Abstract
Purpose: To examine trends in patients submitting samples for cell-free DNA screening to determine whether they reflect a shift towards NIPT use in the low-risk population.
Methods: A review of demographic information was performed for all specimens submitted to the Ariosa Diagnostics clinical laboratory for the Harmony® prenatal test between January 1, 2014 and December 30, 2017. The proportions of specimens for patients under 35 years and 35 years and older were compared.
Results: There was a significant increase in the proportion of specimens submitted by patients under 35, from 47.3% in 2014 to 60.3% in 2017 (Chi-square test, p < .001).
Conclusions: The proportion of samples submitted to our laboratory by patients under 35 years has significantly increased in the 4-year subset, which represents the demographics of a diverse group of patients from across the globe. This suggests an increase in uptake of NIPT in the low-risk population.
Objectives and protocol
Non-invasive prenatal testing (NIPT) was initially recommended for women at increased risk of fetal aneuploidy by most professional society guidelines. With the publication of additional clinical studies, offering NIPT to all pregnant women was supported by subsequent guidelines. In addition, provider and public awareness of NIPT has increased over time. This study provides data on the proportion of patients under 35 years of age electing NIPT in a well-established commercial laboratory. We demonstrate an international trend over a 4-year time period with an increasing proportion of samples submitted by patients under 35 years.
Introduction
Non-invasive prenatal testing (NIPT) for trisomy 21 by the analysis of cell-free DNA (cfDNA) in maternal plasma was introduced in 2011 and immediately recognized as a significant advance in prenatal screening. By 2014, NIPT was offered in over 60 countries in six continents [Citation1]. A search of PubMed with the phrases “noninvasive/non-invasive prenatal” or “cell free/cell-free DNA prenatal” reveals a doubling of the number of scientific publications around the topic of NIPT published in 2017 versus 2011, reflecting a heightened interest in the scientific and medical communities [Citation2]. Information about NIPT is accessible to the public through the internet and other forms of digital media with an increase in internet searches on the subject of NIPT from 2011 to 2017 [Citation3].
Initially, most professional society guidelines recommended NIPT for women at increased risk of fetal aneuploidy, a reflection of the study populations in early validation studies [Citation4–Citation6]. In 2013, the American College of Medical Genetics issued a position statement addressing utilization of NIPT in clinical practice without restriction by clinical indication or maternal age [Citation7]. Clinical studies by Nicolaides et al. and Bianchi et al. demonstrated the accuracy of NIPT in the general pregnancy population [Citation8,Citation9] and were followed by the landmark NEXT (Noninvasive Examination of Trisomy) study in 2015, which demonstrated superior performance of targeted cfDNA screening compared to traditional first trimester screening for fetal trisomy in the general pregnancy population [Citation10]. Subsequent guidelines included this evidence in support of offering NIPT to all pregnant women, regardless of maternal age or risk status [Citation11–Citation13].
NIPT has seen rapid adoption with studies showing a concomitant decrease in both the number of serum screening tests and invasive diagnostic procedures [Citation14,Citation15]. In this study, we examine international trends in the age of patients choosing NIPT for fetal aneuploidy screening to explore whether they are consistent with a shift towards use of NIPT in the low-risk pregnancy population. We review the demographic information provided with samples submitted for cfDNA screening to a well-established commercial laboratory over a 4-year period.
Materials and methods
We performed a retrospective review of demographic information for all specimens submitted to the Ariosa Diagnostics, Inc. CLIA-certified and CAP-accredited laboratory for the Harmony® prenatal test between 1 January 2014 and 30 December 2017. The test includes screening for trisomies 21, 18 and 13 with optional assessment for sex chromosome aneuploidy. Maternal age and country of origin were obtained from test requisition forms and sub-grouped by categories of 35 years and older or under 35 years. Given the limited clinical information provided to the laboratory, maternal age under 35 years was chosen as representative of a low-risk pregnancy.
Results
Specimens were received from the United States (US) and over 65 other countries. Out of 903,789 specimens, the proportion of specimens submitted by patients under 35 years significantly increased across the study period, from 47.3% in 2014 to 60.3% in 2017 (Chi-square test, p < .001) (). In December 2014, the proportion of specimens from patients under 35 reached 50%; this steadily increased to 60.3% by the end of 2017.
Figure 1. (a) Proportion of samples by age group and time, US and outside of the US. (b) Proportion of samples by age group and time, outside of US only.
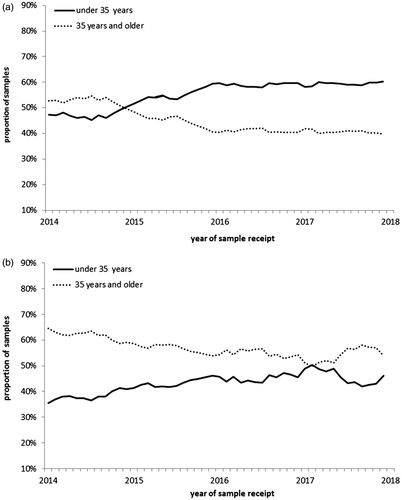
In the subset of specimens submitted from countries other than the US, the proportion of patients under 35 also increased, 35.5% to 46.2% from January 2014 to December 2017 (Chi-square test, p < .001) but did not reach 50% (). This trend primarily reflects countries in Europe. There was no statistically significant change in the proportions in Asia and South America over the study period. In Asia the proportions of the two age groups remained relatively equal (mean of 49.0% under 35 and 51.0% 35 years and older) whereas in South America, the proportion of specimens from patients under 35 remained consistently lower (mean of 32.7%).
Discussion
The group of samples in this study was received from over 65 countries and represents the demographics of a diverse group of patients. This study clearly demonstrates an international trend over a 4-year time period, with an increasing proportion of samples submitted by patients under 35 years of age. The data suggests an increase in use of NIPT in low-risk pregnancies.
These trends are likely due to many factors including the publication of clinical studies such as the NEXT study, the inclusion of NIPT in professional society guidelines, increased provider and public awareness across a broader segment of the pregnancy population, and a growing number of national and regional health plans supporting access to NIPT [Citation16].
Trends outside of the US were less striking and may reflect different healthcare systems, patient and provider preferences, and costs. Until now, most of these patients have paid for NIPT out-of-pocket; however testing is now being implemented into routine prenatal care in many European countries. Belgium and the Netherlands recently introduced NIPT to all pregnant women as an alternative to first trimester combined screening through national healthcare-funded prenatal screening programs [Citation17,Citation18]. It is reasonable to presume that other countries within and outside of Europe will consider broader NIPT implementation as barriers with cost and other factors are resolved.
It should be noted that our analysis used maternal age as the main study parameter. Currently, clinical indications provided with the specimen are used for billing purposes and may be incomplete or subject to bias. A proportion of the women under 35 may have high-risk indications, e.g. positive serum screen results or fetal ultrasound findings. We might expect this proportion to remain stable—or even decline with the decreased use of serum screening [Citation14] and not contribute to the trend observed; however, this could be confirmed in a more comprehensive study with medical records review. This study also focused on one commercial test offering, which could be impacted by a shift in client base over time.
Although our maternal age data supports an increase in NIPT uptake in the low-risk pregnancy population, there is still a disproportionate amount of specimens from women 35 years and over. In our data set, the proportion of NIPT specimens from women under 35 reached 60.3% by the end of 2017. However, approximately 83% of live births in the USA and 77% in the United Kingdom in 2016 were from women under 35 [Citation19,Citation20]. A large portion of the low-risk pregnancy population is therefore still not currently utilizing NIPT. However, as barriers with access and cost are resolved, utilization of NIPT in the low-risk population will likely continue to change over time.
Disclosure statement
All authors are employees of Roche Sequencing Solutions, Inc.
References
- Allyse M, Minear MA, Berson E, et al. Non-invasive prenatal testing: a review of international implementation and challenges. Int J Womens Health. 2015;7:113–126.
- PubMed. Bethesda: US National Library of Medicine, National Center for Biotechnology Information. 1996 [cited 2018 Apr 25], Available from: https://www.ncbi.nlm.nih.gov/pubmed/
- Google. Menlo Park. 1998. [cited 2018 Apr 25]. Available from: https://trends.google.com/trends/
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532–1534.
- Devers PL, Cronister A, Ormond KE, et al. Noninvasive prenatal testing/noninvasive prenatal diagnosis: the position of the National Society of Genetic Counselors. J Genet Counsel. 2013;22:291.
- Benn P, Borell A, Chiu R, et al. Position statement from the aneuploidy screening committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn. 2013;33:622–629.
- Gregg AR, Gross SJ, Best RG, et al. ACMG statement on noninvasive prenatal screening for fetal aneuploidy. Genet Med. 2013;15:395–398.
- Nicolaides KH, Syngelaki A, Ashoor G, et al. Noninvasive prenatal testing for fetal trisomies in a routinely screened first-trimester population. Am J Obstet Gynecol. 2012;207:374e1–374e6.
- Bianchi DW, Parker RL, Wentworth J, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799–808.
- Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589–1597.
- Benn P, Borrell A, Chiu RW, et al. Position statement from the Chromosome Abnormality Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn. 2015;35:725–734.
- Committee Opinion No. 640: Cell-Free DNA Screening For Fetal Aneuploidy. Obstet Gynecol. 2015;126:e31–e37.
- Gregg AR, Skotko BG, Benkendorf JL, et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2016;18:1056–1065.
- Larion S, Warsof SL, Romary L, et al. Use of the combined first-trimester screen in high- and low-risk patient populations after introduction of noninvasive prenatal testing. J Ultrasound Med. 2015;34:1423–1428.
- Hui L, Muggli EE, Halliday JL. Population-based trends in prenatal screening and diagnosis for aneuploidy: a retrospective analysis of 38 years of state-wide data. BJOG. 2016;123:90–97.
- “Roche’s Ariosa Harmony Noninvasive Prenatal Test Gets Coverage From Large Private Insurers in US.” GenomeWeb. [cited 2016 May 3]. Available from: https://www.genomeweb.com/molecular-diagnostics/roches-ariosa-harmony-noninvasive-prenatal-test-gets-coverage-large-private.
- Oepkes D, Page-Christiaens GC, Bax CJ, et al. Trial by Dutch laboratories for evaluation of non-invasive prenatal testing. Part I-clinical impact. Prenat Diagn. 2016;36:1083–1090.
- Institut national d’assurance maladie-invalidite. [cited 2017 June 30]. Available from: http://www.riziv.fgov.be/fr/nouvelles/Pages/remboursement-test-prenatal-non-invasif.aspx.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1–55.
- Office for National Statistics. Statistical bulletin: births in England and Wales. 2016 [cited 2018 Apr 25]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthsummarytablesenglandandwales/2016.
