Abstract
Objectives: To investigate factors associated with obtaining results on repeat cell-free DNA testing for fetal trisomy after an initial sample with insufficient fetal fraction.
Methods: A series of clinical laboratory samples was queried to identify patients with multiple samples drawn for the Harmony® prenatal test. Maternal demographics, gestational age, timing of sampling, and repeat test outcome were reviewed. Multivariate logistic regression analysis was used to determine the odds ratio of obtaining a result.
Results: Two thousand nine hundred six unique pregnancies were identified with a sample submitted for repeat testing after an initial test with an insufficient fetal fraction. Overall, 53% obtained a result on the second draw. The odds of obtaining a result were associated with interval time between draws (per day, OR 1.040, 95% CI 1.031–1.051) and maternal weight (per kg, OR 0.988, 95% CI 0.985–0.991) but not maternal age, gestational age at initial draw, IVF status, or twin versus singleton pregnancy.
Conclusions: The probability of obtaining a result with repeat cell-free DNA testing decreases with higher maternal weight and increases with the interval between draws. Waiting longer before collecting a repeat sample increases the probability of obtaining a result but should be considered in the context of the gestational age of the pregnancy and the clinical indication for testing.
Introduction
The provision of options for genetic screening and testing is part of standard prenatal care in most developed countries [Citation1]. As a highly sensitive and specific screen for fetal trisomy [Citation2], cell-free DNA (cfDNA) testing is now widely offered as one option. For any prenatal test, measures to ensure the quality of each individual test performed should be in place. Analyses that do not meet quality standards do not produce a result and need to be repeated. For first trimester combined screening, accurate nuchal translucency measurement is central to quality results [Citation3]. The First- and Second-Trimester Evaluation of Risk (FASTER) trial documented a 7% rate of failed or suboptimal imaging [Citation4]. Similarly, cfDNA screening requires a minimum fetal fraction, the proportion of cfDNA in maternal blood that derives from the pregnancy [Citation5]. In the Noninvasive EXamination of Trisomy (NEXT) study, 1.7% of cfDNA tests had insufficient or unmeasurable fetal fraction [Citation6].
Many factors, biological and technical, may be associated with “no-result” rates. For both ultrasound and cfDNA testing, maternal obesity is a significant factor [Citation7–12]; however, cfDNA tests that do not yield a result have drawn particular attention due to concerns that they may indicate an increased risk for fetal aneuploidy [Citation6].
cfDNA testing was not repeated as part of the NEXT study and limited published data exists to inform a decision to obtain a repeat specimen versus pursue alternative testing [Citation8,Citation11,Citation13]. Consequently, there is a lack of consensus among professional societies around the clinical management of these patients. Guidelines range from the position statement of the International Society of Prenatal Diagnosis, which recommends reappraisal based on clinical factors such as gestational age and the presence of ultrasound findings [Citation1], to that of the American College of Medical Genetics, which advices against repeat testing [Citation14]. The latter’s conclusion rests on uncertainty over whether repeat collection would overcome a low fetal fraction.
The objectives of this study were to investigate factors associated with the probability of obtaining a result on repeat blood draw after an initial cfDNA result was not reported due to an insufficient fetal fraction and to consider how this information could be used in the clinical management of these pregnancies. The interval between draws was investigated as well as maternal weight, gestational age, twin pregnancy, and in vitro fertilization, factors that have been associated with no results on the first draw in other studies [Citation8,Citation11,Citation12,Citation15,Citation16].
Materials and methods
The data for this study were derived from samples submitted to the Ariosa Diagnostics Inc. clinical laboratory in San Jose, CA, USA between April 2015 and September 2016. A consecutive series of clinical laboratory samples was queried to identify patients with multiple samples drawn during the same pregnancy for the Harmony® prenatal test. Patients consented to the clinical testing ordered. The Harmony test is a cfDNA test that uses DANSR assays and a fetal-fraction optimized algorithm to determine the probability of trisomy 13, trisomy 18, and trisomy 21 [Citation17,Citation18]. Fetal fraction measurement leverages single nucleotide polymorphisms (SNPs), using relative quantitation at selected loci [Citation19]. A minimum of 4% fetal fraction is required in the sample for reporting probability scores.
The query captured maternal age, maternal weight (when available), gestational age, timing of sampling, method of conception, and number of fetuses. Samples from the same patient were assumed to be in the same pregnancy if they were drawn within a 90-day period. After the ascertainment of linked samples by clinical staff, the information was deidentified before further aggregate data analysis was performed in pregnancies for which the first blood sample had insufficient fetal fraction for analysis.
Test outcome for subsequent draws was categorized as “result provided” if a probability score was reported or “no result provided” if a probability score was not reported due to insufficient fetal fraction or failure to meet other quality control thresholds. A logistic regression model was built to determine the probability of obtaining a result upon repeat testing. Statistical analyses were performed in R (v3.1.3) [Citation20].
Results
Querying 428,707, consecutive samples identified 2904 patients with 2906 unique pregnancies redrawn after an initial sample with an insufficient fetal fraction. Two patients had tests with no results in more than one pregnancy. The characteristics of the redrawn pregnancies are summarized in . Mean maternal weight was 96 kg. Mean gestational age at the time of first sampling was 12.3 weeks. IVF and twin pregnancies comprised 12.5 and 8.6%, respectively, of the pregnancies studied.
Table 1. Characteristics of 2906 pregnancies redrawn after an initial sample with insufficient fetal fraction.
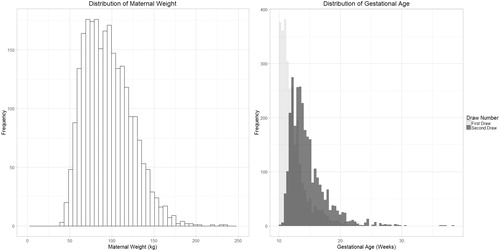
Mean gestational age at the time of the second draw was 14.6 weeks with a wider distribution than the gestational age on the first draw []. Repeat testing provided a result on the second draw in 1540 pregnancies (53%). Mean maternal weight was 100 kg in pregnancies that did not obtain a result upon redraw compared to 92.3 kg in those that obtained a result (p values <.001 using a two-sided t-test).
Figure 1. Distribution of maternal weight and gestational age at the time of first and second draws. Maternal weight is at the time of first draw and was available for 2466 pregnancies.
Multivariate logistic regression analysis including all variables demonstrated that the odds of obtaining a result decreased with maternal weight and increased with interval time between draws []. There was no significant contribution by maternal age, gestational age at initial sampling IVF status, number of fetuses. The final model included only maternal weight and days between draws. The odds ratio of obtaining a result was 1.040, (95% CI 1.031–1.051) for the interval between draws and 0.988 (95% CI 0.985–0.991) for maternal weight. This means that for every day the redraw interval increases, we expect to see a 4% increase in the odds of obtaining a result. In contrast, for every kg increase in maternal weight, we expect to see a 1.2% decrease in the odds of obtaining a result.
Table 2. Results of multivariate regression analysis demonstrating the contribution of specific factors to obtaining a result with repeat testing.
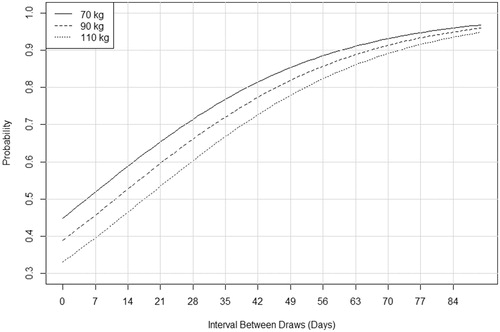
To generate a representation that communicates the magnitude of this increase in a context that is more familiar to clinicians, the final regression model was used to predict the probability of obtaining a result []. Predictions were based on an interval between draws, holding maternal weight constant at 70, 90, and 110 kg, as examples.
Figure 2. Predicted probability of obtaining a result by interval between draws. The interval is given in days and is calculated from the day the initial sample was drawn (not when it was reported). Probabilities are plotted for three maternal weight values: 70, 90, 110 kg.
This study was intended to investigate factors associated with obtaining a result upon the second draw; however, in this dataset, we observed 246 pregnancies in which a patient was redrawn more than once. A result was reported in 125 of the third samples (51%), a rate comparable to that of second samples. When these pregnancies were divided into two groups by gestational age at the time of the third draw, the rate of reported results was 48% in the group 20 weeks and less and 66% in the group greater than 20 weeks [], a difference that was not statistically significant based on Pearson’s chi-squared test (p values >.05). In the group of third-draw samples, 20 weeks and less, the average gestational age at the time of draw was 15.9 weeks for samples receiving a result compared to 14.2 weeks for the second draw samples receiving a result.
Table 3. Proportion of results obtained in pregnancies with multiple repeat tests.
Discussion
We have identified a large series of pregnancies with samples obtained for repeat cfDNA testing after an initial sample had an insufficient fetal fraction and demonstrated that the odds of obtaining a result upon repeat testing decreases with maternal weight and increases with the interval between draws. A model based on the odds ratios generates a figure that provides predicted probabilities of obtaining a result and may help inform a clinical decision as to whether and when to redraw for repeat testing [].
Previous studies have investigated maternal and pregnancy factors associated with fetal fraction [Citation8–12]. Both fetal fraction and the odds of receiving a result on a first draw have been shown to decrease with maternal weight and increase with gestational age [Citation8–12,Citation21]. Although it seems reasonable that these factors would also impact repeat testing, there has been a less systematic evaluation of their influence on repeat test outcome. In an earlier study of 135 redrawn samples, we grouped patients into bins by weight and observed a decreasing proportion of results with increasing maternal weight [Citation8]. Kinnings et al. [Citation11] took a similar approach using BMI but found no apparent trend. They also concluded that there was no effect of the interval between draws but acknowledged that the distribution of their 381 samples across the interval bins was not sufficient. The size of the current study, with close to 3000 redrawn patients, confirms an association between the odds of obtaining a result upon redraw and both maternal weight and interval between draws. This is consistent with the recently reported study by Benn et al., who also used logistic regression analysis in another large series of samples redrawn for a range of reasons [Citation13]. Interestingly, twin pregnancy and IVF pregnancy have also been associated with a lower fetal fraction on the first draw in previous studies [Citation12,Citation15,Citation16], but neither of these influenced the odds of receiving a result on repeat testing in this study. This could have a biological basis – the underlying reasons for a lower fetal fraction in these groups have yet to be fully understood – or could be related to the specific patient population with repeat testing in this study.
An average increase in the fetal fraction of 0.1% per week between 10 and 21 weeks of gestation and 1% per week after 21 weeks has been demonstrated [Citation8,Citation11]; however, this is an average across a population and significant variation between pregnancies was observed. In the current study, the relatively high proportion of samples that yielded a result even within the shortest interval of time and the only modest increase in the probability of a result thereafter suggests that changes in a fetal fraction over time may be more complex in individual pregnancies. It is also possible that nonbiological factors such as specimen handling issues may be contributing to a lower fetal fraction in some initial draws.
About 53% of patients overall receiving a result upon repeat testing in this cohort is consistent with two previously reported clinical laboratory series, both of which reported rates of 56% in smaller cohorts of patients redrawn due to insufficient fetal fraction [Citation8,Citation11]. Somewhat higher rates (63–64%) have been reported in three studies that included samples redrawn for a broader range of reasons than just fetal fraction [Citation10,Citation12,Citation13]. Our analysis also demonstrates that these rates will differ between studies depending on the average weight and gestational age of the cohort. The predicted probability of a result based on our model will range from less than 40% to greater than 70% depending on the specific situation.
The probabilities presented in support offering repeat testing to most patients but show that waiting before redrawing will offer only a minor benefit. For example, a 70 kg woman has a 60% probability of obtaining a result if redrawn 2 weeks after the initial draw but waiting for two additional weeks only increases this figure to approximately 70%. Although waiting before redrawing a sample will increase the probability of obtaining a result, in general, the clinical utility of doing so must be considered. Patients that are drawn early and would not consider CVS or do not have this option may choose to wait. Most patients in this series were redrawn sufficiently early to make amniocentesis a reasonable option with the average gestational age of second sample collection being less than 15 weeks in pregnancies that received results. For pregnancies that are later in gestation or pregnancies with other risk factors such as a positive serum screen, a better choice might be to redraw immediately. Interestingly, we observed a surprising number of patients in this series that were redrawn after 20 weeks of gestation or redrawn multiple times. We do not have access to the clinical history in these cases and there is a possibility that gestational age was misreported in some; however, we advocate for careful counseling in such circumstances.
The use of a large clinical laboratory database, such as the one queried here, permits the analysis of a large cohort, which increases the strength of the logistic regression model, but reduces flexibility in study design. The retrospective nature of this study limits the study population to patients that choose repeat testing. The proportion of patients choosing repeat testing will vary by region and prevailing clinical practice; however, some of the cases not redrawn may include pregnancy losses or aneuploid pregnancies that instead proceed to diagnostic testing due to other considerations such as abnormal ultrasound findings or other screening results. Since chromosome abnormalities, such as triploidy and trisomy 18, that have been reported in association with no results on cfDNA screening are also associated with early pregnancy loss and significant ultrasound findings [Citation12,Citation22], the findings of this study may be best applied after the demonstration of a normal fetal ultrasound. We also do not have information about fetal karyotype or pregnancy outcome in the pregnancies that were included in this analysis as this information is rarely provided to the laboratory and the study was not intended to investigate aneuploidy risk.
In conclusion, repeat cfDNA testing after an initial sample with insufficient fetal fraction is an option for clinical management and can be considered in the context of the individual pregnancy. The probability of receiving a result with repeat testing is influenced by maternal weight and interval between draws but will be more than 50% for most women. The decision of whether to redraw a sample would optimally take into account clinical factors such as ultrasound and other screening results, maternal factors, gestational age, and parental preferences for follow-up testing. The information that is presented in this study may assist in this counseling and timing of a redraw.
Disclosure statement
All authors are employees of Roche Sequencing Solutions Inc.
References
- Benn P, Borrell A, Chiu RW, et al. Position statement from the Chromosome Abnormality Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn. 2015;35:725–734.
- Gil MM, Accurti V, Santacruz B, et al. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2017;50:302–314.
- Kagan KO, Wright D, Etchegaray A, et al. Effect of deviation of nuchal translucency measurements on the performance of screening for trisomy 21. Ultrasound Obstet Gynecol. 2009;33:657–664.
- Malone FD, Canick JA, Ball RH, et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N Engl J Med. 2005;353:2001–2011.
- Canick JA, Palomaki GE, Kloza EM, et al. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat Diagn. 2013;33:667–674.
- Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589–1597.
- Thornburg LL, Mulconry M, Post A, et al. Fetal nuchal translucency thickness evaluation in the overweight and obese gravida. Ultrasound Obstet Gynecol. 2009;33:665–669.
- Wang E, Batey A, Struble C, et al. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013;33:662–666.
- Ashoor G, Poon L, Syngelaki A, et al. Fetal fraction in maternal plasma cell-free DNA at 11–13 weeks’ gestation: effect of maternal and fetal factors. Fetal Diagn Ther. 2012;31:237–243.
- Dar P, Curnow KJ, Gross SJ, et al. Clinical experience and follow-up with large scale single-nucleotide polymorphism-based noninvasive prenatal aneuploidy testing. Am J Obstet Gynecol. 2014;211:527.e1–527.e17.
- Kinnings SL, Geis JA, Almasri E, et al. Factors affecting levels of circulating cell-free fetal DNA in maternal plasma and their implications for noninvasive prenatal testing. Prenat Diagn. 2015;35:816–822.
- Revello R, Sarno L, Ispas A, et al. Screening for trisomies by cell-free DNA testing of maternal blood: consequences of a failed result. Ultrasound Obstet Gynecol. 2016;47:698–704.
- Benn P, Valenti E, Shah S, et al. Factors associated with informative redraw after an initial no result in noninvasive prenatal testing. Obstet Gynecol. 2018;132:428–435.
- Gregg AR, Skotko BG, Benkendorf JL, et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2016;18:1056–1065.
- Sarno L, Revello R, Hanson E, et al. Prospective first-trimester screening for trisomies by cell-free DNA testing of maternal blood in twin pregnancy. Ultrasound Obstet Gynecol. 2016;47:705–711.
- Lee TJ, Rolnik DL, Menezes MA, et al. Cell-free fetal DNA testing in singleton IVF conceptions. Hum Reprod. 2018;33:572–578.
- Sparks AB, Struble CA, Wang ET, et al. Noninvasive prenatal detection and selective analysis of cell-free DNA obtained from maternal blood: evaluation for trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206:319.e1–319.e9.
- Juneau K, Bogard PE, Huang S, et al. Microarray-based cell-free DNA analysis improves noninvasive prenatal testing. Fetal Diagn Ther. 2014;36:282–286.
- Schmid M, White K, Stokowski R, et al. Accuracy and reproducibility of fetal-fraction measurement using relative quantitation at polymorphic loci with microarray. Ultrasound Obstet Gynecol. 2018;51:813–817.
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. Available from: https://www.R-project.org.
- Livergood MC, Lechien KA, Trudell AS. Obesity and cell-free DNA “No Calls”: is there an optimal gestational age at time of sampling? Am J Obstet Gynecol. 2017;216:413.e1–413.e9.
- Langlois S, Johnson J, Audibert F, et al. Comparison of first-tier cell-free DNA screening for common aneuploidies with conventional publically funded screening. Prenat Diagn. 2017;37:1238–1244.
