpherson et al. found no difference in heart rate and a small subclinical difference in systolic blood pressure in 11 term babies of mothers treated with labetalol versus placebo [Citation7]. Plouin et al. found no difference in heart rate and blood pressure in offsprings of mothers treated with labetalol versus methyldopa for mild to moderate hypertension [Citation8]. Hjertberg et al. found no difference in neonatal heart rate and blood pressure up to seven days after birth comparing hydralazine and labetalol for mothers with moderate to severe hypertension [Citation9]. However, in a well-described cohort of preterm infants exposed to labetalol in utero Heida et al. found no association with heart rate <100 bpm in the first 48 h after birth but higher incidence of hypotension irrespective of dosage and route of administration. Differentiation between labetalol versus patent ductus arteriosus (PDA) effects was difficult since PDA appeared independently associated with hypotension. Absence of a dose dependent effect suggested that the observed association with labetalol may be due to other factors such as maternal disease severity rather than pharmacological effects of labetalol [Citation10].
In our case-control study, with detailed measurements of heart rate and blood pressure, but also cerebral oxygenation, an expected physiological increase in mean MABP was seen during the first day [Citation20] but significance was only reached in the noHDP group. Also, the significant increase of rScO2 and decrease of cFTOE during the first day in the noHDP group was not seen in both HDP groups. A possible explanation for this, besides the small sample size, could be disease severity with a less effective regulation of the vascular tone in preterm infants exposed to maternal HDP [Citation21] and/or central/peripheral vasodilating effects of other vaso-active drugs (i.e. MgSO4, methyldopa, dihydralazine sulphate). Prevalence of hypotension and inotropic support being not different between groups remains a favorable finding since both conditions are associated with adverse neurodevelopmental outcome [Citation22]. However, whether the lack of increase of blood pressure and rScO2 in the first 24 h in both HDP groups has any effect on long term outcome remains unknown.
Using trend monitoring of cerebral oxygenation as an estimator of cerebral blood flow after birth, cerebral oxygen delivery and consumption remained stable and without dose correlation following foetal exposure to labetalol. In particular, no compromised oxygenation at risk for hypoxic brain injury (rScO2<40–45%) was seen [Citation18]. Comparable to this important finding, stable foetal cerebral blood flow during and after intravenous maternal labetalol treatment in HDP is described by others in two studies where no alterations in foetal blood flow measured by 133Xenon isotope clearance method and foetal Doppler ultrasound parameters were found [Citation23,Citation24].
Although not significant, possibly due to small sample size, a remarkable trend of higher mean rScO2 and lower mean cFTOE in both groups with HDP was observed in the first 12 h of life, in contrast to our hypothesis. Verhagen et al. [Citation14] described lower cFTOE values in nine preterm neonates prenatally exposed to labetalol and/or MgSO4 versus no exposure but could not differentiate between drugs and maternal disease severity. This current study demonstrates labetalol exposure by itself is not associated with differences in preterm cerebral haemodynamics when compared to exposure to other antihypertensive treatment.
The trend of higher mean rScO2 and lower mean cFTOE could be explained by occurrence of brain sparing in almost half of the HDP infants as a result of longstanding hypoxic state in utero. Although still within normal values, some important remarks regarding oxygenation of the immature brain of preterm infants with brain sparing and exposure to labetalol and other antihypertensive drugs have to be made. Postnatal cerebral vasodilation after foetal brain sparing as described by Scherjon et al. [Citation25] together with the possible effect of vaso-active antihypertensive drugs could have a negative effect on the autoregulatory capacity of the brain in these immature infants [Citation26]. Pressure passive cerebral circulation in infants of mothers with antihypertensive medication is possible. If autoregulatory mechanisms fail during blood pressure instability, both cerebral hypoxia and cerebral hyperoxia render the foetal and neonatal brain at risk for the occurrence of intraventricular haemorrhage [Citation27]. Continuously monitored rScO2 and MABP can be used to estimate the autoregulatory capacity of the brain [Citation28]. Caicedo showed in his model that infants of mothers treated with labetalol have evidence of decreased autoregulatory capacity but these results need to be confirmed [Citation29].
We are aware of limitations in this case-control study. First, power calculation was not performed but up to date, this is the largest study in literature describing neonatal cerebral oxygenation after foetal labetalol exposure. Using small sample sizes and three defined groups we were able to elucidate the specific effects of labetalol on cerebral circulation. Secondly, labetalol blood levels were not measured to give information about pharmacokinetic effects of labetalol. We studied the first 24 h of life in the assumption labetalol concentration and thus pharmacodynamic effects would be highest in that time frame. Finally, the effect of labetalol on maternal blood pressure was not investigated and disease severity was based on treatment given to the mother.
In conclusion, we report a group of preterm neonates with foetal labetalol exposure carefully matched with two control groups in which haemodynamic vital parameters and cerebral oxygenation and extraction were monitored. Clinically important negative effects of labetalol in preterm newborns on heart rate, blood pressure, cerebral oxygenation and extraction were not seen. Within the dose range and patient group described, maternal treatment with labetalol to prolong pregnancy is not associated with clinically important short term neonatal side effects concerning cerebral haemodynamics in the transitional phase.
Declaration of interest
No declaration of interest is reported.
Acknowledgements
The authors acknowledge the children and families who participated in this project.
References
- Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens 2014;4:97–104
- Magee LA, Pels A, Helewa M, et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens 2014;4:105–45
- Baum T, Sybertz EJ. Pharmacology of labetalol in experimental animals. Am J Med 1983;75:15–23
- Saotome T, Minoura S, Terashi K, et al. Labetalol in hypertension during the third trimester of pregnancy: its antihypertensive effect and pharmacokinetic-dynamic analysis. J Clin Pharmacol 1993;33:979–88
- Haraldsson A, Geven W. Half-life of maternal labetalol in a premature infant. Pharm Weekbl Sci 1989;11:229–31
- Meidahl Petersen K, Jimenez-Solem E, Andersen JT, et al. β-Blocker treatment during pregnancy and adverse pregnancy outcomes: a nationwide population-based cohort study. BMJ Open 2012;2:e001185
- Macpherson M, Broughton Pipkin F, Rutter N. The effect of maternal labetalol on the newborn infant. Br J Obstet Gynaecol 1986;93:539–42
- Plouin PF, Breart G, Maillard F, et al. Comparison of antihypertensive efficacy and perinatal safety of labetalol and methyldopa in the treatment of hypertension in pregnancy: a randomized controlled trial. Br J Obstet Gynaecol 1988;95:868–76
- Hjertberg R, Faxelius G, Lagercrantz H. Neonatal adaptation in hypertensive pregnancy – a study of labetalol vs hydralazine treatment. J Perinat Med 1993;21:69–75
- Heida KY, Zeeman GG, Van Veen TR, Hulzebos CV. Neonatal side effects of maternal labetalol treatment in severe preeclampsia. Early Hum Dev 2012;88:503–7
- Stevens TP, Guillet R. Use of glucagon to treat neonatal low-output congestive heart failure after maternal labetalol therapy. J Pediatr 1995;127:151–3
- Olsen KS, Beier-Holgersen R. Foetal death following labetalol administration in pre-eclampsia. Acta Obstet Gynecol Scand 1992;71:145–7
- Haraldsson A, Geven W. Severe adverse effects of maternal labetalol in a premature infant. Acta Paediatr Scand 1989;78:956–8
- Verhagen EA, Kooi EM, van den Berg PP, Bos AF. Maternal antihypertensive drugs may influence cerebral oxygen extraction in preterm infants during the first days after birth. J Matern Foetal Neonatal Med 2013;26:871–6
- Krediet TG, Valk L, Hempenius I, et al. Nitric oxide production and plasma cyclic guanosine monophosphate in premature infants with respiratory distress syndrome. Biol Neonate 2002;82:150–4
- Scherjon SA, Smolders-DeHaas H, Kok JH, Zondervan HA. The “brain-sparing” effect: antenatal cerebral Doppler findings in relation to neurologic outcome in very preterm infants. Am J Obstet Gynecol 1993;169:169–75
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular haemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529–34
- van Bel F, Lemmers P, Naulaers G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology 2008;94:237–44
- Naulaers G, Meyns B, Miserez M, et al. Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. A validation study in piglets. Neonatology 2007;92:120–6
- Cunningham S, Symon AG, Elton RA, et al. Intra-arterial blood pressure reference ranges, death and morbidity in very low birthweight infants during the first seven days of life. Early Hum Dev 1999;56:151–65
- Antonios TF, Raghuraman RP, D’Souza R, et al. Capillary remodeling in infants born to hypertensive pregnancy: pilot study. Am J Hypertens 2012;25:848–53
- Dempsey EM, Barrington KJ. Evaluation and treatment of hypotension in the preterm infant. Clin Perinatol 2009;36:75–85
- Jouppila P, Kirkinen P, Koivula A, Ylikorkala O. Labetalol does not alter the placental and foetal blood flow or maternal prostanoids in pre-eclampsia. Br J Obstet Gynaecol 1986;93:543–7
- Baggio MRF, Martins WP, Calderon ACS. Changes in foetal and maternal doppler parameters observed during acute severe hypertension treatment with hydralazine or labetalol: a randomized controlled trial. Ultrasound Med Biol 2011;37:53–8
- Scherjon SA, Oosting H, Kok JH, Zondervan HA. Effect of foetal brainsparing on the early neonatal cerebral circulation. Arch Dis Child Foetal Neonatal Ed 1994;71:F11–15
- Greisen G. Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev 2005;81:423–8
- Alderliesten T, Lemmers PMA, Smarius JJM, et al. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular haemorrhage. J Pediatr 2013;162:698–704.e2
- Caicedo A, De Smet D, Naulaers G, et al. Cerebral tissue oxygenation and regional oxygen saturation can be used to study cerebral autoregulation in prematurely born infants. Pediatr Res 2011;69:548–53
- Caicedo A, Thewissen L, Naulaers G, et al. Effect of maternal use of labetalol on the cerebral autoregulation in premature infants. Adv Exp Med Biol 2013;789:105–11
