Abstract
Background and objectives: To explore if intravenous iron isomaltoside (Monofer®) leads to a better relief of fatigue than current treatment practice with oral iron in women suffering from severe fatigue after postpartum hemorrhage.
Materials and methods: This is a subanalysis of a single-center, open-label, randomized controlled trial conducted in women suffering from postpartum hemorrhage. Participants were randomized 1:1 to 1200 mg iron isomaltoside or current treatment practice with oral iron. We measured fatigue by the Multidimensional Fatigue Inventory (MFI) and Edinburgh Postnatal Depression Scale, and determined hematological parameters. The subanalysis includes all participants with a high fatigue score (MFI physical fatigue score >15) at inclusion. The primary endpoint was aggregated change in physical fatigue score from inclusion to 12 weeks postpartum with a predefined minimum clinically relevant difference of 1.8. The trial is registered at ClinicalTrials.gov (identifier: NCT01895218).
Results: A total of 85 women had a high fatigue score at inclusion. The aggregated change in physical fatigue score was −2.3 (confidence interval 95%: −3.3; −1.3) (p < .0001) in favor of iron isomaltoside. Significant differences in other fatigue and depression scores and hematological parameters were observed and all in favor of iron isomaltoside. There were no differences in side effects between the groups.
Conclusions: In women suffering from severe fatigue after postpartum hemorrhage, a single dose of iron isomaltoside is associated with a statistically significant and clinically relevant reduction in aggregated physical fatigue within 12 weeks after delivery, when compared to current treatment practice with oral iron and with a similar safety profile.
Introduction
Globally, 30% of all women in the fertile age suffer from anemia and during pregnancy, where the iron demands are considerably increased and anemia is observed in more than 40% [Citation1]. Postpartum hemorrhage (PPH), defined as a blood loss of more than 500 mL, may be a severe complication after delivery. PPH can cause aggravated anemia leading to fatigue and decreased quality of life (QoL) and even maternal death. In low income countries, PPH is the leading cause of maternal mortality and globally it is the primary cause of nearly one quarter of all maternal deaths [Citation2]. Postpartum anemia is often treated with oral iron supplementation and/or red blood cell transfusion. In Denmark, red blood cell transfusion is only indicated for severe symptoms of anemia and hemoglobin (Hb) values below 7 g/dL (4.3 mmol/L) [Citation3]. Thus, postpartum anemia is primarily treated with oral iron. However, oral iron may be a challenge because of intolerance, lack of compliance, and limited absorption especially when the iron need is high [Citation4,Citation5]. In other therapeutic areas, intravenous (IV) iron is considered more effective, better tolerated, and improves QoL to a greater extent than oral iron [Citation5–7]. Iron isomaltoside (Monofer®, Pharmacosmos, Holbaek, Denmark) is one of the newer IV iron formulations available. Clinical efficacy and safety data is available for iron isomaltoside administered to patients with iron deficiency anemia requiring iron therapy [Citation8–16], nonanemic patients undergoing cardiac surgery [Citation17], and women with PPH [Citation18,Citation19]. In a previous trial including 196 women with PPH, a single dose of iron isomaltoside was associated with a statistically significant reduction in aggregated physical fatigue within 12 weeks compared to current treatment practice with oral iron, however below the prespecified criteria of clinical superiority [Citation18].
We here present data from a subpopulation of women with PPH from this previously reported trial [Citation18]. The objective was to compare the efficacy and safety of iron isomaltoside with current treatment practice with oral iron in women with PPH and who suffered from severe fatigue measured by the Multidimensional Fatigue Inventory (MFI) within 48 hours after delivery.
Materials and methods
Trial design
This is a subanalysis of a randomized, comparative, open-label, single-center trial conducted in Denmark from May 2013 to December 2014 in women suffering from PPH. The trial protocol and other related documents were approved by relevant competent authorities and the local ethics committee (the Committees on Health Research Ethics for the Capital Region of Denmark, approval date: 12 April 2013, approval number: H-4-2013-019). The trial was conducted in accordance with good clinical practice and the Declaration of Helsinki. Informed consent was obtained in writing prior to any trial-related activities.
The trial is registered at ClinicalTrials.gov (identifier: NCT01895218). The participants attended six visits: a baseline/treatment visit within 48 hours after the delivery at the hospital before discharge and five visits at home (day 3, and week 1, 3, 8, and 12 after baseline). The assessments performed included fatigue measured by the MFI, symptoms of depression measured by the Edinburgh Postnatal Depression Scale (EPDS), laboratory assessments, and adverse events (AEs). The participants were randomized 1:1 to receive either IV iron isomaltoside or current treatment practice with oral iron. The randomization was stratified by bleeding volume (700–1000 mL or >1000 mL).
Permuted block randomization with a block size of four was used to randomize the participants. The participants were randomized by using the built-in randomization module in the electronic Case Report Form (eCRF) system eClinicalOS (CFR 21 part 11 compliant and provided by Merge Healthcare, Morrisville, NC). When the participants’ data had been entered, a unique randomization number was generated; identifying which treatment the participant was allocated to.
Participants
The main trial took place at the Department of Obstetrics, Rigshospitalet, University of Copenhagen, Denmark. Women ≥18 years of age with PPH ≥700 and ≤1000 mL or PPH >1000 mL and Hb >6.5 g/dL measured >12 hours after delivery, and who were willing to provide written informed consent were considered eligible to participate in the trial. The exclusion criteria included multiple births, peripartum red blood cell (RBC) transfusion, and history of multiple allergies. All exclusion criteria are listed in the published trial protocol [Citation20]. The present subanalysis includes the participants of the main trial with a high fatigue score (MFI physical fatigue score >15) within 48 hours after delivery.
Any concomitant medication or treatment deemed necessary to provide adequate supportive care was allowed throughout the trial except erythropoiesis stimulating agent treatment, and in addition participants receiving iron isomaltoside was not allowed to take any iron supplementation other than investigational drug as this could influence the outcome measures of the trial.
Interventions
The enrolled participants received either 1200 mg iron isomaltoside as an IV infusion diluted in 100 mL 0.9% sodium chloride and given over at least 15 minutes or current treatment practice with oral iron.
Endpoints
The primary endpoint was the aggregated change in physical fatigue score measured by the MFI from baseline to week 12. A subanalysis was also performed on women who delivered by the vaginal route. The secondary endpoints were the physical fatigue symptoms at day 3, week 1, 3, 8, and 12, other fatigue symptoms at day 3, week 1, 3, 8, and 12, postpartum depression symptoms measured by the Edinburgh Postnatal Depression Scale (EPDS) at week one, three, eight, and 12, Hb, p-ferritin, transferrin saturation (TSAT), and reticulocyte count at day 3, week 1, 3, 8, and 12, time to postpartum lactogenesis and discontinuation of breastfeeding, proportion of women who received one or more allogenic RBC transfusions and the number of units of RBC transfused per transfused woman during the trial, and safety (AEs).
The MFI is a 20-item scale evaluating five dimensions of fatigue: general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. The obtainable score within each subscale ranges from four (absence of fatigue) to 20 (maximum fatigue). The MFI has high feasibility, reliability, and validity in chronically anemic and postpartum women [Citation21]. The EPDS detects symptoms of depression in puerperal women during the previous 7 days [Citation22]. The maximum score is 30 and a score of 10 or higher indicates possible depression.
Statistical methods
The safety analysis set (n = 85) included randomized participants who received the trial drug. The full analysis set (FAS) population (n = 85) included randomized participants who received the trial drug, had a baseline physical fatigue score, and had at least one postbaseline physical fatigue score.
The aggregated change in physical fatigue score was calculated as the area under the curve (AUC) of the change from baseline to week 12, using the trapezoidal method adjusted for the observation period. The primary endpoint was analyzed using an analysis of covariance model (ANCOVA), with treatment and bleeding volume (700–1000 mL, >1000 mL) as factors and baseline physical fatigue score as covariate.
Continuous secondary endpoints were analyzed by a mixed model for repeated measurements (MMRM) including visit, treatment-by-visit, and bleeding volume (700–1000 mL, >1000 mL) as factors, baseline (for parameter where the baseline value is measured), and participant as random effect. A variance component covariance was used to model the within-subject errors and the estimation method was a restricted maximum likelihood-based approach. In addition, the aggregated postnatal depression score was calculated as the AUC of the score from week one to week 12, using the trapezoidal method adjusted for the observation period, and analyzed as the primary endpoint. Time to lactogenesis and time to discontinuation of breastfeeding were compared between treatments by a log-rank test. The baseline characteristics, endpoints on proportion of participants, and safety data were described descriptively. All tests were two-tailed and the significance level was 0.05.
The statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). The AEs were coded by system organ class and preferred term using Medical Dictionary for Regulatory Activities body system version 16.0.
Results
Out of the 200 women enrolled in the main trial [Citation18], 85 women had a baseline physical fatigue score >15. These participants in the main trial were randomized 1:1 to the iron isomaltoside group (41 participants) or oral iron (44 participants). Demographics and baseline characteristics are summarized in . Overall baseline characteristics were comparable between the treatment groups ().
Table 1. Participant demographics and baseline characteristics.
All 41 participants in the iron isomaltoside group were administered the full dose of 1200 mg iron at baseline. Participants in the oral iron group had a mean (standard deviation, SD) daily dose of 155 (68) mg oral iron.
Fatigue and depression scores
The primary efficacy endpoint of the trial was AUC of change in physical fatigue score from baseline to week 12 assessed by the MFI. The difference estimate between the two treatments groups in aggregated change in physical fatigue score from baseline to week 12 was −2.3 (confidence interval (CI) 95%: −3.3; −1.3) (p < .0001) in favor of iron isomaltoside (). In both treatment groups, the aggregated change in physical fatigue score decreased at each visit from baseline to week 12, and the decrease was statistically significantly higher in the iron isomaltoside group as compared to the oral iron group at all time points (). A subanalysis was also performed on women who delivered by vaginal route. The difference estimate between the two treatments groups was −2.2 (95%CI: −3.6; −0.8) (p < .003) in favor of iron isomaltoside.
Table 2. Aggregated change in physical fatigue score.
All measured fatigue scores (physical fatigue, general fatigue, reduced activity, mental fatigue, and reduced motivation) decreased continuously in both treatment groups during the 12 weeks with significantly lower scores (low scores indicate a low degree of fatigue) in the iron isomaltoside group, except for physical fatigue at week 1, reduced activity at day 3 and week 1, and reduced motivation at week 1, 3, and 12 ().
Figure 1. Fatigue and depression scores. Results are shown as mean scores of the Multidimensional Fatigue Inventory (MFI) and Edinburgh Postnatal Depression Scale (EPDS) in the iron isomaltoside and oral iron groups from baseline to 12 weeks postpartum.
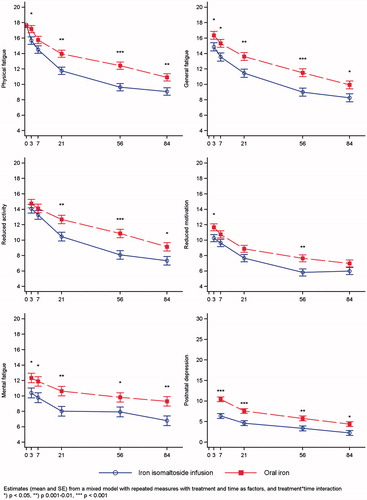
Depression scores (EPDS score for postnatal depression) decreased continuously in both treatment groups during the 12 weeks with significantly lower scores (low scores indicate a low degree of depression) in the iron isomaltoside group from week 1 and onwards (). The difference estimate between the two treatments groups in postnatal depression score from week one to week 12 was −2.39 (95%CI: −3.62; −1.16) (p = .0002) in favor of iron isomaltoside.
Hematological parameters
One week after baseline, the increase from baseline in Hb concentration was greater in the iron isomaltoside group compared with the oral iron group, reaching statistically significance at week 1, 3, and 12 (p < .03) (). Reticulocyte count increased within the first week in both treatment groups with a significantly higher increase observed in the iron isomaltoside group (p ≤ .02) ().
Figure 2. Hemoglobin, p-ferritin, transferrin saturation, and reticulocyte count over time by treatment group.
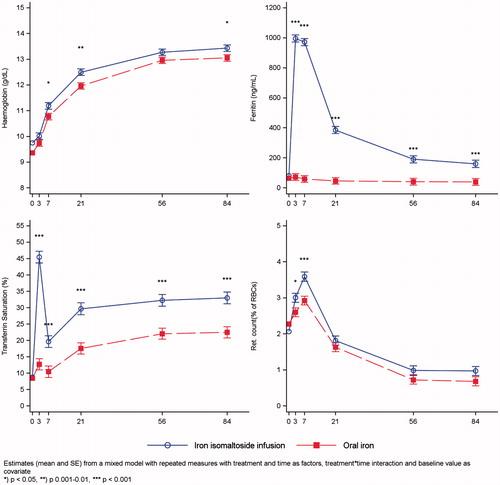
P-ferritin increased significantly in the iron isomaltoside group whereas it remained unchanged with oral iron (p ≤ .0003) (). TSAT increased in both treatment groups with a significantly higher increase observed in the iron isomaltoside group (p ≤ .0003) ().
Lactogenesis and breastfeeding
There was no significant difference in the median time to lactogenesis between the treatment groups (81 h for iron isomaltoside versus 86 h for oral iron; p = .13) or time to discontinuation of breastfeeding (p = .57). None of the participants in the iron isomaltoside group and one (3%) of the women in the oral iron group had discontinued breastfeeding at week 12 postpartum.
RBC transfusion
Participants in both treatment groups received RBC transfusions. One participant (2%) in the iron isomaltoside group received two units, and three participants (7%) in the oral iron group received two units (two participants) or four units (one participant).
Safety
A total of 13 serious adverse events (SAEs) occurred during the trial (six SAEs in four participants in the iron isomaltoside group and seven SAEs in six participants in the oral iron group). All SAEs were mild and assessed by the investigator not to be related to trial drug, and for all SAEs the participant recovered. Hence, no serious adverse drug reactions occurred.
In the iron isomaltoside group, seven AEs in five participants were assessed to be possibly related or related to investigational product (myalgia, pyrexia, infusion site reaction, puncture site swelling, infusion site irritation, infusion site discoloration, and phlebitis), while in the oral iron group, 13 AEs in 11 participants were assessed to be possibly related to investigational product (four events of hemorrhoids and nine events of constipation).
Two participants (5%) in the iron isomaltoside group and one participant (2%) in the oral iron group had a p-phosphate levels <2 mg/dL 1 week after baseline. In all cases, the phosphate level increased to above 2 mg/dL at the subsequent visit. These events were a-symptomatic, not reported as AEs, and assessed as nonclinically significant.
Discussion
We analyzed a subpopulation of 85 women with PPH and a high fatigue score (physical fatigue score >15) within 48 hours after delivery and compared the effect of a single infusion of 1200 mg iron isomaltoside to current treatment practice with oral iron on aggregated change in physical fatigue 12 weeks postpartum. The difference estimate between the two treatments groups was −2.3 (p < .0001), showing that treatment with iron isomaltoside lead to a significantly higher decrease in physical fatigue when compared to current treatment practice with oral iron. This finding is consistent with the main trial [Citation18]. In the main trial, there was an estimated difference of −0.97 in physical fatigue score between the treatment groups which was less than the predefined minimum clinically relevant difference of 1.8 required for claiming superiority [Citation18]. However, in this subanalysis, the estimated difference of −2.3 reached the predefined target of 1.8, and superiority was confirmed for iron isomaltoside compared with current treatment practice with oral iron in women suffering from severe fatigue after PPH. Similar results were obtained when including only women who delivered by the vaginal route. Other fatigue scores also improved significantly with iron isomaltoside compared with current treatment practice with oral iron.
Postnatal depression decreased continuously in both treatment groups with significantly lower scores in the iron isomaltoside group from week one and onwards. Thus, treatment of iron deficiency may decrease depression. This is supported by a randomized, double-blind, placebo-controlled trial of nonanemic women suffering from postpartum depression [Citation23]. Early iron supplementation (1 week after delivery) in women suffering from postpartum depression significantly improved the iron stores and caused a significant improvement in postpartum depression with a 42.8% improvement rate during 6 weeks.
In general, greater improvements in hematological parameters were observed in the iron isomaltoside group compared with the oral iron group and the treatment differences persisted until 12 weeks. This indicates that treatment with iron isomaltoside leads to a better hematopoietic response than current treatment practice with oral iron.
Iron isomaltoside administration was well tolerated and no serious adverse drug reactions were observed. More participants in the oral iron group experienced related AEs then in the iron isomaltoside group (25 versus 12%).
Women with iron deficiency with or without anemia may suffer from various symptoms including fatigue, reduced quality of life, headache, and dizziness. All these symptoms can be debilitating, especially when caring for the newborn. Thus, it is highly important to treat women suffering from PPH and anemia, and the drug profile of iron isomaltoside seems optimal as these women that need fast correction of their iron deficit and are unlikely to attend the hospital for multiple IV iron infusions of lower dose IV iron. In a previous subsample of the main trial, we found a transient increase in iron concentration in breast milk 3 days after treatment with IV iron isomaltoside [Citation24]. After 1 week, however, the level did not differ from women treated with oral iron, and mean iron concentrations were within the normal range in all samples.
Besides treating the women with IV iron after PPH it is also important to prevent the PPH from occurring. Previous trials have shown a link between low Hb levels during pregnancy and potential risk of PPH [Citation25–27]. Frass (2015) reported that 29.1% of anemic women developed PPH during cesarean delivery due to uterine atony [Citation27] and it is suggested that low iron stores may lead to decreased uterine blood flow or low uterine muscle strength contributing to inefficient uterine contractions and blood loss [Citation25]. Therefore, it is also important to ensure that women are not anemic during pregnancy; anemia is highly prevalent in pregnant women with a global prevalence of more than 40% [Citation1]. Treatment with iron isomaltoside is currently restricted to the second and third trimester and further clinical trials of iron isomaltoside administered to pregnant women are warranted.
In conclusion, IV iron isomaltoside is a relevant alternative to oral iron supplementation in women suffering from severe fatigue after postpartum hemorrhage. A single dose of 1200-mg IV iron isomaltoside is associated with a statistically significant and clinically relevant reduction in aggregated physical fatigue, as well as a significant reduction in other fatigue scores, and postnatal depression within 12 weeks after delivery when compared to current treatment practice with oral iron and with a similar safety profile.
Acknowledgements
The authors gratefully acknowledge all the investigators and trial personal for their contribution to the trial, the statistical support from Jens-Kristian Slott Jensen, Slott Stat, and the medical writing support from Eva-Maria Damsgaard Nielsen.
Disclosure statement
Lars Lykke Thomsen is employed by Pharmacosmos A/S, Charlotte Holm serves on advisory board for Pharmacosmos A/S. The institution (Department of Obstetrics, Juliane Marie Centre, Rigshospitalet, University of Copenhagen) received a fee per participant. Eva-Maria Damsgaard Nielsen is employed by Pharmacosmos A/S. Jens Langhoff-Roos did not have further conflict of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- WHO Global Database on Anaemia. Worldwide prevalence of anaemia 1993–2005. Atlanta: Center for Disease Control and Prevention; 2008.
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage; 2012.
- Sundhedsstyrelsen [Internet]. National klinisk retningslinje om Indikation for transfusion Med blodkomponenter; [cited 2017 Dec 21]. Available from: https://sundhedsstyrelsen.dk/da/udgivelser/2014/∼/media/EEA1EA90C15E4A97B9E786D2850B3664.ashx
- Goldberg ND. Iron deficiency anemia in patients with inflammatory bowel disease. Clin Exp Gastroenterol. 2013;6:61–70.
- KDIGO clinical practice guideline for anemia in chronic kidney disease [Internet]; 2012. Vol. 2; [cited 2017 Dec 21]. Available from: http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO-Anemia%20GL.pdf
- Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211–222.
- Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545–1553.
- Hildebrandt PR, Bruun NE, Nielsen OW, et al. Effects of administration of iron isomaltoside 1000 in patients with chronic heart failure. A pilot study. TATM. 2010;11:131–137.
- Wikström B, Bhandari S, Barany P, et al. Iron isomaltoside 1000: a new intravenous iron for treating iron deficiency in chronic kidney disease. J Nephrol. 2011;24:589–596.
- Reinisch W, Staun M, Tandon RK, et al. A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). Am J Gastroenterol. 2013;108:1877–1888.
- Reinisch W, Altorjay I, Zsigmond F, et al. A 1-year trial of repeated high-dose intravenous iron isomaltoside 1000 to maintain stable hemoglobin levels in inflammatory bowel disease. Scand J Gastroenterol. 2015;50:1226–1233.
- Bhandari S, Kalra PA, Kothari J, et al. A randomized, open-label trial of iron isomaltoside 1000 (Monofer®) compared with iron sucrose (Venofer®) as maintenance therapy in haemodialysis patients. Nephrol Dial Transplant. 2015;30:1577–1589.
- Kalra PA, Bhandari S, Saxena S, et al. A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney disease patients with anaemia. Nephrol Dial Transplant. 2016;31:646–655.
- Birgegård G, Henry D, Glaspy J, et al. A randomized, noninferiority trial of intravenous iron isomaltoside versus oral iron sulfate in patients with nonmyeloid malignancies and anemia receiving chemotherapy: The PROFOUND Trial. Pharmacotherapy. 2016;36:402–414.
- Dahlerup JF, Jacobsen BA, van der Woude J, et al. High-dose fast infusion of parenteral iron isomaltoside is efficacious in inflammatory bowel disease patients with iron-deficiency anaemia without profound changes in phosphate or fibroblast growth factor 23. Scand J Gastroenterol. 2016;51:1332–1338.
- Derman R, Roman E, Modiano MR, et al. A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia. Am J Hematol. 2017;92:286–291.
- Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer®) reduces postoperative anaemia in preoperatively non-anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double-blind placebo-controlled clinical trial (the PROTECT trial). Vox Sang. 2015;109:257–266.
- Holm C, Thomsen LL, Norgaard A, et al. Single-dose intravenous iron infusion or oral iron for treatment of fatigue after postpartum haemorrhage: a randomized controlled trial. Vox Sang. 2017;112:219–228.
- Holm C, Thomsen LL, Norgaard A, et al. Single-dose intravenous iron infusion versus red blood cell transfusion for the treatment of severe postpartum anaemia: a randomized controlled pilot study. Vox Sang. 2017;112:122–131.
- Holm C, Thomsen LL, Norgaard A, et al. Intravenous iron isomaltoside 1000 administered by high single-dose infusions or standard medical care for the treatment of fatigue in women after postpartum haemorrhage: study protocol for a randomised controlled trial. Trials. 2015;16:5.
- Jansen AJ, Essink-Bot ML, Duvekot JJ, et al. Psychometric evaluation of health-related quality of life measures in women after different types of delivery. J Psychosom Res. 2007;63:275–281.
- Gibson J, McKenzie-McHarg K, Shakespeare J, et al. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119:350–364.
- Sheikh M, Hantoushzadeh S, Shariat M, et al. The efficacy of early iron supplementation on postpartum depression, a randomized double-blind placebo-controlled trial. Eur J Nutr. 2017;56:901–908.
- Holm C, Thomsen LL, Norgaard A, et al. Iron concentration in breast milk normalised within one week of a single high-dose infusion of iron isomaltoside in randomised controlled trial. Acta Paediatr. 2017;106:256–260.
- Kavle JA, Stoltzfus RJ, Witter F, et al. Association between anaemia during pregnancy and blood loss at and after delivery among women with vaginal births in Pemba Island, Zanzibar, Tanzania. J Health Popul Nutr. 2008;26:232–240.
- Jaleel R, Khan A. Post-partum haemorrhage – a risk factor analysis. Mymensingh Med J. 2010;19:282–289.
- Frass KA. Postpartum hemorrhage is related to the hemoglobin levels at labor: observational study. Alex J Med. 2015;51:333–337.
