Abstract
Background: Boys born small for gestational age (SGA) are at increased risk of testicular dysgenesis syndrome, and girls born SGA face the risk of polycystic ovary syndrome later in life. Our aim was to study whether neonates born SGA have an altered profile of steroid hormones at birth.
Materials and methods: A total of 168 singletons (99 boys, 69 girls) born at 32.0–36.9 gestational weeks were recruited to a population-based, university hospital, single-center study. Of these, 31 infants (17 boys, 14 girls) were born SGA. The concentrations of dehydroepiandrosterone sulfate (DHEAS), androstenedione, testosterone, dihydrotestosterone, estrone, estradiol, cortisone, and cortisol were analyzed in umbilical cord serum with mass spectrometry.
Results: Girls born SGA had higher levels of androstenedione than girls born appropriate for gestational age (AGA) (4.0 versus 2.6 nmol/L, p = .002). Boys born SGA had lower levels of estrone than boys born AGA (33 822 versus 62 471 pmol/L, p = .038). Infants born SGA had lower levels of cortisone than infants born AGA, both in girls (340 versus 579 nmol/L, p = .010) and in boys (308 versus 521 nmol/L, p = .045). Furthermore, boys born SGA had a higher cortisol/cortisone ratio than boys born AGA (0.41 versus 0.25, p = .028). Gestational age correlated with DHEAS (boys r = 0.48, p = .000, girls r = 0.35, p = .013), and cortisol (boys r = 0.48, p = .000, girls r = 0.29, p = .039).
Conclusions: In moderate-to-late preterm infants born SGA, we observed a different steroid hormone profile in cord serum. Girls born SGA show increased levels of androstenedione and boys born SGA show decreased levels of estrone in cord serum, which could be related to placental aromatase deficiency in intrauterine growth restriction.
Introduction
Prenatal growth is important for an individual’s future health. Low birth weight and/or short birth length can be caused by prematurity or intrauterine growth retardation. Individuals with low birth weight are at increased risk of developing hypertension, impaired glucose tolerance, and dyslipidemia as adults [Citation1]. In addition, boys with low birth weight have an increased risk of testicular dysgenesis syndrome, characterized by cryptorchidism, hypospadias, and testicular cancer in adult life [Citation2,Citation3]. Girls, on the other hand, tend to have an earlier onset of puberty than girls with normal birth weight [Citation4].
In a previous study, we found elevated levels of estradiol and dihydrotestosterone (DHT) in men born small for gestational age (SGA) [Citation5]. Whether newborn boys born SGA also have an altered steroid profile is not known.
The association between low birth weight and sex steroids has not been fully clarified. During pregnancy, the placenta produces large amounts of estriol. The main substrate for estriol is dehydroepiandrosterone sulfate (DHEAS), synthesized by the fetal adrenals. Regardless of gestational age, the fetal adrenal gland undergoes involution within the first week of life, with a decline in DHEAS secretion [Citation6].
The placental enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) protects the fetus from high maternal cortisol levels. Furthermore, in preterm infants, both 11β-HSD2 activity and cortisone concentration in umbilical venous blood correlate with birth weight standard deviation score (SDS) [Citation7]. Preterm infants exhibit a global deficit in mineralocorticoids, glucocorticoids, and adrenal androgens at birth [Citation8]. Term infants born after intrauterine growth retardation also have lower levels of both cortisone and cortisol in cord blood [Citation9].
In term neonates, there is evidence for a gender difference in androgens in cord blood, with significantly higher testosterone and DHT concentrations in boys [Citation10,Citation11] but similar levels of dehydroepiandrosterone (DHEA) and androstenedione [Citation11].
The combination of placental and fetal steroid synthesis and metabolism gives cord blood a unique steroid profile. Measuring steroid hormones in cord blood can therefore be challenging, and using adequate methods is of outmost importance [Citation12]. Previous studies using immunoassay have often shown much higher androgen levels than those using gas chromatography-tandem mass spectrometry (GC-MS/MS), or liquid chromatography-tandem mass spectrometry (LC-MS/MS) [Citation12,Citation13].
In the present study, we assayed androgens, estrogens, and glucocorticoids in cord serum in moderate-to-late preterm infants, using highly sensitive GC-MS/MS and LC-MS/MS. Our aims were to investigate whether infants born SGA have an altered steroid hormone profile at birth.
Materials and methods
Patients
The study population was recruited prospectively as a population-based cohort at the two delivery wards at Sahlgrenska University Hospital in Gothenburg. One hundred sixty-eight singletons (99 boys, 69 girls) born from September 2002 to June 2004 at gestational week 32.0–36.9 were included. In all infants, gestational age had been estimated by ultrasonography performed at gestational week 16–18. Infants with serious medical conditions, malformations, or chromosomal anomalies were not included. No boy had hypospadias, three boys (3%) had cryptorchidism on one side and two of these underwent orchiopexy. However, preterm boys are at increased risk of having cryptorchidism and the prevalence is in the same range as in a large cohort by Jensen et al. [Citation14]. Boys conceived by intracytoplasmic sperm injection (ICSI) have previously been shown to have decreased testosterone levels [Citation15]. Due to inconsistencies in antenatal charts, and thereby difficulties in distinguishing ICSI from standard in vitro fertilization, we excluded all infants conceived by assisted reproduction technologies. As infants born to mothers with diabetes mellitus are known to have higher insulin values in umbilical venous blood, and sex hormone-binding globulin correlates negatively to insulin, these infants were also excluded [Citation16]. Thirty-one (17 boys, 14 girls) out of the 168 infants were born SGA, defined as birth weight or birth length below −2 standard deviation scores (SDS), according to the Swedish reference for newborns [Citation17]. Of these 31 infants, 16 (eight boys, eight girls) were SGA by birth weight and birth length, six (three boys, three girls) were SGA by birth weight only, and nine (six boys, three girls) were SGA by birth length only. Twenty-seven (14 boys, 13 girls) out of 168 infants were born to mothers whose pregnancy was complicated by preeclampsia. Of these, 14 infants (seven boys, seven girls) were born SGA (by birth weight or birth length).
Auxology
Birth weight and birth length were measured with the infant in a supine position, using digital infant scales and measuring tape.
Blood sampling
Umbilical venous blood was collected directly after birth and immediately chilled to 4 °C. The blood was centrifuged within 24 h, and thereafter, serum was frozen and stored at −80 °C until hormone determination. In previous studies, we have shown that sex steroids were not affected by long-term storage up to a decade or repeated thaw/freeze cycles [Citation18,Citation19].
Mothers
Maternal data were collected from antenatal charts at maternity centers. The median maternal age at delivery was 30.2 years. Mothers of girls born SGA were older than mothers of girls born appropriate for gestational age (AGA) (median 32.1 versus 29.3 years, p = .009), but no other differences in maternal age were found between groups. The following pregnancy complications were recorded if present: hypertension (blood pressure > 140/90 mm Hg), preeclampsia (blood pressure > 140/90 mm Hg and proteinuria after 20 weeks’ gestation), and HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count). Twenty-seven mothers had developed preeclampsia; among them, four had HELLP syndrome.
Antenatal betamethasone had been administered to mothers of 36 infants (20 boys, 16 girls) and nine of these (four boys, five girls) were born SGA (by birth weight or birth length). Since betamethasone had been given to all women at risk of delivering before gestational age 34 weeks, and at different time intervals before the actual delivery, we were unable to calculate whether prenatally administered betamethasone had an effect on levels of androgens, estrogens, or glucocorticoids in umbilical cord serum.
Hormone assays
Serum concentrations of testosterone, DHT, estrone, and estradiol were simultaneously determined by GC-MS/MS [Citation20]. Limit of detection (LOD) was 0.1 nmol/L for testosterone, 27 pmol/L for DHT, 9 pmol/L for estrone, and 2 pmol/L for estradiol. Total coefficient of variation (CV) for testosterone was 16% at 0.3 nmol/L and <10% for >1.5 nmol/L; for DHT, CV was 15% at 55 pmol/L and 10% at 200 pmol/L; for estrone, it was 11% at ≥100 pmol/L; and for estradiol, 6% at ≥ 36 pmol/L. Fourteen boys (one SGA, 13 AGA) and 61 girls (12 SGA, 49 AGA) had DHT levels below LOD.
Serum concentrations of DHEAS, androstenedione, cortisone, and cortisol were simultaneously determined by LC-MS/MS (Ankarberg-Lindgren, manuscript in preparation). LOD was 0.1 µmol/L for DHEAS, 0.1 nmol/L for androstenedione, 1.8 nmol/L for cortisone, and 11 nmol/L for cortisol. Total CV for DHEAS was < 7% at ≥1.0 µmol/L, for androstenedione it was 13% at ≥2.0 nmol/L, for cortisone 15% at 60 nmol/L, and for cortisol 7% at 240 nmol/L and 19% at 680 nmol/L. Four AGA boys and two AGA girls had cortisol levels below LOD.
Statistical analyses
The data are presented as median and range. Hormone determinations below LOD were set to LOD/2. All correlation analyses were performed using Spearman’s nonparametric rank correlation. The Mann–Whitney U-test was used for comparisons between groups.
For statistical analyses, we used the software IBM SPSS Statistics for Windows, version 22.0 (IBM Corp, Armonk, NY, USA).
Ethical considerations
The study was approved by the Ethics Committee of the Medical Faculty of the University of Gothenburg (approval number Ö-562–01). Informed consent was obtained from the parents of the participants.
Results
Auxological and obstetric data
Girls born SGA had lower gestational age than girls born AGA. Infants of both genders born SGA were more often delivered by cesarean section than infants born AGA. For detailed auxological data, see .
Table 1. Auxological and endocrinological data at birth, comparing boys and girls born small for gestational age (birth weight or birth length) with those born appropriate for gestational age.
Androgens and estrogens in cord serum
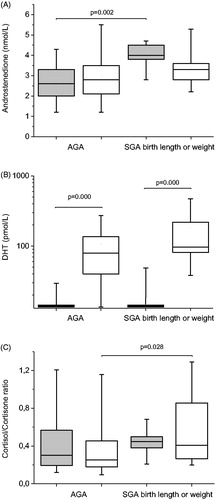
shows sex steroid levels in boys and girls born SGA and AGA. Girls born SGA showed significantly elevated levels of androstenedione compared to girls born AGA ( and ). On the other hand, boys born SGA showed significantly decreased levels of estrone compared to boys born AGA (). Other differences between the SGA and AGA groups were small but gender differences were evident. Boys and girls had similar levels of DHEAS, androstenedione, estrone, and estradiol, whereas boys had significantly higher testosterone and DHT levels compared to girls, in both the SGA and AGA groups (). In the case of DHT, the difference between boys and girls was striking in both groups ().
Figure 1. Hormone concentrations for androstenedione (panel A), dihydrotestosterone (DHT) (panel B), and cortisol/cortisone ratio (panel C), in umbilical cord serum in moderate-to-late preterm infants. Boys and girls born small for gestational age (SGA), as measured by birth length and/or birth weight, are compared with those born appropriate for gestational age (AGA). Box plots show 5th, 25th, 50th, 75th, and 95th percentiles. Gray boxes represent girls, and white boxes represent boys.
In boys, gestational age correlated with DHEAS (r = 0.48, p = .000), androstenedione (r = 0.39, p = .000), estrone (r = 0.24, p = .017), and estradiol (r = 0.28, p = .006). Birth weight correlated with DHEAS (r = 0.43, p = .000), estrone (r = 0.31, p = .002), and estradiol (r = 0.22, p = .030), and birth weight SDS showed an inverse correlation with DHT (r = −0.23, p = .022). Birth length correlated with DHEAS (r = 0.36, p = .001) and estrone (r = 0.29, p = .004), while birth length SDS showed an inverse correlation with testosterone (r = −0.24, p = .018) and DHT (r = −0.28, p = .006).
In girls, gestational age correlated with DHEAS (r = 0.35, p = .013). Androstenedione showed an inverse correlation with birth weight (r = −0.29, p = .040), birth weight SDS (r = −0.33, p = .020), birth length (r = −0.32, p = .022), and birth length SDS (r = −0.39, p = .005).
Glucocorticoids in cord serum
No gender differences were seen in cortisone levels (). However, infants born SGA had lower levels of cortisone than infants born AGA (). Furthermore, boys born SGA had a higher cortisol/cortisone ratio than boys born AGA ().
In boys, gestational age correlated with cortisone (r = 0.28, p = .009), cortisol (r = 0.48, p = .000), and cortisol/cortisone ratio (r = 0.24, p = .023). Birth weight correlated with both glucocorticoids (cortisone r = 0.28, p = .008, cortisol r = 0.37, p = .000), as did birth length (cortisone r = 0.28, p = .008, cortisol r = 0.38, p = .000).
In girls, gestational age correlated with cortisol (r = 0.29, p = .039). Birth weight correlated with cortisone (r = 0.29, p = .038).
Discussion
To our knowledge, the present study is the first to show elevated concentrations of androstenedione in cord serum in girls born SGA and decreased levels of estrone in boys born SGA among moderate-to-late preterm infants. Furthermore, boys born SGA had a higher cortisol/cortisone ratio compared to boys born AGA.
Infants of both genders had similar levels of DHEAS. As in a previous study, DHEAS correlated with gestational age [Citation21] and, in boys only, also with size at birth.
During pregnancy, androstenedione is mainly produced by the conversion of dehydroepiandrosterone (DHEA), and via aromatase, further converted to estrogens in the placenta. In this study, SGA girls had higher levels of androstenedione than AGA girls. Placental aromatase is deficient in preeclampsia and placental ischemia [Citation22]. Seven of 14 (50%) of the girls born SGA in our study were born after preeclamptic pregnancies, compared to only six of 55 (11%) of the girls born AGA. We therefore speculate that a relative lack of aromatase due to placental deficiency may explain the high androstenedione seen in the cord serum of girls born SGA. In boys, androstenedione correlated with gestational age. Previous studies show conflicting results, with one finding a correlation [Citation8], and the other no correlation [Citation11] for infants of both genders.
There are few studies on neonatal testosterone concentrations in moderate-to-late preterm infants determined by mass spectrometry methods. In this study, we found significantly higher cord serum levels of testosterone in boys than girls, both in the SGA and in the AGA group. This finding, as well as the overall testosterone levels found in this study, is supported by previous studies in term infants [Citation11,Citation23].
DHT is important for the sexual differentiation of the male genitalia during embryogenesis. Its role in female fetuses is less clear. Boys in our study had higher DHT concentrations than girls, as expected from a previous study [Citation11]. Cord serum concentrations of DHT were lower than previously reported for prepubertal girls, but in line with previous results for boys [Citation20]. Despite using a highly sensitive GC-MS/MS for determining DHT, we still found that 14 of the 99 boys (14.1%) and 61 of the 69 girls (88.4%) had DHT concentrations under LOD. The SGA boys in our study had a tendency toward higher levels of DHT than the AGA boys, consistent with the weak inverse correlation between DHT, birth weight SDS, and birth length SDS. In a previous study, our group showed elevated levels of DHT in young adult men born SGA [Citation5], a disturbance that may have already started in utero.
During pregnancy, estrogens are synthesized mainly by the placenta using androgen precursors [Citation24]. We found no gender difference in cord serum levels of estrone or estradiol, which is consistent with a previous study [Citation25]. Boys born SGA had lower estrone levels compared to boys born AGA. With the help of aromatase, androstenedione acts as a precursor for the synthesis of estrone. In preeclampsia, placental aromatase can be attenuated [Citation22]. Boys born SGA were to a higher extent born after preeclamptic pregnancies (seven of 17, 41%) than boys born AGA (seven of 82, 9%), so we can speculate that low estrone levels in boys born SGA may be caused by a lack of aromatase. In boys but not girls, estrone and estradiol correlated with gestational age, whereas a previous study found this correlation in both genders [Citation25].
Cortisone and cortisol have previously been shown to increase with gestational age [Citation8], which was confirmed in our study for boys only; for girls, only cortisol increased. Infants born SGA had lower cortisone but similar cortisol levels compared to AGA infants. Our findings are partly in line with a previous study showing lower levels of both cortisone and cortisol in both umbilical artery and venous blood in infants born after intrauterine growth retardation, compared to controls [Citation9]. Placental 11β-HSD 2 converts maternal cortisol to biologically inactive cortisone and serves as a functional barrier, protecting the fetus from cortisol exposure. The cortisol/cortisone ratio may serve as an indirect marker of 11β-HSD 2 enzyme activity. Previous studies have shown an attenuated placental 11β-HSD 2 activity after intrauterine growth retardation [Citation9] and a positive relation between birth weight SDS and placental 11β-HSD 2 activity [Citation7]. A lower 11β-HSD 2 activity supports our finding of a higher cortisol/cortisone ratio in boys born SGA.
The strengths of our study are the population-based design, the dating of all pregnancies using ultrasound, and the use of reliable mass spectrometry methods for steroid determinations. We consistently used only umbilical venous blood for hormone determinations, which is important, since levels of unconjugated steroids differ between umbilical arterial and venous blood [Citation26].
Weaknesses of our study are first of all that fewer girls than boys were included. We also lacked term infants for comparison. We did not adjust our data for maternal habits, such as smoking, which could potentially affect steroidogenesis in the placenta and fetus [Citation27].
In conclusion, our study is the first to report increased levels of androstenedione in cord serum in girls born SGA and decreased levels of estrone in boys born SGA among moderate-to-late preterm infants. This may be caused by attenuated aromatase activity in the placenta following intrauterine growth restriction. We further showed an increased cortisol/cortisone ratio in cord serum in boys born SGA. The mechanisms behind these observations remain to be further evaluated, but our findings could be the first signs of a lifelong disturbance of the steroid biosynthesis in infants born SGA, leading to future health issues in both genders.
Acknowledgements
We would like to thank all the children and their families who participated in the study. We are grateful to registered nurses Kerstin Bredin, Annette Ödman, and Marie Sellhed for data collection and Patient Care. We would finally like to thank Mats X. Andersson, Monika Eriksson, and Tove Hellqvist for hormone determinations.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Barker DJ, Hales CN, Fall CH, et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67.
- Main KM, Jensen RB, Asklund C, et al. Low birth weight and male reproductive function. Horm Res Paediatr. 2006;65:116–122.
- Gatti JM, Kirsch AJ, Troyer WA, et al. Increased incidence of hypospadias in small-for-gestational age infants in a neonatal intensive care unit. BJU Int. 2001;87:548–550.
- Deng X, Li W, Luo Y, et al. Association between small fetuses and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Health. 2017;14:1377.
- Allvin K, Ankarberg-Lindgren C, Fors H, et al. Elevated serum levels of estradiol, dihydrotestosterone, and inhibin B in adult males born small for gestational age. J Clin Endocrinol Metab. 2008;93:1464–1469.
- Ben-David S, Zuckerman-Levin N, Epelman M, et al. Parturition itself is the basis for fetal adrenal involution. J Clin Endocrinol Metab. 2007;92:93–97.
- Kajantie E, Dunkel L, Turpeinen U, et al. Placental 11 beta-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J Clin Endocrinol Metab. 2003;88:493–500.
- Travers S, Martinerie L, Boileau P, et al. Alterations of adrenal steroidomic profiles in preterm infants at birth. Arch Dis Child Fetal Neonatal Ed. 2018;103:F143–F151.
- Dy J, Guan H, Sampath-Kumar R, et al. Placental 11beta-hydroxysteroid dehydrogenase type 2 is reduced in pregnancies complicated with idiopathic intrauterine growth Restriction: evidence that this is associated with an attenuated ratio of cortisone to cortisol in the umbilical artery. Placenta. 2008;29:193–200.
- Morisset AS, Dubé MC, Drolet R, et al. Androgens in the maternal and fetal circulation: association with insulin resistance. J Matern Fetal Neonatal Med. 2013;26:513–519.
- Lundell AC, Ryberg H, Vandenput L, et al. Umbilical cord blood androgen levels in girls and boys assessed by gas chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol. 2017;171:195–200.
- Hollier LP, Keelan JA, Hickey M, et al. Measurement of androgen and estrogen concentrations in cord blood: accuracy, biological interpretation, and applications to understanding human behavioral development. Front Endocrinol (Lausanne). 2014;5:64.
- Herruzo AJ, Mozas J, Alarcón JL, et al. Sex differences in serum hormone levels in umbilical vein blood. Int J Gynecol Obstet. 1993;41:37–41.
- Jensen MS, Wilcox AJ, Olsen J, et al. Cryptorchidism and hypospadias in a cohort of 934,538 Danish boys: the role of birth weight, gestational age, body dimensions, and fetal growth. Am J Epidemiol. 2012;175:917–925.
- Mau Kai C, Main KM, Andersen AN, et al. Reduced serum testosterone levels in infant boys conceived by intracytoplasmic sperm injection. J Clin Endocrinol Metab. 2007;92:2598–2603.
- Simmons D. Interrelation between umbilical cord serum sex hormones, sex hormone-binding globulin, insulin-like growth factor I, and insulin in neonates from normal pregnancies and pregnancies complicated by diabetes. J Clin Endocrinol Metab. 1995;80:2217–2221.
- Niklasson A, Albertsson-Wikland K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 2008;8:8.
- Ankarberg-Lindgren C, Norjavaara E. Changes of diurnal rhythm and levels of total and free testosterone secretion from pre to late puberty in boys: testis size of 3 ml is a transition stage to puberty. Eur J Endocrinol. 2004;151:747–757.
- Ankarberg-Lindgren C, Norjavaara E. Twenty-four hours secretion pattern of serum estradiol in healthy prepubertal and pubertal boys as determined by a validated ultra-sensitive extraction RIA. BMC Endocr Disord. 2008;8:10.
- Ankarberg-Lindgren C, Dahlgren J, Andersson MX. High-sensitivity quantification of serum androstenedione, testosterone, dihydrotestosterone, estrone and estradiol by gas chromatography-tandem mass spectrometry with sex- and puberty-specific reference intervals. J Steroid Biochem Mol Biol. 2018;183:116–124.
- Kari MA, Raivio KO, Stenman UH, et al. Serum cortisol, dehydroepiandrosterone sulfate, and steroid-binding globulins in preterm neonates: effect of gestational age and dexamethasone therapy. Pediatr Res. 1996;40:319–324.
- Perez-Sepulveda A, Monteiro LJ, Dobierzewska A, et al. Placental aromatase is deficient in placental ischemia and preeclampsia. PLoS One. 2015;10:e0139682.
- Keelan JA, Mattes E, Tan H, et al. Androgen concentrations in umbilical cord blood and their association with maternal, fetal and obstetric factors. PLoS One. 2012;7:e42827.
- Pasqualini JR. Enzymes involved in the formation and transformation of steroid hormones in the fetal and placental compartments. J Steroid Biochem Mol Biol. 2005;97:401–415.
- Hickey M, Hart R, Keelan JA. The relationship between umbilical cord estrogens and perinatal characteristics. Cancer Epidemiol Biomarkers Prev. 2014;23:946–952.
- Pašková A, Pařízek A, Hill M, et al. Steroid metabolome in the umbilical cord: is it necessary to differentiate between arterial and venous blood?. Physiol Res. 2014;63:115–126.
- Adamcová K, Kolátorová L, Chlupáčová T, et al. Changes to fetal steroidogenesis caused by maternal smoking. Physiol Res. 2017;66:S375–S386.
