Abstract
Objective: To evaluate neonatal outcomes of pregnancies complicated by early-onset preeclampsia (PE) and compare these outcomes to those of gestational age matched neonates born to mothers whose pregnancy was not complicated by early-onset PE.
Methods: We analyzed the outcome in 97 neonates born to mothers with early-onset PE (24–32 weeks amenorrhea at diagnosis) and compared it to that of 680 gestational age-matched neonates born between 25–36 weeks due to other etiologies and admitted to the Neonatal Intensive Care Unit (NICU) of a tertiary referral hospital in the Netherlands. We used Chi-square test, Wilcoxon test, and logistic regression analyses.
Results: Neonates born to PE mothers had a higher perinatal mortality (13% vs. 7%, p = 0.03) and infant mortality (16% vs. 9%, p= 0.03), a 20% lower birth weight (1150 vs. 1430 g, p<0.001), were more often SGA (22% vs. 9%, p < 0.001) and had more neonatal complications as compared to neonates born to mothers without PE.
Conclusions: Overall adverse perinatal outcome is significantly worse in neonates born to mothers with early-onset PE. The effect of early-onset PE on perinatal mortality seems partially due to SGA. Whether these differences are due to uteroplacental factors or intrinsic neonatal factors remains to be elucidated.
Introduction
In the Netherlands, yearly about 12,000 women (6–7%) deliver before 37 weeks gestation, of which 1500 deliver before 32 weeks gestation. Preterm delivery is an important cause of perinatal mortality and morbidity [Citation1], mainly due to neurological sequelae. The percentage of medically induced preterm deliveries is rising at a steeper rate than spontaneous preterm deliveries. Pregnancies that are complicated by hypertensive disorders, contribute to this rise. About 16% of pregnancies in the Netherlands are complicated by pregnancy induced hypertension (PIH) and 2% by preeclampsia (PE) [Citation1]. PE is characterized by hypertension with proteinuria. Pregnancies complicated by PE contribute to maternal and perinatal mortality and morbidity [Citation2,Citation3]. All reported studies recommended intensive fetal surveillance for early detection of fetal compromise. Delivery of the fetus and placenta remain to date the only causal treatment. There is a broad agreement to terminate pregnancy when maternal or fetal conditions are altered or once 34 weeks of gestation are reached [Citation4]. But early-onset PE poses a management dilemma. Delivery may benefit the mother, but may harm a premature fetus. Expectant management in women with early-onset PE before 34 weeks’ gestation may reduce neonatal complications and stay in a Neonatal Intensive Care Unit (NICU), [Citation4,Citation5] but could aggravate the maternal condition [Citation3,Citation4].
It is uncertain whether neonatal outcome in PE mothers is determined by prematurity itself, by PE complications or other main factors. Gestational hypertension occurring early in pregnancy is associated with a higher rate of small for gestational age (SGA) infants and lower gestational age at delivery when compared to late gestational hypertension in previous studies [Citation6]. SGA is often caused by uteroplacental insufficiency, which is associated with PIH. In addition, pregnancies complicated by early-onset PE are associated with the highest rate of SGA neonates comparing to PIH, suggesting the influence of the pathophysiology of early-onset PE on fetal outcome in hypertensive diseases [Citation7]. Maternal lifestyle [Citation8], congenital malformations and chronic maternal (kidney) disease [Citation9] are also etiologic factors of SGA. Less is known of the impact of early-onset PE on perinatal mortality. Previous studies have shown differences in the prevalence of perinatal mortality most likely as a result of methodological etiology or power [Citation10,Citation11].
Therefore, the aim of this study was to address neonatal outcome in women with early-onset PE and compare these outcomes to those of gestational age matched premature neonates born to women not affected by early-onset PE.
Materials and methods
All medical records of singleton pregnancies with early-onset PE (gestational age 24–32 weeks at diagnosis for the current study) admitted to the Obstetric High Care (OHC) unit of the Radboud University Medical Center Nijmegen (Netherlands) between January 2008 and December 2011 were studied. We excluded pregnancies complicated by congenital anomalies that have a significant impact on survival and neonatal complications.
The definitions of hypertension and proteinuria used were those described in Dutch guidelines and the guideline of the Royal College of Obstetricians and Gynecologists produced by the National Collaborating Center for Women’s and Children’s Health (NCC-WCH) on behalf of the National Institute of Health and Care Excellence (NICE) [Citation12]. Briefly, PE is defined as a combination of blood pressure ≥140/90 mmHg and proteinuria ≥0.3 g total protein within a 24-h urine collection, or ratio of protein to creatinine ≥0.03 g/mmol.
Perinatal outcome was defined as a composite of mortality and variables of neonatal morbidity. Mortality was divided into perinatal mortality, occurring within the first 28 days of life, and infant mortality, occurring within the first year of life. We defined perinatal complications as infant respiratory distress syndrome (IRDS), use of surfactant therapy, clinical sepsis with positive blood culture, stage I, II or III necrotizing enterocolitis [Citation13] (NEC), grades III or IV intraventricular hemorrhage [Citation14] (IVH) and grades II, III or IV periventricular leukomalacy [Citation15] (PVL) diagnosed by utrasonographic examination. Stage I–V retinopathy of prematurity (ROP) was defined as abnormal vasoproliferation and tortuosity, retinal ridge, scarring and retinal detachment. Pneumothorax was diagnosed by radiographic findings. Furthermore, we registered Apgar scores after 1 and 5 minutes and the neonatal birth weight. We defined SGA as a birth weight below the 10th percentile of the new Dutch reference curves for birth weight by gestational age corrected for parity and gender [Citation16].
Statistical methods
To determine the effect of early-onset PE on the endpoints described earlier, we made a comparison between neonates born to PE mothers and to non-PE mothers, which we defined as controls (non-PE neonates). Patients admitted for suspected fetal distress or placental abruption were excluded. We used the Radboud University Medical Center NICU-registration between January 2008 and December 2011 to collect the data from the control group that consisted of gestational age-matched neonates at delivery.
Differences between groups were evaluated with the Chi-square test for dichotomous and with the Wilcoxon test for continuous variables. Odds ratios and adjusted odds ratios were calculated using logistic regression analysis. Statistical analysis was performed using SPSS 22.0 statistical software (IBM Corp., Armonk, NY), a p values of <0.05 was considered significant.
This study did not need approval of our ethical committee because it was a retrospective collection of data in women who received standard management at the hospital.
Results
Maternal characteristics
We registered 109 patients with early-onset PE of which 78% were nulliparous. Mean gestational age at diagnosis was 29 ± 2 weeks. Mean maternal age was 30 ± 5 years (17–41 years) and the ethnic origin of 81% of this study group was Dutch. There were no maternal deaths reported. According to the hospital guidelines, temporizing treatment was given to PE mothers consisting of antepartum corticosteroids (81% of cases) to accelerate fetal lung maturation in addition to antihypertensive treatment. The median of days gained by temporizing management was 6 days (range 1–52 days). All mothers delivered after 25 weeks amenorrhea.
Neonatal characteristics
A total of 109 neonates were born to early-onset PE mothers and were admitted to the NICU department. Three neonates were excluded from statistical analysis because of missing data. There was one case of fetal death. A further eight neonates were excluded because of severe congenital anomalies, as microcephaly (1), gastrointestinal atresia (1), diaphragmatic hernia (1), chromosomal anomalies (1), lung hypoplasia (1), tuberous sclerosis (1), congenital hypothyroidism (1) and colpocephaly (1). The perinatal outcome of the remaining 97 neonates was analyzed ().
Table 1. Perinatal outcome.
The control group consisted of 739 gestational age-matched neonates born with other etiologies that were admitted to the NICU during the same study period. Fifty-nine neonates were excluded from analysis because of severe congenital anomalies. Most prevalent anomalies were gastrointestinal atresia (10), microcephaly (5), diaphragmatic hernia (3), chromosomal anomalies (5), renal diseases (8) and lung hypoplasia (3). Perinatal outcome of the remaining 680 neonates was analyzed ().
Perinatal outcome
While the median gestational age at delivery was similar, the birth weight of early-onset PE neonates was significantly lower as compared to non-PE neonates (1150 g vs. 1430 g, p < 0.001). PE neonates were more often SGA at birth as compared to non-PE neonates (22% vs. 9%, p < 0.001). Perinatal mortality (13% vs. 7%, p = 0.03) and infant mortality (16% vs. 9%, p = 0.03) of PE neonates were significantly higher as compared to controls. Using logistic regression analysis, the odds ratio for perinatal mortality was 2.038 (95% CI: 1.06–3.92) when comparing the PE group with the controls cases, and odds ratio for infant mortality was 1.962 (95% CI: 1.06–3.62). Fifteen neonates died in the PE group, of which thirteen in the first 28 days after delivery.
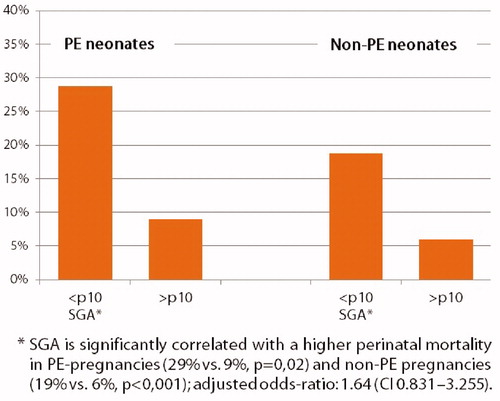
Perinatal mortality was further evaluated by SGA subgroup analysis (). In PE pregnancies, perinatal mortality was 29% in infants born SGA compared to 9% of infants born non SGA (p = 0.02). In non-PE infants, perinatal mortality was also higher in the SGA group (19% vs. 6%, p< 0.001). Using logistic regression analysis the adjusted effect of PE correcting for SGA revealed an odds ratio of 1.640 (95% CI: 0.831–3.235) for perinatal mortality, indicating that the difference of 0.4 in OR is due to SGA. A comparison of these analyses suggests that the effect of PE on perinatal mortality is partially due to SGA. In the control group, SGA infants also showed a higher perinatal mortality.
Perinatal mortality was also analyzed according to sex. In both PE- and non-PE pregnancies, gender had no significant influence on the perinatal mortality rate (male 7% vs. female 17%, p = 0.17 in PE pregnancies and male 8% vs. female 7%, p = 0.60, in non-PE pregnancies).
Furthermore, the incidence of some neonatal complications was increased in PE neonates. In particular, IRDS (62% vs. 50%, p = 0.045) and sepsis (43% vs. 30%, p< 0.01) occurred more often in PE neonates than in non-PE neonates. No differences between groups were found for pneumothorax, PVL, IVH or ROP ().
Table 2. Neonatal complications.
Discussion
This study shows that neonatal outcome in pregnancies complicated by early-onset PE differs from that of age-matched NICU neonates born to mothers not affected by PE. Early-onset PE neonates are more often SGA at birth, and perinatal and infant mortality and morbidity are increased. These results demonstrate the adverse effects of early-onset PE on the overall perinatal outcome and demonstrate the impact of early-onset PE as a proxy for perinatal survival.
Neonatal growth
Neonatal outcome in pregnancies complicated by PE is mostly related to gestational age at delivery, [Citation17,Citation18] and gestational age is the strongest predictor of perinatal mortality and morbidity [Citation19]. This study demonstrates that early-onset PE is a strong variable for perinatal outcome in premature neonates. Previous studies suggest that maternal hypertension and PE cause a reduced utero-placental perfusion and, consequently, impaired fetal growth [Citation20,Citation21]. The former could be explained by the lack of spiral artery remodeling that leads to high velocity pulsatile blood flow and syncytiotrophoblast damage. In established severe disease, acute atherosis leads to reduced utero-placental perfusion. The concomitant raised blood pressure, related to increased release of sFlt-1, could be interpreted as a fetal attempt to raise maternal blood pressure in order to increase perfusion pressure [Citation22]. The present study shows that PE neonates born between 25 and 36 weeks’ gestation have a 20% lower birth weight and a twofold higher rate of SGA (22% vs. 9%) than preterm infants born with similar gestational ages without maternal early-onset PE. These findings are in accordance with previous reports [Citation7,Citation10,Citation21,Citation23]. For example, Jelin et al. [Citation10], in a cohort of 235 women affected by early onset PE, also found a threefold higher rate of SGA neonates as compared to non-PE women (18% vs. 6%). Whether this is related to the time of onset of PE was studied in a Norwegian population by Rasmussen et al. [Citation23]. They compared the effect of early- versus late-onset PE on birth weight and found an 11–23% reduced birth weight in early-onset PE neonates. This is in accordance with our study, where we report a 20% reduction.
Perinatal mortality
Regarding perinatal mortality rate, early-onset PE is associated with an almost twofold increase in perinatal death as compared to non-PE pregnancies in our study cohort. The increased perinatal mortality is congruent with the increased rate of SGA. SGA is correlated with a higher mortality rate and is an important predictor for perinatal survival [Citation24]. In the present study cohort, early-onset PE pregnancies are associated with a higher mortality rate (29%) in infants born SGA compared to SGA infants in the non-PE group (19%). Regression analysis showed the effect of early-onset PE on perinatal mortality is mainly due to SGA. Besides, there could be common causes of SGA and mortality that we cannot make visual for bias correction. Explaining by the ‘birth weight paradox’ in Hernández-Diaz et al. [Citation25], adjustment for birth weight is unwarranted when the analytical goal is to estimate overall effects of perinatal variables on infant mortality. Even for estimating direct effects of prenatal variables, adjustment for birth weight may be invalid when there is an unmeasured common cause of SGA and mortality.
Jelin et al. [Citation10] described a 9% decrease in perinatal mortality in pregnancies complicated by PE as compared to the control group of other preterm deliveries (11% vs. 18%, p = 0.008) refuting previous studies that demonstrated no difference in perinatal survival [Citation26]. One explanation for this decrease could be the relative high incidence of preterm premature rupture of membranes in the control group. Preterm premature rupture of membranes carries the highest neonatal morbidity of all etiologies of preterm labor [Citation27]. Withagen et al. [Citation11], who evaluated temporizing treatment in PE-pregnancies to assess its effect on neonatal outcome, found a 33% lower birth weight and higher rate of SGA neonates (52% vs. 3.2%) in PE pregnancies as compared to gestational age-matched non-PE control group. No significant difference was found in perinatal or infant mortality. The perinatal mortality rate in PE pregnancies in the present study population is higher (13% vs. 7%) compared to that of Withagen et al. and may be explained by the shorter mean temporization treatment (8 days vs. 14 days). In accordance with our study, Withagen et al. also observed a better prognosis in infants born to mothers without early-onset PE.
Perinatal morbidity
In this study, infants born to mothers with early-onset PE were more likely to develop IRDS, with 62% of infants affected versus 50% in the control group, which is in accordance with others [Citation10,Citation28]. The postnatal course of early-onset PE neonates was more often complicated by sepsis (43% vs. 30%) compared to non-PE neonates. The increased risk of IRDS could at least be partially related to the higher frequency of cesarean delivery (66% vs. 35%, p <0.001) in women with PE [Citation29,Citation30], despite the treatment of ante partum corticosteroids in 81% of cases. Witlin et al. [Citation28] concluded in a cohort of 195 PE pregnancies that respiratory distress syndrome was inversely related to gestational age at delivery and directly related to cesarean delivery. Werner et al. [Citation30] also reported increased odds of respiratory distress and sepsis in preterm neonates in cesarean compared with vaginal delivery.
Our study has some limitations, because it is a retrospective study. Therefore, our results should be interpreted with caution. However, the results show a worsened perinatal outcome in early-onset PE. In 2001, Withagen et al. [Citation11] showed the benefit of temporizing treatment in neonatal outcome in PE pregnancies. As of 2016, despite all efforts, non-PE neonates still have a better outcome than infants born to mothers with early-onset PE. A previous attempt to address this issue in a randomized controlled trial was initiated in the Netherlands in which the risks of maternal complications by temporizing treatment in women with early-onset PE and the subsequent benefits for the neonate at 24 to 32 weeks’ gestation were weighed [Citation31]. Due to disappointing inclusion, the study did not meet inclusion targets and was canceled.
In summary, this study shows that neonatal outcome of infants born to mothers with early-onset PE is worse than that of infants born to mothers with other etiologies of preterm labor. Specifically, these infants were more often SGA and their mortality and neonatal complication rates were greater. This information can be used in the counseling of women with early-onset PE. Whether these differences are attributed to SGA alone or another yet unsolved pathophysiological mechanism remains unclear and warrant additional investigation.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Iams J. Prevention of preterm birth. N Engl J Med 1998;338:54–6.
- Murphy DJ, Stirrat GM. Mortality and morbidity associated with early-onset preeclampsia. Hypertens Pregnancy 2000;19:221–31.
- Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol 2007;196:514 e1–9.
- Swamy MK, Patil K, Nageshu S. Maternal and perinatal outcome during expectant management of severe pre-eclampsia between 24 and 34 weeks of gestation. J Obstet Gynaecol India 2012;62:413–8.
- Magee LA, Yong PJ, Espinosa V, et al. Expectant management of severe preeclampsia remote from term: a structured systematic review. Hypertens Pregnancy 2009;28:312–47.
- Xiong X, Demianczuk NN, Saunders LD, et al. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol 2002;155:203–9.
- Ferrazzani S, Luciano R, Garofalo S, et al. Neonatal outcome in hypertensive disorders of pregnancy. Early Hum Dev 2011;87:445–9.
- Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol 2002;100:369–77.
- Dejmek J, Solansk y I, Podrazilova K, Sram RJ. The exposure of nonsmoking and smoking mothers to environmental tobacco smoke during different gestational phases and fetal growth. Environ Health Perspect 2002;110:601–6.
- Jelin AC, Cheng YW, Shaffer BL, et al. Early-onset preeclampsia and neonatal outcomes. J Matern Fetal Neonatal Med 2010;23:389–92.
- Withagen MI, Visser W, Wallenburg HC. Neonatal outcome of temporizing treatment in early-onset preeclampsia. Eur J Obstet Gynecol Reprod Biol 2001;94:211–5.
- National Institute for Health and Clinical Excellence. Hypertension in pregnancy: diagnosis and management. London: NICE; 2010.
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179–201.
- Allan WC, Volpe JJ. Periventricular-intraventricular hemorrhage. Pediatr Clin North Am 1986;33:47–63.
- de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res 1992;49:1–6.
- Visser GH, Eilers PH, Elferink-Stinkens PM, et al. New Dutch reference curves for birthweight by gestational age. Early Hum Dev 2009;85:737–44.
- Haddad B, Deis S, Goffinet F, et al. Maternal and perinatal outcomes during expectant management of 239 severe preeclamptic women between 24 and 33 weeks’ gestation. Am J Obstet Gynecol 2004;190:1590–5. discussion 5–7.
- Bombrys AE, Barton JR, Habli M, Sibai BM. Expectant management of severe preeclampsia at 27(0/7) to 33(6/7) weeks’ gestation: maternal and perinatal outcomes according to gestational age by weeks at onset of expectant management. Am J Perinatol 2009;26:441–6.
- Shear RM, Rinfret D, Leduc L. Should we offer expectant management in cases of severe preterm preeclampsia with fetal growth restriction? Am J Obstet Gynecol 2005;192:1119–25.
- Villar J, Carroli G, Wojdyla D, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol 2006;194:921–31.
- Kovo M, Schreiber L, Elyashiv O, et al. Pregnancy outcome and placental findings in pregnancies complicated by fetal growth restriction with and without preeclampsia. Reprod Sci 2015;22:316–21.
- Cetin I, Huppertz B, Burton G, et al. Pregenesys pre-eclampsia markers consensus meeting: What do we require from markers, risk assessment and model systems to tailor preventive strategies? Placenta 2011;32 Suppl:S4–S16.
- Rasmussen S, Irgens LM. Fetal growth and body proportion in preeclampsia. Obstet Gynecol 2003;101:575–83.
- Copper RL, Goldenberg RL, Creasy RK, et al. A multicenter study of preterm birth weight and gestational age-specific neonatal mortality. Am J Obstet Gynecol 1993;168:78–84.
- Hernandez-Diaz S, Schisterman EF, Hernan MA. The birth weight “paradox” uncovered? Am J Epidemiol 2006;164:1115–20.
- Friedman SA, Schiff E, Kao L, Sibai BM. Neonatal outcome after preterm delivery for preeclampsia. Am J Obstet Gynecol 1995;172:1785–8. discussion 8-92.
- Johanzon M, Odesjo H, Jacobsson B, et al. Extreme preterm birth: onset of delivery and its effect on infant survival and morbidity. Obstet Gynecol 2008;111:42–50.
- Witlin AG, Saade GR, Mattar F, Sibai BM. Predictors of neonatal outcome in women with severe preeclampsia or eclampsia between 24 and 33 weeks’ gestation. Am J Obstet Gynecol 2000;182:607–11.
- Yeekian C, Jesadapornchai S, Urairong K, et al. Comparison of maternal factors and neonatal outcomes between elective cesarean section and spontaneous vaginal delivery. J Med Assoc Thai 2013;96:389–94.
- Werner EF, Han CS, Savitz DA, et al. Health outcomes for vaginal compared with cesarean delivery of appropriately grown preterm neonates. Obstet Gynecol 2013;121:1195–200.
- Duvekot JJSE, Van der Wilk EC, Hop WCJ, Temporise or terminate pregnancy in women with severe preeclampsia at 28-34 weeks. 2013.