Abstract
Aim: To measure serum hepcidin in late pregnancy and in cord blood, and to analyze relationship between hepcidin, interleukin-6, and biomarkers of fetal iron status.
Materials and methods: Data from 15 uncomplicated singleton pregnancies were analyzed longitudinally in trimester 3 (T3) and at birth.
Results: In T3, S-ferritin (median 14 µg/L) and transferrin (median 4.0 g/L) indicated low iron status, whereas the median soluble transferrin receptor (sTfR) was 4.0 mg/L, i.e. within the reference interval. Median T3 S-hepcidin was 7.8 ng/mL. Later on in cord blood, ferritin concentration (180 µg/L) were significantly higher, transferrin concentration (1.8 g/L) were significantly lower, and both sTfR (4.7 mg/L) and S-hepcidin concentrations (30.5 ng/mL) were significantly higher than maternal T3 concentrations. At the same time, cord blood interleukin-6 indicated an activated acute-phase reaction. In T3, after logarithmic transformation, there was a significant correlation between S-hepcidin and both S-ferritin (r = 0.691) and sTfR (r = −0.825). There was also a significant correlation between S-ferritin and both sTfR (r = −0.729) and transferrin (r = 0.549) in T3.
Conclusions: Although S-ferritin, S-hepcidin, and sTfR were correlated during pregnancy, these relationships were not apparent in umbilical cord blood. Further, cord blood interleukin-6 indicated an activated acute-phase response, and sTfR, which is known to be unaffected by inflammation, indicated a low iron status in cord blood. Thus, instead of representing an enhanced iron status, the data appear to suggest that hepcidin and ferritin in cord blood may be influenced by the low-grade acute-phase response that occurs during delivery.
Keywords:
Introduction
Iron deficiency is the most common nutrient deficiency globally, and is a serious health problem, especially for women of reproductive age. Iron deficiency anemia during pregnancy has been linked to low birth weight, premature birth, and negative effects on the child’s neuropsychological development [Citation1]. Even early in pregnancy, maternal hemoglobin (Hb) levels have different effects on health [Citation2]. One factor in the development of iron deficiency in pregnancy is the extraordinary need for iron. Based on the iron required for the fetus, for the placenta, for the increase in maternal red cell mass, together with the basal iron losses from the mother, it has been estimated that as much as 1000 mg of iron is needed to cover for the whole pregnancy [Citation3]. The regulatory peptide hepcidin has attracted considerable interest due to its key role in iron metabolism. Hepcidin is secreted into the blood, mainly by hepatocytes, and production is induced by inflammation [Citation4], mainly via interleukin-6 (IL-6) [Citation5], and by increase in circulating iron concentration. Increased production of hepcidin promotes internalization and degradation of ferroportin, which leads to reduced uptake of iron across the intestinal mucosa and reduced release of iron from body stores [Citation6].
Due to the major impact of iron on maternal and fetal health, it is of vital importance that we gain further understanding of the important role of hepcidin in neonates, and its relation to maternal iron status. The present study had an observation-oriented approach with the aim of investigating S-hepcidin and iron status during late pregnancy and in umbilical cord blood. Since the predominant changes in iron metabolism during pregnancy occur in late pregnancy, this study focused on the transition between trimester 3 and parturition [Citation3].
Materials and methods
Ethics
The study was conducted according to the Ethical Principles for Medical Research Involving Human Subjects (Helsinki, Finland, June 1964, as amended by the 64th WMA General Assembly, Fortaleza, Brazil, in October 2013). The study protocol was approved by the Ethics Review Board in Gothenburg (registration nos. 241-09 and 402-08). The pregnant women who participated gave their informed written consent before being enrolled in the study.
General protocol
The study was designed as a longitudinal study involving healthy, pregnant Caucasian women attending antenatal clinics in the county of Västra Götaland in western Sweden. Sampling of maternal blood was done in pregnancy trimester 3, and umbilical cord blood was sampled at birth. Data on the child’s birth weight and length were also collected at birth. No maternal blood was drawn at birth.
Subjects
The study population consisted of 15 healthy, pregnant Swedish women, all Caucasian. They were all enrolled at the official region Maternal Healthcare Centers, one in the town of Trollhättan (population approx. 47,000) and the other in Bengtsfors (population approx. 3000). Data were collected in connection with the visits at the Maternal Healthcare Centers during weeks 33–38 (trimester 3) and later on in connection with parturition at the maternity ward of Northern Älvsborg County Hospital. Weight and height in trimester 1, i.e. when attending the first regular check-up, were acquired retroperspectively from the journals kept at the Maternal Healthcare Centers. All deliveries were performed at the same hospital. All women attending their regular check-up at the participating Maternal Healthcare Centers between February 2010 and July 2011 were asked to participate in the study. Thus, no randomization in selecting the women was carried out.
Birth outcomes
Data on gestational age at delivery, birth weight, length at birth, and mode of delivery were obtained from patient files at the maternity wards. Gestational age was either calculated from information on the expected date of delivery, or based on the gestational age according to the ultrasound, or the first day of the last menstrual period.
Laboratory analysis
Blood samples were collected from the pregnant women at the antenatal clinics by venous puncture. Cord blood was sampled before placental expulsion and after clamping of the umbilical cord at the maternity ward (Northern Älvsborg County Hospital). Samples destined for analysis of transferrin, S-ferritin, and soluble transferrin receptor (sTfR) were sent directly after sampling to the accredited reference laboratory (Clinical Chemistry Laboratory, Northern Älvsborg Hospital) according to the ISO/IEC 15 189 Standard for Medical Laboratories. The reference values for S-ferritin, sTfR, and transferrin are 5–148 µg/L, 1.9–4.4 mg/L, and 1.9–3.3 g/L, respectively.
In addition to the abovementioned analyses, some extra serum was drawn, centrifuged, and stored at −80 °C until analysis for interleukin-6 (IL-6) and hepcidin at the one time. Serum interleukin-6 was determined using the commercially available human IL-6 Quantikine HS high-sensitivity enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems GmbH, Wiesbaden, Germany). The sensitivity and assay range are 0.11 pg/mL and 0.156–10 pg/mL, respectively. The intra- and interassay coefficient of variation (CV) is 7.4% and 6.5%, respectively, for IL-6 at a concentration of 5.5 pg/mL.
Hepcidin in serum was determined using a commercial peptide enzyme immunoassay (EIA) according to the manufacturer’s instructions (Hepcidin-25 human EIA kit; Bachem, Inc, Bubendorf, Switzerland) with a typical sensitivity of 1.5 ng/mL. Samples giving readings outside the standard curve region were diluted and reanalyzed. A lower limit of detection has not been defined by the manufacturer, but they report a detection range of 0–25 ng/mL. Both the intra- and interassay variation were <10%.
Analysis and statistics
Descriptive data are presented as median and interquartile range (IQR). Data were checked for normality of distribution using the Kolmogorov–Smirnov test. Most variables were significantly skewed. Consequently, data were log-transformed before statistical analysis. No significant deviances from Gaussian distribution were found after log-transformation. For ease of interpretation, untransformed data are presented throughout. Possible differences over time were tested for statistical significance by Student’s paired t-test. Associations were tested using Pearson’s correlation test. All p values are two-tailed and were considered to be statistically significant if p < .05. Statistical analyses were performed using IBM© SPSS statistics for Windows 22.0.0.0 (IBM Corp, Armonk, NY).
Results
Gestational age and laboratory measurements are shown in . Median body mass index when attending the first regular check-up in trimester 1 at the Maternal Healthcare Center was 24.4 kg/m2 (interquartile range [IQR] 23.3–26.3). All pregnancies were singleton and uncomplicated. The median age of the women in trimester 3 was 29.4 years (IQR 27.5–31.6). Nine of the women had more than 3 years of college education, five women were high school graduates (i.e. 3 years of high school education) and one woman had 2 years of high school education. All but one mother denied smoking tobacco during pregnancy. Eight out of 15 had been taking some kind of iron supplementation during the 6 months preceding the pregnancy. Hemoglobin concentration was not analyzed at trimester 3 or in cord blood, but from the journals kept at the Maternal Healthcare Centers, Hb at trimester 1 was retroperspectively acquired, giving that one woman was anemic (<114 g/L).
Table 1. Longitudinal data (n = 15)Table Footnotea.
Eleven women had vaginal deliveries and four had elective cesarean sections. The proportion of women with low iron stores (S-ferritin ≤20 μg/L) in trimester 3 was 73% (11/15). Twenty-seven percent of the women had sTfR concentrations above 4.4 mg/L in trimester 3.
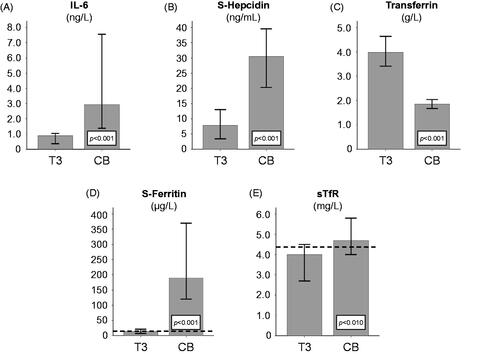
In comparison with serum collected in trimester 3, all 15 cord blood levels were significantly higher regarding IL-6 and S-ferritin, and significantly lower regarding transferrin. Median IL-6 and S-ferritin levels in cord blood were 3.3 (p = .001) and 15.7 (p = .001) times higher, respectively, than in maternal serum during trimester 3.
In 13 out of 15 cord blood samples, S-hepcidin concentrations were significantly higher than in trimester 3 sera, indicating an enhanced iron status. However, cord blood sTfR concentrations (12 out of 15) were significantly higher than in trimester 3 serum, thus indicating a significant reduction in iron status.
Relative to maternal serum concentrations in trimester 3, median S-hepcidin and sTfR concentrations in cord blood were 4.8 and 1.4 times higher, respectively ().
In contrast, the median transferrin concentration was 2.2 times lower in cord blood than in trimester 3 serum samples (p = .001) ( and ).
Figure 1. Changes over time. The different panels illustrate longitudinal time course changes in (A) interleukin-6 (IL-6), (B) S-hepcidin, (C) transferrin, (D) S-ferritin, and (E) soluble transferrin receptor (sTfR) throughout pregnancy. Bars show median values and whiskers show the 95% confidence interval (CI). The dotted horizontal lines in panel D indicate the cutoff for exhausted iron stores based on S-ferritin (12 µg/L) and the dotted horizontal lines in panel E indicate the lower concentration of the transferrin receptor (TfR) reference interval (4.4 mg/L). sTfR: soluble transferrin receptor; IL-6: interleukin-6; CB: cord blood; T3: trimester 3.
Median birth weight was 3700 g (IQR 3305–3855 g) and median length of the offspring was 50 cm (IQR 50–51 cm). Nine babies were boys and six were girls. Apgar score at 1 min ranged from 8 (n = 1) to 10, with a median at 9.
The bivariate analysis of trimester 3 data showed that there were significant correlations between log S-hepcidin concentrations and the logarithmic transformed variables S-ferritin (r = 0.691, p = .004), sTfR (r = −0.825, p = .001), and IL-6 (r = −0.539, p = .038). In trimester 3, there were also correlations between S-ferritin and both sTfR (r = −0.729, p = .002) and transferrin (r = 0.549, p = .034) ( and ).
Figure 2. Correlations during trimester 3 of pregnancy. The different panels illustrate the correlation coefficients for (A) log soluble transferrin receptor (sTfR) versus log S-ferritin; (B) log transferrin versus log S-ferritin; (C) log IL-6 versus log S-hepcidin; (D) log sTfR versus log S-hepcidin; and (E) log S-ferritin versus log S-hepcidin.
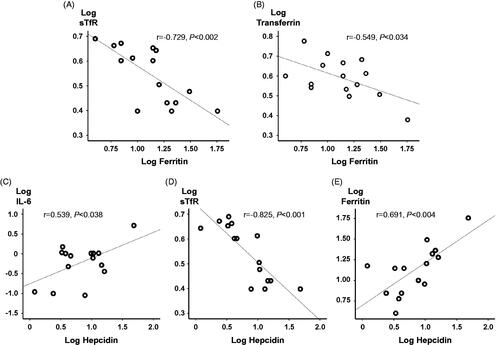
Table 2. Correlations in biomarkers sampled in trimester 3 and in cord blood. Pearson correlations with p values.
In cord blood neither log S-hepcidin nor log S-ferritin was associated with any other biomarker. However, log IL-6 was negatively correlated with log S-Fe (r = −0.580, p = .023) and log TSAT (r = −0.579, p = .024), and there was a positive correlation between log sTfR and log transferrin (r = 0.765, p = .001).
There was no significant association between mode of delivery (i.e. vaginal delivery or cesarean section) and any of the cord blood biomarkers; nor were there any significant differences in any of the cord blood biomarkers regarding mode of delivery.
Discussion
This study emphasizes on the important biochemical changes that occur in the transition from late pregnancy to parturition, by reporting on longitudinal measurements of S-hepcidin, sTfR, and S-ferritin sampled in trimester 3 of pregnancy and in cord blood obtained at parturition. It was possible to detect that all deliveries studied, without exception, were associated with activated acute-phase reactions by also including IL-6 in the analysis. This was despite the fact that all deliveries were uncomplicated. Although 73% of the women in trimester 3 had S-ferritin concentrations indicating low iron stores (≤20 μg/L), we found that median cord blood ferritin concentrations were more than 15 times higher than maternal concentrations measured in trimester 3. Also, cord blood S-hepcidin concentrations were significantly higher (five times) than maternal serum concentrations in trimester 3, whereas the cord blood transferrin concentrations were less than half the serum levels in trimester 3. These high cord blood concentrations of hepcidin and ferritin, and also the decrease in transferrin, could be interpreted as showing a considerably enhanced iron status.
However, at the same time, cord blood sTfR was also enhanced, thus indicating the opposite, i.e. a significantly low iron status in cord blood.
Also, besides being proxy for iron stores, S-hepcidin, S-ferritin, and transferrin (the latter being downregulated in acute-phase reactions) are highly influenced by an acute-phase response [Citation5,Citation7–9]. In contrast, this is not the case with sTfR, since it is known to be unaffected by inflammation [Citation10] or by gestation per se [Citation11]. Accordingly, sTfR has advantages over S-ferritin regarding assessment of iron status in pregnancy [Citation12].
Furthermore, the final stage of pregnancy, i.e. parturition, can be considered to be a low-grade inflammatory process due to ruptured fetal membranes and an influx of leukocytes (mainly macrophages and neutrophils) into cervical and endometrial tissues [Citation13]. This influx of leukocytes results in upregulated expression of cytokines and prostaglandins, which in turn is a necessity for cervical ripening and, later on, uterine contraction [Citation14]. Vaginal delivery has been shown to promote production of various cytokines, including IL-6 [Citation15]. Since cesarean section itself is an invasive procedure, it is likely that cesarean section, as all other surgical procedures, would also induce an inflammatory reaction [Citation4].
Consequently, the synthesis of cytokines during labor and delivery appears to be a contributory factor behind the upregulation of hepcidin and ferritin expression in cord blood. And, since it was observed that every cord blood IL-6 concentration was significantly higher than that in maternal serum samples in trimester 3, we conclude that hepcidin and ferritin in cord blood are not as related to iron status as they are to an activated low-grade acute-phase reaction. This has also been proposed to apply to maternal S-ferritin and S-hepcidin at delivery [Citation16,Citation17]. Even so, no correlations between IL-6 and hepcidin or ferritin concentrations could be detected in the present study. Since lacking evidence of a correlation is not the same as having evidence of absence of a correlation, this could possibly have been due to the small sample size.
Apart from being a measure of tissue iron deficiency, sTfR can also be a quantitative measure of erythropoietic activity [Citation18]. Since several physiological changes occur in pregnancy, the high sTfR concentrations observed in cord blood may not only indicate low iron status, but possibly also increased erythropoiesis. Also, there are reports that iron store depletion induces an increase in sTfR that is higher than the sTfR concentration observed in noniron-deficient pregnancies, particularly at the time of labor [Citation19], which also speaks in favor of sTfR being a proxy of iron stores in the present trial setting.
The high concentrations of hepcidin and ferritin measured here in cord blood are in accordance with previous observations [Citation20–30]. Nevertheless, there are a handful of previous studies [Citation21,Citation24,Citation31,Citation32] involving measurements of S-hepcidin, sTfR, and S-ferritin sampled in late pregnancy as well as in cord blood. However, only two of these studies did include IL-6 [Citation31,Citation32]. Together with these studies, the present study emphasizes the important biochemical changes in iron status as well as acute-phase reactions that occur in the transition from late pregnancy to parturition in the cord blood. Nevertheless, it is important to keep in mind that the fetal and maternal circulations are separate from each other and that hepcidin is not believed to cross the placenta. Thus, cord blood results must be interpreted in terms of fetal status. A positive correlation between S-hepcidin and S-ferritin in nonpregnant individuals has been confirmed by several studies. Thus, our results are the first to show that this relationship also exists during pregnancy. In cord blood, however, no correlation between hepcidin and ferritin was seen. The lack of any correlation in our study agrees with the results of Briana et al. [Citation33]. Although Rehu et al. [Citation23] demonstrated that none of the cord blood iron parameters were associated with maternal hepcidin, there have also been reports of an association between cord blood hepcidin and cord blood ferritin [Citation23,Citation34].
One limitation of the study was the small sample size, but even so, the results are rather unequivocal in that all the women except one showed the same changes in iron status biomarkers. Also, there were no dropouts, and the biochemical analyses were complete in all women.
Taken together, all the data indicate that care should be taken in the interpretation of the interrelationship between iron biomarkers, including hepcidin, in cord blood. Although the hierarchies of iron supply during pregnancy most likely benefit the fetus at the expense of the mother, it should be noted that these findings come from a population that was fairly iron-replete, and they may not apply to an anemic cohort. Given the observational nature of this study and the importance of iron metabolism during pregnancy, larger and designated studies are needed to confirm these findings.
Acknowledgements
We thank the women who participated for their cooperation. We also acknowledge Dr Leif Inganäs and Dr Johan Robinson of the neonatal ward, Northern Älvsborg Hospital, Trollhättan, for their valuable technical assistance. We also thank Martin Gellerstedt, PhD, Lecturer in Statistics, University West, for sharing his expertise in medical statistics.
Disclosure statement
None of the authors has any financial interests or other conflicts of interests regarding this manuscript. The funding sponsors had no role in the design of the study; in the collection, analysis, or interpretation of data; or in the writing of the manuscript.
Additional information
Funding
References
- Rioux FM, Lindmark G, Hernell O. Does inadequate maternal iron or DHA status have a negative impact on an infant’s functional outcomes? Acta paediatr. 2006;95(2):137–144.
- Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71(5 Suppl): 1280S–1284S.
- Hallberg L. Iron balance in pregnancy. In: Berger H, editor. Vitamins and minerals in pregnancy and lactation Nutrition Workshop Series. Vol. 16. New York: Vevey/Raven Press: Nestlé Nutrition Workshop Series; 1988. p. 115–127.
- Hoppe M, Lönnerdal B, Hossain B, et al. Hepcidin, interleukin-6 and hematological iron markers in males before and after heart surgery. J Nutr Biochem. 2009;20(1):11–16.
- Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276.
- Leong WI, Lönnerdal B. Hepcidin, the recently identified peptide that appears to regulate iron absorption. J Nutr. 2004;134(1):1–4.
- Nemeth E, Valore EV, Territo M, et al. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101(7): 2461–2463.
- Hulthén L, Lindstedt G, Lundberg PA, et al. Effect of a mild infection on serum ferritin concentration – clinical and epidemiological implications. Eur J Clin Nutr. 1998;52(5):376–379.
- Ritchie RF, Palomaki GE, Neveux LM, et al. Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 1999;13(6):273–279.
- Ferguson BJ, Skikne BS, Simpson KM, et al. Serum transferrin receptor distinguishes the anemia of chronic disease from iron deficiency anemia. J Lab Clin Med. 1992;119(4):385–390.
- Sweet DG, Savage GA, Tubman R, et al. Cord blood transferrin receptors to assess fetal iron status. Arch Dis Child Fetal Neonatal Ed. 2001;85(1):F46–F48.
- Akesson A, Bjellerup P, Berglund M, et al. Serum transferrin receptor: a specific marker of iron deficiency in pregnancy. Am J Clin Nutr. 1998;68(6):1241–1246.
- Young A, Thomson AJ, Ledingham M, et al. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66(2): 445–449.
- Terzidou V. Preterm labour. Biochemical and endocrinological preparation for parturition. Best Pract Res Clin Obstet Gynaecol. 2007;21(5):729–756.
- Marchini G, Berggren V, Djilali-Merzoug R, et al. The birth process initiates an acute phase reaction in the fetus-newborn infant. Acta paediatr. 2000;89(9): 1082–1086.
- Lee S, Guillet R, Cooper EM, et al. Maternal inflammation at delivery affects assessment of maternal iron status. J Nutr. 2014;144(10):1524–1532.
- Hedengran KK, Nelson D, Andersen MR, et al. Hepcidin levels are low during pregnancy and increase around delivery in women without iron deficiency – a prospective cohort study. J Matern Fetal Neonatal Med. 2016;29(9):1506–1508.
- Skikne BS, Flowers CH, Cook JD. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood. 1990;75(9):1870–1876.
- Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta. 2003;329(1–2):9–22.
- Kulik-Rechberger B, Kościesza A, Szponar E, et al. Hepcidin and iron status in pregnant women and full-term newborns in first days of life. Ginekol Pol. 2016;87(4):288–292.
- Garcia-Valdes L, Campoy C, Hayes H, et al. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int J Obes. 2015; 39(4):571–578.
- Altinkaynak S, Alp H, Bastem A, et al. Serum ferritin and hemoglobin levels of mothers and their newborns. Turk J Pediatr. 1994;36(4):289–293.
- Rehu M, Punnonen K, Ostland V, et al. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol. 2010;85(4):345–352.
- Yang A, Zhao J, Lu M, et al. Expression of hepcidin and ferroportin in the placenta, and ferritin and transferrin receptor 1 levels in maternal and umbilical cord blood in pregnant women with and without gestational diabetes. Int J Environ Res Public Health. 2016;13(8).
- Basu S, Kumar N, Srivastava R, et al. Maternal and cord blood hepcidin concentrations in severe iron deficiency anemia. Pediatr Neonatol. 2016;57(5): 413–419.
- Ervasti M, Sankilampi U, Luukkonen S, et al. Maternal pro-hepcidin at term correlates with cord blood pro-hepcidin at birth. Eur J Obstet Gynecol Reprod Biol. 2009;147(2):161–165.
- Lao TT, Loong EP, Chin RK, et al. Relationship between newborn and maternal iron status and haematological indices. Biol Neonate. 1991;60(5): 303–307.
- Bratlid D, Moe PJ. Hemoglobin and serum ferritin levels in mothers and infants at birth. Eur J Pediatr. 1980;134(2):125–127.
- Pavelka R, Kofler E, Linkesch W, et al. Serum ferritin in pregnancy at term and in newborn (author’s transl). Padiatr Padol. 1981;16(4):443–450.
- Kelly AM, MacDonald DJ, McDougall AN. Observations on maternal and fetal ferritin concentrations at term. Br J Obstet Gynaecol. 1978;85(5):338–343.
- Cao C, Pressman EK, Cooper EM, et al. Prepregnancy body mass index and gestational weight gain have no negative impact on maternal or neonatal iron status. Reprod Sci. 2016;23(5):613–622.
- Lee S, Guillet R, Cooper EM, et al. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res. 2016;79(1–1):42–48.
- Briana DD, Boutsikou T, Baka S, et al. Perinatal role of hepcidin and iron homeostasis in full-term intrauterine growth-restricted infants. Eur J Haematol. 2013; 90(1):37–44.
- Lorenz L, Herbst J, Engel C, et al. Gestational age-specific reference ranges of hepcidin in cord blood. Neonatology. 2014;106(2):133–139.
