Abstract
Objective: Asphyxia is a major cause of disabilities in term-born infants. Here we have explored the value in HIE (hypoxic-ischemic-encephalopathy) of using a combination of serum pro-oxidant/antioxidant balance (PAB) assay for predicting the prognosis of asphyxia.
Method: Ninety term neonates with asphyxia were enrolled and followed up for two years. Serum PAB, demographic/biochemical characteristics of mothers, and their neonates were determined. The Denver II test was used to assess outcomes.
Results: Of the 90 asphyxiated neonates, 47 (52.2%) had a normal outcome and 43 babies (47.8%) had abnormal outcome. Serum PAB levels in neonates with normal and abnormal outcomes were 17.1 ± 9.23 and 48.27 ± 41.30 HK, respectively. A combination of HIE intensity and PAB, compared to other indicators, had a higher predictive-value (95.2%) for outcomes in asphyxiated babies.
Conclusion: We demonstrate that PAB in combination with HIE grade may have a better predictive value for the prognosis of asphyxiated babies and predicting future neurologic problems in asphyxiated term infants.
Introduction
Perinatal asphyxia is associated with a lack of oxygen and perfusion in several organs [Citation1]. Asphyxia remains a major clinical concern [Citation2], although several investigations have been carried out in the past decades. According to the World Health Organization data, 3% of infants (3.6 million per annum) suffer from moderate to severe asphyxia in developing countries; 23% of these infants (840 000 people) died, and almost the same number of infants with asphyxia have serious complications [Citation3]. It has been reported that asphyxia, associated with severe prematurity, is the second leading cause of neonatal mortality (20%) in Ghaem Hospital in Mashhad [Citation4]. The diagnosis of severe asphyxia is determined by Apgar scores, arterial blood gases, and symptoms of HIE (hypoxic ischemic encephalopathy) [Citation3,Citation5,Citation6]. The incidence of HIE in newborns with perinatal asphyxia is 50% to 60% [Citation7].
Moderate to severe HIE is a major cause of mortality and morbidity and permanent neurological disability in survived newborns [Citation8]. Perinatal asphyxia can cause severe hypoxic-ischemic injury in the newborn organs and cause fatal or serious long-term complications. Therefore determining the prognosis of asphyxia in order to prevent and treat before its complications develop is a priority objective in infants and children [Citation9]. There is no gold standard for the diagnosis of HIE, but the following parameters are currently used to assess it: Apgar score of 0–3 in the fifth minute after birth, excessive umbilical artery acidemia, abnormal electronic fetal monitoring during labor, measuring scalp pH along with the presence of meconium in the amniotic fluid, multisystem involvement within 72 h after delivery and early imaging evidence of acute non-focal brain damage and encephalopathy [Citation10].
Currently, there are no biomarkers to effectively predict outcome of perinatal asphyxia. A combination of different methods is utilized in the diagnosis of perinatal asphyxia [Citation11]. It has been suggested that asphyxia is associated with oxidative stress [Citation12]. An increased in oxidants and decreased in antioxidants is involved in the pathogenesis of several diseases including neonatal asphyxia. Free radicals (−O2, H2O2, OH−, etc.) are produced by metabolic processes or external sources, and these have the ability to react with DNA and proteins. Antioxidants can offset these effects [Citation13]. The evaluation of the activity of oxidants and antioxidants simultaneously by using tetramethyl benzoyl (TMB) could provide a method for assessing the balance [Citation14]. Therefore the aim of present investigation was to evaluate the predictive value of the serum PAB assay in infants with asphyxia.
Materials and methods
Population
The current prospective, observational study was conducted between January 2008 and May 2015, in Ghaem Hospital, as a tertiary referral hospital, in which approximately 3000 child are born annually. The inclusion Criteria were: (1) those with fetal distress (late deceleration, lack of heart rate variability, FHR < 100); (2) those associated with thick meconial amniotic fluid plus hypotonia, bradycardia, or respiratory distress; (3) neonates with an Apgar score < 4 within the first minute or an Apgar score < 7 within the first 5 minutes; (4) neonates requiring cardiopulmonary resuscitation more than 1 minute using oxygen and IPPV (intermittent positive pressure ventilation); (5) Blood pH < 7.2 and base deficit (BD) < −12.
Initially 98 neonates were eligible for the study but 8 of these were excluded due to incomplete data. The exclusion criteria included: Congenital malformations, metabolic disturbances, congenital or perinatal infections, or maternal chorioamnionitis.
According to the criteria of Sarnat, HIE was classified as mild (Grade I) if the neonate was hyperalert, hyperexcitable, with normal muscle tone and no seizures; as moderate (Grade II) if the infant was hypotonic with decreased movements and often seizures; and severe (Grade III) if the infant was stuporous, flaccid without primitive reflexes and usually with seizures.
Informed parental consent was obtained. The study protocol was approved by the Ethics Committee of Mashhad University of Medical Sciences.
Biochemical analysis and anthropometric measurements
Anthropometric parameters were recorded for all individuals. Blood samples were collected from all asphyxiated infants immediately after delivery. The PAB assay, pH, BD, PCO2, PO2, O2 saturation measurement were carried out for all the subjects. A comprehensive clinical examination was used to evaluate all subjects at the day of their born, 3 and 7 days. Neurologic examination was performed by same neonatologists.
Pro-oxidant/antioxidant balance (PAB) assay
The pro-oxidant/antioxidant balance (PAB) assay was performed, as previously described [Citation12]. Briefly, 400 μL of TMB (3,3′,5,5′-tetramethylbenzidine, Fluka), which was prepared in DMSP, was added in 20 mL of acetate buffer [0.05 M buffer, pH 4.5]. 70 μL of fresh chloramine T (100 mM) solution was added in the mix followed by incubation for 2 h. for standard control, different proportions of 250 μM hydrogen peroxide (Darmstadt, Germany) were prepared by mixing with 3 mM uric acid (in 10 mM NaOH). The mix was transferred to a 96-well plate. Then 100 μL of 2 N HCl was added and measured in an ELISA plate reader at 450 nm with a reference wavelength of 620 nm. A standard curve was constructed from the values derived using standard samples. The values of the serum PAB were expressed in arbitrary HK units, which represent the percentage of hydrogen peroxide in standard solution.
Denver Developmental Screening Test
Infants were followed up at 6, 12, 18, 24 months for assessment of developmental assessment. The Denver Developmental Screening Test (DDST), is known as the Denver Scale, which is used for screening cognitive and behavioral problems in preschool children.
The tests cover four general functions: personal social (such as smiling), fine motor adaptive (such as grasping and drawing), language, and gross motor. A child fails a Denver screen if he or she has a delay in any of the above domains. If the child has a delay in one or two domains, he/she will be considered as a mild or moderate developmental, respectively. If he/she has a problem in 3 or more domains, he/she will be considered as severe developmental delay [Citation15].
Statistical analysis
Data were analyzed using SPSS-20 software (IBM, Chicago, IL). The values were expressed as mean ± SD. Student’s t-test, Kruskal–Wallis test, and Mann–Whitney U test were used. Parametric and non-parametric correlations were assessed using Pearson’s correlation coefficients and Spearman’s correlation coefficients, respectively. For determining the predictive value of method, regression models were applied. Moreover, we drew the receiver-operating characteristic (ROC) curve to evaluate the sensitivity and specificity of HIE, PAB, and other indicators for determining outcomes in asphyxiated babies. p value < 0.05 was considered statistically significant.
Results
During the follow-up period, 90 asphyxiated newborns were enrolled, in which 47 of cases (52.2%) had normal outcomes and 43 (47.8%) had abnormal outcomes. As shown in , the statistically significant differences were detected between two groups with respect to PAB (p < 0.0001), fifth minute Apgar score (p < 0.0001), white blood cell count of the neonate (p = 0.033), duration of ventilation (p = 0.017), and pH at the first hour after birth (p < 0.0001). However no significant differences were identified with respect to birth weight, first minute Apgar score, neutrophils, lymphocytes, platelet count, first hour base excess (BE), and first hour HCO3− ().
Table 1. Clinical characteristics of asphyxiated newborns with normal and abnormal outcome.
Additionally, the final outcome of newborns after 6, 12, 18, and 24 months was also studied. This analysis showed that 52.2% of cases had normal outcomes and 47.8% had abnormal outcomes. The degree of HIE was significantly related to prognosis, and with an increased severity of HIE. Infants without HIE had no cases of mild to severe developmental delay and neonatal mortality was observed in 1 case. In infants with HIE Grade I, 3 cases (42%) had mild developmental delay, 2 cases (28%) had moderate developmental delay, 1 had (14%) severe developmental delay, and 1 baby (14%) died. In infants with HIE Grade II, 9 baby (33%) had mild developmental delay, 4 babies (14%) had moderate developmental delay, 5 babies (18%) had severe developmental delay, and 5 babies (18%) died. In infants with HIE III, 1 baby (4%) had mild developmental delay, 1 baby (4%) had moderate developmental delay, 5 babies (2%) had severe developmental delay, and 17 babies (70%) were died.
Chi-square test showed that there was a significant difference between two groups about the degree of HIE (p < 0.0001) and the need for mechanical ventilation (p < 0.0001). The values of these variables were higher in asphyxiated babies with abnormal outcome. Interestingly, no significant differences were observed for sex and method of delivery ().
Table 2. Comparison of maternal and neonatal parameters between the two groups of asphyxiated infants with normal and abnormal outcomes unite: n (%).
Moreover, PAB, degree of HIE, fifth-minute Apgar score, and first hour pH were the most important determinants of prognosis for infants with asphyxia, as detected by regression models and ROC curve (, Supplementary Figure S1). As shown in and , the combination of PAB and severity of HIE had more than 95% predictive value, compared to other indicators, for determine the prognosis of birth asphyxia (; Supplementary Figure S1).
Figure 1. ROC curve for HIE, PAB, and other indicators for determining outcomes in asphyxiated babies.
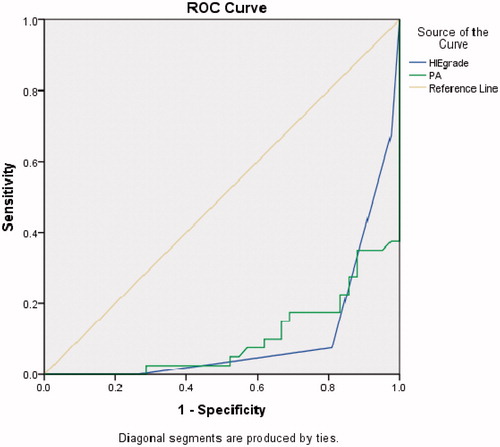
Table 3. Comparison the combination of PAB, severity of HIE, first hour pH, and fifth minute Apgar score in order to determine the prognosis of developmental delay.
Discussion
To the best of our knowledge, this is the first study evaluating and testing a simple, rapid, and cheap method to measure the balance between pro-oxidant and antioxidant simultaneously for assessing the prognosis of birth asphyxia. We found that PAB, fifth minute Apgar score, and first hour pH are important determinants for the prognosis of asphyxiated infants and the serum PAB assay may be used for evaluating the prognosis of birth asphyxia. Moreover, we observed that asphyxiated babies with abnormal outcomes had an increased in serum PAB and lower fifth minute Apgar score and pH, which is in line with our observation [Citation2]. In particular, Bijari et al. show that pH and bicarbonate levels of neonatal Apgar score at five minutes in neonatal asphyxia were less than healthy babies [Citation16]. Furthermore, there was a difference for duration of ventilation between groups. This could suggest that a longer time was needed for mechanical ventilation in asphyxiated neonatal was related to unusual consequences. Withdrawal of gas exchange in placenta or lungs is the main cause of hypoxemia, hypercapnia, and perinatal asphyxia. Thus, the current therapeutic strategies, including supportive procedures, such as oxygen therapy and mechanical ventilation, blood pressure regulations, keeping normoglycemia, hydrotherapy, and induced hypothermia are required to reduce neurological problems [Citation17].
WBC count was statistically different between our studied groups. In particular, an increase in the number of peripheral leukocytes in the first 96 h of life indicates abnormal neuromotor outcome in asphyxia [Citation18]. Our data showed that the number of neutrophils, lymphocytes, and platelets at the first day of life did not change. It has been shown that blood biophysical profile changes, such as changes in the structure and function of erythrocytes, leukocytes and thrombocytes may occur due to asphyxia [Citation19,Citation20]. Thrombocytopenia may be present at the second day of life but at the tenth day of life it will become normal. Brucknerová et al. [Citation21] and Phelan et al. [Citation22] demonstrated that thrombocytopenia was not associated with acute asphyxia at birth and was not a sensitive marker for mild acute asphyxia. The study of Brucknerová et al. reported that the number of leukocytes was not different between healthy term infants and asphyxiated newborns [Citation21]. This discrepancy can be explained at least in part by population: the comparison in the Brucknerová study was between healthy and asphyxiated newborns, while in our study all of newborns were asphyxiated. Also, it is possible that WBC in newborn with bad prognosis is higher than normal prognosis. Furthermore, our findings suggested that BE and bicarbonate levels in the first hour of life were not different between two groups. Portman and colleagues revealed that the score made from a combination of factors including cardiotocography impairment, umbilical artery BE, and low Apgar score at five minutes were a predictor of outcomes of asphyxia [Citation23]. Another study by Carter et al. showed positive predictive value of these combined factors [Citation24].
Moreover, we observed a statistically significant correlation between the severity of HIE and long-term prognosis of these children. In the group of asphyxiated newborns with normal outcome, 32.5% of cases were without HIE, 60% were HIE Grade I, 7.5% were HIE Grade II. In asphyxiated newborns with abnormal outcomes 16.66%, 54.76%, and 26.19% of cases were Grade I, Grade II, and Grade III, respectively. Additionally, babies without HIE did not have developmental delay. In particular, 42%, 28%, and 14% of cases with Grade I were mild, moderate, and severe developmental delay respectively. Nine Infants with HIE Grade II had mild developmental delay, 4 babies had moderate developmental delay, 5 babies had severe developmental delay, and 5 babies died. Regarding cases with HIE Grade III, 1 baby had mild developmental delay, 1 baby had moderate developmental delay, 5 babies had severe developmental delay, and 17 babies died. George et al. evaluated the state of mental and motor development in 48 term newborns with HIE. Among 41 babies with HIE Grade I babies, 15 infants (36%) had normal development and only 4 baby (9.8%) had mental retardation. Among other 7 babies with HIE Grade II or III, 2 babies had shown mental retardation, 3 babies had average intelligence, and 2 children had low intelligence. The most common mental health problems at all stages of HIE were hyperactivity (23%) and physical disorders (17%). The vision disorders, cerebral palsy and mental problems were seen in children with HIE Grade II and III [Citation25]. Also HIE grade, and need for ventilation had a significant differences between two groups of asphyxiated newborns with normal and abnormal outcomes. Consistent with the findings of other studies, severe developmental delay was related to cerebral abnormalities during prenatal and HIE grade [Citation26,Citation27]. Moreover, gender and mode of delivery were different with respect to outcome of both normal and abnormal groups, which is in agreement with previous studies [Citation28]. Our team was previously showed that gestational age and modes of delivery were not significantly different between two groups of asphyxiated and healthy babies [Citation3]. A retrospective study (1999–2000) in Thailand examined the risk factors for neonatal HIE in 17 706 hospitalized newborns. The results showed 84 infants were born with birth asphyxia, and first and fifth minute Apgar, male gender, cord prolapse, post-term delivery, and delivery with instruments were the major risk factors for HIE, but only fifth-minute Apgar score had significant relation with HIE [Citation29].
In conclusion, we demonstrated that PAB ratio, fifth minute Apgar score, WBC count, and pH during the first hour of life were significantly different between asphyxiated newborns with normal versus abnormal outcome. PAB, HIE, fifth-minute Apgar score, and first hour pH were the most important determinants to predict the prognosis of birth asphyxia. A combination of PAB and HIE degrees had more predictive value in compare to other indexes for prognosis of asphyxia. Further studies are needed to confirm the emerging data and value of PAB assay for identification of high-risk infants and assess the asphyxiated term infants as a predictive method.
Declaration of interest
The authors have no conflict of interest to disclose.
This work was supported by grant from Mashhad University of Medical Science.
References
- Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–58
- Boskabadi H, Boroujeni AN, Mostafavi-Toroghi H, et al. Prooxidant-antioxidant balance in perinatal asphyxia. Indian J Pediatr 2014;81:248–53
- Boskabadi H, Tavakol Afshari J, Maamouri G, et al. Association between serum interleukin-6 levels and severity of perinatal asphyxia. Asian Biomed 2010;4:79–85
- Boskabadi H, Parvini Z, Tahereh B, Asiyeh M. Study of the causes and predisposing factors in neonatal mortality in Ghaem Hospital (March 2009 to May 2010). Iranian J Obstet Gyn Infer 2012;14:21–6
- Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev 2001;7:56–64
- Low JA, Pickersgill H, Killen H, Derrick EJ. The prediction and prevention of intrapartum fetal asphyxia in term pregnancies. Am J Obstet Gynecol 2001;184:724–30
- Collaborators MDS. Causes of neonatal and child mortality in India: nationally representative mortality survey. Lancet 2010;376:1853
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 2010;86:329–38
- Golubnitschaja O, Yeghiazaryan K, Cebioglu M, et al. Birth asphyxia as the major complication in newborns: moving towards improved individual outcomes by prediction, targeted prevention and tailored medical care. EPMA 2011;2:197–210
- Hankins GD, Speer M. Defining the pathogenesis and pathophysiology of neonatal encephalopathy and cerebral palsy. Obst Gyn 2003;102:628–36
- Ghosh B, Mittal S, Kumar S, Dadhwal V. Prediction of perinatal asphyxia with nucleated red blood cells in cord blood of newborns. Int J Gynaecol Obstet 2003;81:267–71
- Alamdari DH, Paletas K, Pegiou T, et al. A novel assay for the evaluation of the prooxidant–antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clin Bioch 2007;40:248–54
- Bae S-C, Kim S-J, Sung M-K. Impaired antioxidant status and decreased dietary intake of antioxidants in patients with systemic lupus erythematosus. Rheumatology Int 2002;22:238–43
- Zeraati AA, Mirfeizi Z, Sanati I, et al. Assessment of correlation between prooxidant-antioxidant balance and nephritis in patients with systemic lupus erythematosus. Med J Mash Uni Med Sci 2014;57:684–9
- Frankenburg WK, Dodds J, Archer P, et al. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics 1992;89:91–7
- Bahman Bijari B, Farahmandinia Z, Hazeghi A. Predictive value of nucleated red blood cell counts in cord and peripheral blood of asphyxiated term neonates in the first week of life. JSSU 2010;17:330–6
- Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70
- Morkos A, Hopper A, Deming D, et al. Elevated total peripheral leukocyte count may identify risk for neurological disability in asphyxiated term neonates. J Perinatol 2007;27:365–70
- Bracci R, Perrone S, Buonocore G. The timing of neonatal brain damage. Biol Neonate 2006;90:145–55
- Curtin WM, Shehata BM, Khuder SA, et al. The feasibility of using histologic placental sections to predict newborn nucleated red blood cell counts. Obstet Gynecol 2002;100:305–10
- Brucknerová I, Ujházy E, Dubovický M, Mach M. Early assessment of the severity of asphyxia in term newborns using parameters of blood count. Interdiscip Toxicol 2008;1:211
- Phelan JP, Kirkendall C, Korst LM, Martin GI. Nucleated red blood cell and platelet counts in asphyxiated neonates sufficient to result in permanent neurologic impairment. J Maternal Fetal Neonatal Med 2007;20:377–80
- Portman RJ, Carter BS, Gaylord MS, et al. Predicting neonatal morbidity after perinatal asphyxia: a scoring system. Am J Obstet Gynecol 1990;162:174–82
- Carter BS, McNabb F, Merenstein GB. Prospective validation of a scoring system for predicting neonatal morbidity after acute perinatal asphyxia. J Pediatrics 1998;132:619–23
- George B, Padmam M, Nair M, et al. Hypoxic ischemic encephalopathy developmental outcome at 12 years. Indian Pediatrics 2009;46:s67–70
- Menkes JH, Sarnat HB, Maria BL. Child neurology. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2006
- Volpe JJ. Neurology of the newborn. Philadelphia: Elsevier Health Sciences; 2008
- Hatami G, Motamed N, Darvishi Z. Outcomes and survival of neonates with hypoxic ischemic encephalopathy (HIE) in a university hospital in Bushehr port 1999–2006. ISMJ 2006;9:36–44
- Itoo BA, Al-Hawsawi ZM, Khan AH. Hypoxic ischemic encephalopathy. Incidence and risk factors in North Western Saudi Arabia. Saudi Med J 2003;24:147–53
