Abstract
Objective: Nucleated-red-blood-cells (NRBC) count in umbilical cord of newborns is been suggested as a sign of birth asphyxia. The present study was conducted to explore the value of NRBC count in prognosis of asphyxiated neonates.
Methods: Sixty-three neonates with asphyxia were followed up for two years. Maternal and neonatal information was recorded follow by clinical and laboratory evaluation. NRBC-level was determined per 100 white-blood-cells (WBC). After discharge, follow-up of asphyxiated infants was performed using Denver II test at 6, 12, 18 and 24 months. Neonates were divided into two groups, with favorable and unfavorable outcome based on developmental delay or death.
Results: We observed that NRBC count with more than 11 per 100 WBC, had sensitivity of 85% and specificity of 90% in predicting complications of asphyxia, while in absolute NRBC count with more than 1554, the sensitivity and specificity were 85% and of 87%, respectively. Combination of NRBC + HIE (hypoxic ischemic encephalopathy) grade had a high-predictive power for determining the prognosis of asphyxia in neonates.
Conclusion: We demonstrate that NRBC/100 WBC and absolute NRCB count can be used as prognostic marker for neonatal asphyxia, which in combination with the severity of asphyxia could indicate high infant mortality, and complications of asphyxia. Further studies in a larger and multi center setting trail are warranted to investigate the value of NRBC and HIE in asphyxiate term infants.
Introduction
Asphyxia may have serious effects on many major and vital organs of neonates and can lead to respiratory distress syndrome, disseminated intravascular coagulation, necrosis of subcutaneous fat, myocardial ischemia, adrenal hemorrhage, metabolic complications, acute tubular necrosis, neurological complications such as brain paralysis, convulsion and reduce in learning [Citation1–3]. Asphyxia is one of the main causes of infant mortality and chronic neurologic disabilities in survived neonates. This condition occurs in 2–10% of deliveries [Citation4,Citation5]. According to the reports of the World Health Organization (WHO) in developing countries, three percent of newborns (6.3 million people) suffer from moderate or severe asphyxia, although 23% of them (840 000 people) die and approximately the same number of subjects confront the complications of asphyxia [Citation2]. Asphyxia is the cause of about one-fifth of neonatal deaths and the second leading cause of neonatal mortality after severe prematurity [Citation6]. Perinatal asphyxia can cause severe hypoxic-ischemic damages in the organs of neonates and cause severe long-term consequences or fatal complications. So, predicting the prognosis of asphyxia for prevention and targeted treatment before the incidence of complications should be given the high priority in neonatology and pediatrics [Citation4,Citation7]. Thus, a reliable marker is required to predict the hospital stay and the prognosis of neonates with perinatal asphyxia [Citation8].
Nowadays, diagnosis of asphyxia is performed by several methods, including: Apgar score, arterial blood gases, HIE signs, NRBCs [Citation9], abnormal symptoms in electronic fetal monitoring during labor, as well as biochemical markers such as lactate, LDH, Peroxidan antioxidant balance [Citation10,Citation11] and heat shock protein [Citation12–14]. However there is no effective indicator for prediction of perinatal asphyxia although a combination of different methods and markers could help in the diagnosis of perinatal asphyxia [Citation15]. It has recently been suggested that the increase in NRBC count in the umbilical vein of newborns can be considered a sign of birth asphyxia [Citation16]. In healthy newborns, NRBC count is reduced by half 12 h after birth, and there are only 20–30 NRBCs/m3 48 h after birth. Although on the third or fourth day of birth, NRBCs are not seen in the blood circulation, but in preterm newborns, small amounts of NRBCs may be seen in the first week of life [Citation17–20]. On the other hand, tissue hypoxia results in increased levels of erythropoietin and erythrocytes. NRBC count reflects high production of erythropoietin; it means that erythropoietin stimulates fetal hematopoietic system, mainly in bone marrow, which increases the production of RBCs [Citation21–23]. Increase in NRBC count is often due to prematurity, ABO or RH blood incompatibility, increase in hematopoiesis followed by chronic diseases, maternal diabetes, preeclampsia, fetal anemia, intrauterine infections, chorioamnionitis and acute or chronic asphyxia [Citation21,24–28]. In our previous study, we performed preliminary diagnosis of perinatal asphyxia based on NRBC count. We observed that NRBC/100 WBC or absolute NRBC count can be considered as simple markers for the evaluating of severity and primary consequences of perinatal asphyxia [Citation11].
Despite extensive effort in the pathophysiology of asphyxia, the severity of asphyxia and its long-term consequences is still remained as a major clinical concern. According to the high prevalence of asphyxia and its problems, in the current study we further evaluated the prognostic value of NRBC and other indicators in neonates with asphyxia.
Materials and methods
Population
The current study was a cohort study with a follow-up of two years that was conducted on 80 asphyxiated term neonates in Mashhad Ghaem Hospital during 2011–2015. The study was undertaken with the approval of the Ethics Committee of the Mashhad University of Sciences.
Assessment of asphyxia and biochemical analysis
Perinatal asphyxia was defined as the presence of at least two of the following conditions: (1) Fetal distress symptoms ((heart rate of less than 100 beats per minute, late decelerations, or an absence of heart rate variability); (2) Thick, meconium stained amniotic fluid and respiratory depression, hypotonia or bradycardia; (3) Apgar score less than 4 in the first minute and less than 7 in the fifth minute; (4) The need for resuscitation for more than one minute with positive pressure ventilation (PPV) and oxygen in the delivery room; (5) Blood pH value of less than 7.20 or a base deficit of at least 12 mmol/L within the first hour after birth. Exclusion criteria were prematurity, intrauterine growth retardation, congenital or perinatal infections, hemolytic anemia, congenital malformations, maternal preeclampsia and diabetes.
In the examination process, neurological function of neonates was evaluated on the first, third and seventh days and severity of Hypoxic-Ischemic Encephalopathy (HIE) was determined based on the Sarnat clinical staging. Hyperexcitability or hyperalerty, irritability, hyperreflexia and lack of convulsion for at least 24 h after birth, were divided as mild HIE or HIE grade I; Being lethargic, hypotonia and decreased reflexes, pupil miosis and convulsions were considered as moderate HIE or HIE grade II and apnea, flaccid paralysis, severe convulsions or coma were considered as sever HIE or HIE grade III.
Maternal characteristics (age, type of delivery), infant characteristics (sex, first-minute Apgar scores, fifth-minute Apgar score, duration of hospitalization, duration of IPPV, duration of oxygen therapy, HIE grades and mechanical ventilation) were collected. WBC, NRBC count, absolute NRBC count and venus blood gas were assessed in all the subjects. NRBC count per 100 WBCs and also absolute NRBC count per cubic millimeter of blood was calculated.
After discharge, they were followed-up using Denver II test at 6, 12, 18 and 24 months. Denver Developmental Screening Test II to assess the growth and development of children from birth to six years old and divided into four categories: personal – social, fine motor, gross motor and language. If the infant had problem in every category, i.e. fine motor skills, gross motor skills, language and personal social, it would be considered as developmental delay for them. If the infant had problem in only one of the categories, it is considered as mild developmental delay, in two categories, as moderate developmental delay, and in three or more categories, as severe developmental delay [Citation29]. A favorable outcome was defined as normal neurologic and good general condition at the end study. Unfavorable outcome was defined as the presence of at least delay in one domain of Denver test or death. Of 80 neonates enrolled in the study, 17 of them were excluded from the study, including 3 due to intrauterine growth restriction, 3 due to congenital or perinatal infections, 1 due to hemolytic anemia, 1 due to congenital malformations, 3 due to maternal preeclampsia and 1 due to maternal diabetes and 5 parents’ lack of cooperation.
Statistical analysis
Data were analyzed using SPSS-20 software (IBM, Chicago, IL). The values were expressed as mean + SD. Student T test, regression model (-2 log likehood, Cox & Snell R Square, Nagelkerke R Square, Hosmer and Lemeshow Tests) were utilized. ROC curve was considered for evaluation of the sensitivity and specificity of NRBC count. p values <.05 was considered statistically significant.
Results
The current study included 63 neonates. The final outcomes of neonates were examined after 6, 12, 18 and 24 months of life. The asphyxiated neonates were divided into two groups: with favorable outcomes (32 neonates, 50.8%) and with unfavorable outcomes (31 neonates, 49.2%). Neonates with unfavorable outcomes included 5 cases (7.9%) with mild developmental delay, 4 (6.3%) with moderate developmental delay, 5 children (7.9%) with severe developmental delay and 17 (27%) who died. The results showed that there were no statistically significant (p > .05) differences between the two groups of asphyxiated neonates with favorable and unfavorable outcomes in terms of first-minute Apgar score, fifth-minute Apgar score, duration of hospital stay, age of mother, duration of IPPV, duration of oxygen therapy, BUN, Sodium, Potassium, blood sugar, first-hour base excess, HCO3 and PCO2. There were statistically significant difference (p <.05) between the two groups of asphyxiated neonates with favorable and unfavorable outcomes in terms of variables such as WBC, NRBC, absolute NRBC count, Cr and first-hour pH ().
Table 1. Comparison of the average of maternal and neonatal clinical parameters in asphyxiated *neonates with favorable and unfavorable outcomes.
As shown in and , a significant relationship was detected between the prognosis and HIE grades and by increasing the HIE grades, unfavorable outcomes were increased. In particular in the group of asphyxiated neonates with favorable outcomes, there were 9 cases (29.3%) with no HIE,19 cases (61.29%) with HIE grade I, 3 cases (9.67%) with HIE grade II and in the group of asphyxiated neonates with unfavorable outcomes, there were 1 case (3.22%) with no HIE grade, 1 case (3.22%) with HIE grade I, 11 cases (35.48%) with HIE grade II and 18 cases (58.06%) with HIE grade III. The results of Chi-square test showed that sex (p = .018) had a statistically significant difference in the two groups of asphyxiated neonates with favorable and unfavorable outcomes; indicating that most of the neonates (67.66%) in the asphyxiated group with unfavorable outcomes were male ().
Table 2. Comparison of some maternal and neonatal variables in the two groups of asphyxiated neonates with favorable and unfavorable outcomes.
Table 3. Comparison of the combination of indicators that predict the risk of unfavorable prognosis.
Moreover our data showed a statistically-significant relationship between NRBC/100 WBC and absolute NRBC count levels and the outcome of asphyxiated neonates (). NRBC count more than 11 per 100 WBC, had sensitivity of 85% and specificity of 90% in predicting complications of asphyxia. Also absolute NRBC count more than 1554, had sensitivity of 85% and specificity of 87% in predicting complications of asphyxia (). Furthermore, regression models showed that variables such as NRBC level, absolute NRBC count, HIE grades and pH were important determinants of the prognosis in predicting complications of asphyxia for neonates with asphyxia ().
Figure 1. ROC curve for comparison of sensitivity and specificity of NRBC and absolute NRBC count in asphyxiated neonates, to determine the prognosis in 24 months.
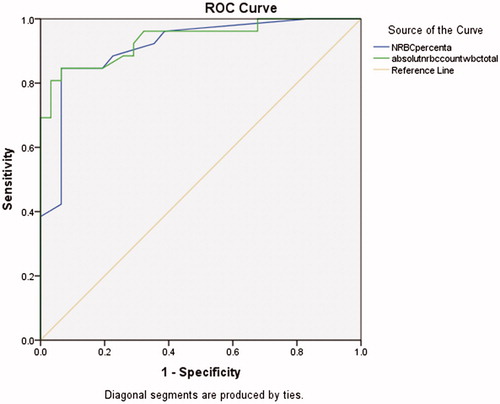
Table 4. Comparison of the final outcomes for neonates at different NRBC and absolute NRBC count levels.
Discussion
We illustrated that NRBC and absolute NRCB count in combination with HIE grade can prognostic the risk of complications of asphyxia. Furthermore, we found that NRBC count with more than 11 per 100 WBC, had sensitivity and specificity of 85% and 90%, respectively in predicting complications of asphyxia. Similar results were also selected for absolute NRBC count.
It has been documented that most of acute and chronic condition could increase NRBC count through boosting the erythropoietic activity [Citation21]. Previous studies have been suggested NRBC as hematopoietic marker in neonates and also its relationship with intrauterine hypoxia [Citation30–32]. Thus, in hypoxic conditions such as in perinatal asphyxia, a compensatory response is created as increased erythropoiesis, and then NRBCs release in the infant's blood circulation. Moreover, it has been suggested that increased NRBC count is not only the marker of perinatal asphyxia, but also predicts the risk of consequences of neurological development [Citation15,Citation21,Citation33,Citation34]. NRBC count is reached to maximum range during six to eight hours after brain damage and will return to its normal range after 36 to 72 h [Citation30]. Although the exact mechanism is unclear, it may be relevant to increase the umbilical cord blood erythropoietin levels in 1 to 4 h after acute asphyxia. Increased secretion of erythropoeitin results in increased release of NRBCs from the bone marrow due to increased mitotic divisions of normoblasts [Citation35,Citation36]. Our data showed that in asphyxiated neonates with unfavorable outcomes, NRBC count/WBC and absolute NRBC count had higher values but pH had lower values. Ghosh et al. found a negative relationship between the NRBCs level, Apgar score and pH of umbilical artery [Citation15]. Although, Rai et al. showed that NRBCs in asphyxiated neonates with unfavorable outcomes was higher [Citation8]. Also, high levels of NRBC had a relationship with severe acidosis, low Apgar scores, low platelet count and poor short-term outcomes [Citation11]. Another study by Niroomanesh and colleagues showed that a reverse relationship between NRBC count in umbilical cord blood and fifth-minute Apgar score and umbilical cord blood pH [Citation37]. Similarly Hanlon-Lundberg et al. revealed that increased in NRBC count was related by a progressive increasing in umbilical acidosis [Citation38]. In line with this data, Ferns and coworkers showed that NRBC count in asphyxiated neonates was markedly higher. These observations provide proofs of concept of NRBC count at birth as a helpful marker in predicting the severity of asphyxia and also prognosis of the neonate in a short-term period [Citation39]. Consistent with previous findings, unfavorable outcomes were increased by increasing HIE grades, which is in line with our previous data [Citation4,Citation40]. Several other studies have also showed the association of HIE grade with neurological development in neonates. Thus, early detection and treatment of neurological development disorders are essential [Citation41–43]. In our study, HIE was also emerged as one of the major causes of morbidity and mortality in asphyxiated neonates. In addition to the value of HIE, several studies, including Minior et al. showed that newborns with low-birth-weight had high NRBC count during the 6 h of life, needed mechanical ventilation or other blood pressure regulators [Citation44]. Our data showed that NRBC and absolute NRBC count levels, HIE grades, first-hour pH and the need for mechanical ventilation are of important indicators of the prognosis in asphyxiated neonates. Also, the combination of NRBC + HIE grade had higher predictive power (94.6%) to determine the prognosis of neonates with asphyxia, which in agreement with previous data [Citation4,11,23,45–47]. Additionally, we observed that NRBC count with more than 11, had sensitivity of 85% and specificity of 90% in predicting the complications of asphyxia, which is in somehow in line with our previous data [Citation11]. Similarly Rai et al. in 2014 suggested that NRBC count with more than 450/mm3 had sensitivity of 90% and specificity of 74.3% for predicting the neurological outcomes at 6 months [Citation8].
In conclusion, our results showed that the combination of NRBC + HIE grade indicators had more than 94% predictive power for determining the prognosis of asphyxiated neonates. Also NRBC count more than 11/100WBC and absolute NRBC/mm3 count more than 1554, increased HIE severity and low first-hour pH, indicated high mortality and morbidity among asphyxiated neonates at the age of two. Moreover our data suggested that neonates who are at the risk of unfavorable outcomes of perinatal asphyxia can be detected at earlier stages through proper fetal and neonatal care during labor, appropriate rehabilitation and resuscitation in the maternity as well as with novel predictive markers, such as the combination of NRBC + HIE grade.
Declaration of interest
The authors have no conflict of interest to disclose. The manuscript has not been and will not be submitted to any other journal while it is under consideration by The Journal of Pediatrics. Also there are no prior publications or submissions with any overlapping information, including studies and patients. This work was supported by grant from Mashhad University of Medical Science.
Acknowledgements
This study is a result of an approved project in Mashhad University of Medical Sciences with No. 940853. Hereby, the authors of the research would like to thank and appreciate Research Deputy, Director of Research and other officials and everyone who helped us in performing this project.
References
- Anceschi M, Piazze J, Vozzi G, et al. Antepartum computerized CTG and neonatal acid-base status at birth. Int J Gynaecol Obstet 1999;65:267–72
- UNICEF. State of the World’s children: celebrating 20 years of the convention on the rights of the child. Unicef; 2009
- Utomo MT. Risk factors for birth asphyxia. Folia Medica Indonesiana 2011;47:211–4
- Boskabadi H, Ashrafzadeh F, Doosti H, Zakerihamidi M. Assessment of risk factors and prognosis in asphyxiated infants. Iran J Pediatr 2015;25:e2006
- Low JA. The role of blood gas and acid-base assessment in the diagnosis of intrapartum fetal asphyxia. Am J Obstet Gynecol 1988;159:1235–40
- Boskabadi H, Parvini Z, Tahereh B, Asiyeh M. Study of the causes and predisposing factors in neonatal mortality in Ghaem Hospital (March 2009 To May 2010). Iran J Obstet Gynecol Infertility 2012;14:21–6
- Golubnitschaja O, Yeghiazaryan K, Cebioglu M, et al. Birth asphyxia as the major complication in newborns: moving towards improved individual outcomes by prediction, targeted prevention and tailored medical care. EPMA J 2011;2:197–210
- Rai R, Tripathi G, Singh D. Nucleated RBC count as predictor of neurological outcome in perinatal asphyxia. Indian Pediatr 2014;51:231–2
- Boskabadi H, Omidian M, Maamouri G, et al. Early diagnosis of perinatal asphyxia by determining serum HSP70 level. Iran J Pediatr 2015;25:e381
- Boskabadi H, Navaei A, mostafavi H, et al. Prooxidant-antioxidant balance in perinatal asphyxia. Indian J Pediatr 2014;81:248–53
- Boskabadi H, Maamouri G, Sadeghian MH, et al. Early diagnosis of perinatal asphyxia by nucleated red blood cell count: a case-control study. Arch Iran Med 2010;13:275–81
- Boskabadi H, Tavakol Afshari J, Maamouri G, et al. Association between serum interleukin-6 levels and severity of perinatal asphyxia. Asian Biomed 2010;4:79–85
- Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev 2001;7:56–64
- Low JA, Pickersgill H, Killen H, Derrick EJ. The prediction and prevention of intrapartum fetal asphyxia in term pregnancies. Am J Obstet Gynecol 2001;184:724–30
- Ghosh B, Mittal S, Kumar S, Dadhwal V. Prediction of perinatal asphyxia with nucleated red blood cells in cord blood of newborns. Int J Gynaecol Obstet 2003;81:267–71
- Martin RJ, Fanaroff AA, Walsh MC. Neonatal-perinatal medicine: diseases of the fetus and infant. St Louis: Elsevier/Mosby; 2011
- Green DW, Mimouni F. Nucleated erythrocytes in healthy infants and in infants of diabetic mothers. J Pediatr 1990;116:129–31
- Oski FA, Naiman JL. Hematologic problems in the newborn. Major Probl Clin Pediatr 1972;4:1
- Phelan JP, Ahn MO, Korst LM, Martin GI. Nucleated red blood cells: a marker for fetal asphyxia? Am J Obstet Gynecol 1995;173:1380–4
- Phelan JP, Kirkendall C, Korst LM, Martin GI. Nucleated red blood cell and platelet counts in asphyxiated neonates sufficient to result in permanent neurologic impairment. J Matern Fetal Neonatal Med 2007;20:377–80
- Hermansen M. Nucleated red blood cells in the fetus and newborn. rch Dis Child Fetal Neonatal Ed 2001;84:F211–F5
- Blackwell SC, Hallak M, Hotra JW, et al. Timing of fetal nucleated red blood cell count elevation in response to acute hypoxia. Biol Neonate 2004;85:217–20
- Bahman Bijari B, Farahmandinia Z, Hazeghi A. Predictive value of nucleated red blood cell counts in cord and peripheral blood of asphyxiated term neonates in the first week of life. JSSU 2010;17:330–6
- Altshuler G. Some placental considerations related to neurodevelopmental and other disorders. J Child Neurol 1993;8:78–94
- Young SA, Crocker DW. Occult congenital syphilis in macerated stillborn fetuses. Arch Pathol Lab Med 1994;118:44–7
- Maier R, Bohme K, Dudenhausen JW, Obladen M. Cord blood erythropoietin in relation to different markers of fetal hypoxia. Obstet Gynecol 1993;81:575–80
- Ruth V, Fyhrquist F, Clemons G, Raivio KO. Cord plasma vasopressin, erythropoietin, and hypoxanthine as indices of asphyxia at birth. Pediatr Res 1988;24:490–4
- Hermansen MC. Potential for brief but severe intrapartum injury among neonates with early-onset seizures and elevated nucleated red blood cell counts. Am J Obstet Gynecol 2001;184:782
- Frankenburg WK, Dodds J, Archer P, et al. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics 1992;89:91–7
- Antonucci R, Porcella A, Pilloni MD. Perinatal asphyxia in the term newborn. JPNIM 2014;3:e030269
- Buonocore G, Perrone S, Gioiaa D, et al. Nucleated red blood cell count at birth as an index of perinatal brain damage. Am J Obstet Gynecol 1999;181:1500–5
- Thilaganathan B, Athanasiou S, Ozmen S, et al. Umbilical cord blood erythroblast count as an index of intrauterine hypoxia. Arch Dis Child Fetal Neonatal Ed 1994;70:F192–F4
- Green DW, Hendon B, Mimouni FB. Nucleated erythrocytes and intraventricular hemorrhage in preterm neonates. Pediatrics 1995;96:475–8
- McCarthy J, Capullari T, Thompson Z, et al. Umbilical cord nucleated red blood cell counts: normal values and the effect of labor. J Perinatol 2006;26:89–92
- Iversen P. Blood flow to the haemopoietic bone marrow. Acta Physiol Scand 1997;159:269–76
- Lichtman MA. Williams hematology. New York: McGraw-Hill; 2006
- Niroumanesh S, Mohebi M. Fetal heart rate pattern and umbilical cord nucleated RBC count. Med J 2009;67:60–4
- Hanlon-Lundberg KM, Kirby RS. Nucleated red blood cells as a marker of acidemia in term neonates. Am J Obstet Gynecol 1999;181:196–201
- Ferns SJ, Bhat BV, Basu D. Value of nucleated red blood cells in predicting severity and outcome of perinatal asphyxia. Indian J Pathol Microbiol 2004;47:503–5
- Robertson CM, Perlman M. Follow-up of the term infant after hypoxic-ischemic encephalopathy. Paediatr Child Health 2006;11:278
- George B, Padmam M, Nair M, Indira M, Syamalan K, Padmamohan J. Hypoxic ischemic encephalopathy developmental outcome at 12 years. Indian Pediatr 2009;46:s67–70
- Perez A, Ritter S, Brotschi B, et al. Long-term neurodevelopmental outcome with hypoxic-ischemic encephalopathy. J Pediatr 2013;163:454–9. e1
- De Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 2010;95:F220–F4
- Minior VK, Shatzkin E, Divon MY. Nucleated red blood cell count in the differentiation of fetuses with pathologic growth restriction from healthy small-for-gestational-age fetuses. Am J Obstet Gynecol 2000;182:1107–9
- Baschat AA, Gungor S, Kush ML, et al. Nucleated red blood cell counts in the first week of life: a critical appraisal of relationships with perinatal outcome in preterm growth-restricted neonates. Am J Obstet Gynecol 2007;197:286. e1–8
- Vatansever Ü, Acuna B, Demİr M, et al. Nucleated red blood cell counts and erythropoietin levels in high-risk neonates. Pediatr Int 2002;44:590–5
- Leikin E, Verma U, Klein S, Tejani N. Relationship between neonatal nucleated red blood cell counts and hypoxic-ischemic injury. Obstet Gynecol 1996;87:439–43
