Abstract
Aim
Patent ductus arteriosus (PDA) is treated with ibuprofen and it is known that the clearance of ibuprofen increases with postnatal age. We aimed to study whether postnatal age-adjusted ibuprofen dosages improve the effectiveness of treatment compared to standard ibuprofen dosages after the first days of life.
Methods
A historical cohort of 207 preterm neonates treated with standard ibuprofen dosages (Group A; 2011–2015) was compared to a prospective cohort of 66 preterm neonates treated with postnatal age-adjusted ibuprofen dosages (Group B; 2015–2016).
Results
Both groups had comparable background characteristics. Treatment was started after median 6 (25–75th percentile: 4–11) and 5 (25–75th percentile: 4–11) days and effectiveness was 33.2 and 44.7% (p = .17) in groups A and B, respectively. No hemodynamically significant PDA was found in 23/49 (46.9%) of the patients born before 28 weeks after adjusted ibuprofen dosages compared to 48/162 (29.6%) after standard ibuprofen dosages (p = .04). There were significantly more reversible side effects with the postnatal age-adjusted ibuprofen dosages (p = .04).
Conclusions
There seems to be a trend to higher effectiveness with the adjusted ibuprofen dosages in preterm neonates before 28 weeks, but it is associated with more reversible side effects.
Introduction
While the ductus arteriosus normally closes spontaneously after term birth, it often remains open in preterm neonates. This condition, known as patent ductus arteriosus (PDA), may result in hemodynamic changes related to a left-to-right shunt, such as pulmonary fluid overload and decreased systemic circulation [Citation1]. This has been associated with prolonged ventilation, chronic lung disease, and necrotizing enterocolitis (NEC). PDA can be treated pharmacologically or by surgical ligation. The best strategy to manage PDA pharmacologically is still being debated internationally [Citation2].
Historically, the NSAID indomethacin was the pharmacological treatment of choice [Citation3]. In 1996, however, Varvarigou et al. showed that ibuprofen was effective to close the ductus arteriosus [Citation4], especially when given during the first three postnatal days. Since then, the effectiveness of ibuprofen for PDA in preterm neonates has been further studied [Citation5,Citation6] and the drug has been registered for intravenous application at a dose of 10, 5, and 5 mg/kg body weight once daily for three consecutive days. Meanwhile, these dosages have been widely implemented as standard of care in the neonatal intensive care unit (NICU). Many trials compared ibuprofen with indomethacin [Citation6,Citation7]. Ibuprofen seems to carry fewer side effects than does indomethacin and is, therefore, often considered to be the first drug in PDA treatment [Citation6].
The optimal timing and dosage of ibuprofen remain unclear. Relatively late treatment with ibuprofen might be effective [Citation8,Citation9]. Awaiting spontaneous closure during the first days of life could prevent overtreatment and potential side effects. In 2008, Hirt et al. showed that ibuprofen clearance importantly increases with postnatal age (PNA) and proposed to increase ibuprofen dosage accordingly after the first days of life [Citation10]. Effectiveness of the increased dosages still needs evaluation in clinical practice.
In this cohort study, we studied the use of ibuprofen dosages that were adjusted to postnatal age. The effectiveness and side effects were compared to the registered ibuprofen dosages.
Materials and methods
All preterm neonates admitted to the level III NICU of the Erasmus UMC-Sophia, Rotterdam, the Netherlands, from September 2011 to November 2016 and who received ibuprofen for PDA treatment were eligible in this before–after cohort study. Neonates with a gestational age above 30 weeks were excluded. Neonates who received paracetamol as a first PDA treatment or ibuprofen dosages that differed from the protocol were excluded. In June 2015, postnatal age-adjusted ibuprofen dosing was introduced as standard of care. Prospectively collected data before June 2015 were analyzed retrospectively. From June 2015 to November 2016 data were prospectively collected.
The primary outcome was the rate of no hemodynamically significant ductus arteriosus (no-hsPDA) after ibuprofen therapy. No-hsPDA is described as a ductus arteriosus smaller than 1.5 mm and left atrium (LA)/aorta (Ao) ratio < 1.4. Secondary outcomes included subgroup analysis of neonates under 26 and 28 weeks of gestation, reported side effects (oliguria, intraventricular hemorrhage (IVH), gastrointestinal bleeding or perforation, and NEC).
Persistent ductus arteriosus
According to our treatment policy, neonates with a gestational age under 26 weeks were routinely screened for hsPDA at the fourth postnatal day. Neonates born between 26 and 28 weeks, echocardiography was indicated at the fourth postnatal day (from June 2015) or within the first week of life (before June 2015). Neonates born after 28 weeks echocardiography was performed based on clinical signs related to hsPDA. If ibuprofen was contraindicated no echocardiography was made because of absent clinical consequences. After three ibuprofen doses, a control echocardiography was performed within 24 h. Echocardiographic examinations were performed by experienced cardiac sonographers or pediatric cardiologists using a Vivid-S6 (GE Health Care, Chicago, IL) two-dimensional color Doppler echocardiographic system equipped with a 10 MHz transducer. For this study, all echocardiographic images were reevaluated afterward by one and the same trained physician (JdeK), who was blinded for patients characteristics and ibuprofen data at the time of evaluation.
Treatment policy was to start ibuprofen in patients with a hsPDA (ductal diameter >2.0 mm, PDA/LPA diameter >0.8, and/or LA/Ao ratio >1.6).
Relative contraindications for start or continuation of ibuprofen treatment were active (intracerebral) hemorrhage, thrombocytopenia ( < 100 × 109/l) or other known clotting disorders, sepsis (positive blood culture), suspected or confirmed NEC, intestinal perforation, liver or kidney function disorders (oliguria < 1.0 ml/kg/h or serum creatinine >110 µmol/l), or severe hyperbilirubinemia. In case of temporary contraindications, ibuprofen could be started or continued later, albeit at the discretion of the attending physician.
Registered dosing protocol (group A)
From September 2011 to June 2015, the registered intravenous ibuprofen dosing protocol was used (10–5–5 mg/kg in three consecutive days). If hsPDA persisted, a second similar course of ibuprofen was administered. If then ductal patency persisted, a third ibuprofen course or a course of paracetamol could be considered [Citation11].
Postnatal age-adjusted dosing (group B)
From June 2015 to November 2016, the dose of ibuprofen per kg bodyweight as suggested by Hirt et al. [Citation10] was used as starting dose. Maintenance doses were adapted by the suggested ongoing increase of ibuprofen clearance during treatment. Hirt et al. used different loading and maintenance dosages according to the postnatal age at start of treatment, being 10–5–5, 14–7–7, and 18–9–9 mg/kg for neonates younger than 70 h, between 70 and 108 h and neonates between 108 and 180 h, respectively [Citation10]. If treatment was started after 192 h, the dosages were increased to 20–10–10 mg/kg. As Hirt et al. did not increase the ibuprofen maintenance dose during treatment, our maintenance doses were increased according to the postnatal age during treatment. This was done because of the expected ongoing increase of ibuprofen clearance during treatment. This would lead to for instance a loading dosage of 14 mg/kg and a maintenance dosage of 9–9 mg/kg. Ibuprofen could be continued for maximally nine consecutive days. If enteral feeding was well tolerated gastroenteral administration of ibuprofen was considered at a dosage similar to the intravenous dosage.
Data analyses
SPSS version 21 (IBM SPSS, Chicago, IL) was used to analyze the data. We used an intention to treat analysis and per-protocol analyses to compare the old versus new protocol and to compare the traditional dosing with the postnatal age-adjusted dosing respectively. Continuous data are presented as median (percentiles) with ranges. Differences between the groups were determined by t-test and Mann–Whitney U test for parametric and nonparametric continuous data, and a chi-square or Fisher’s exact test for categorical data. The postnatal age-adjusted dosages were considered to be as effective as the registered standard regimen if the closure rate is at least the same or higher with a p value of < 0.05.
Sample size
A power analysis for the second part of the study was performed. We considered that an absolute increase in becoming a no-hsPDA rate of at least 20% (increase in rate from 25% to at least 45%) would be of clinical significance. We calculated that a sample size of 183 patients in group A and 61 patients in group B would be necessary to determine a statistically increase of 20% in closure rate in group B at a two-sided alpha level of 5% and with a power of 80%.
Ethical approval
Ibuprofen treatment was considered part of our routine treatment protocol for hsPDA treatment. This observational study was submitted to the institutional medical ethical review board, which waived the need for approval according to the Dutch law (METRIC-2016-126) because ibuprofen dosages and echocardiography were implemented as standard of care. No additional echocardiography, next to those performed for routine clinical practice was permitted.
Results
A total of 314 neonates were eligible for inclusion. Eventually, 273 were included for data analysis in the two groups: group A (N = 207) treated before and group B (N = 66) treated after protocol change (see flowchart, ). The background characteristics between the two cohorts of patients were comparable (). Ibuprofen was started at a median postnatal age of 6 (25th–75th percentile 4–11) days and 5 (25th–75th percentile 4–11) days (p = 0.99), respectively, in group A and group B. In group A, 195 patients received the registered ibuprofen dosage and twelve were treated with the postnatal age-adjusted dosage. In group B six patients received the registered ibuprofen dosage and 60 the new postnatal age-adjusted dosage.
Figure 1. Flowchart. Intention to treat analysis compared group A with group B. Per protocol analysis was × compared with **.
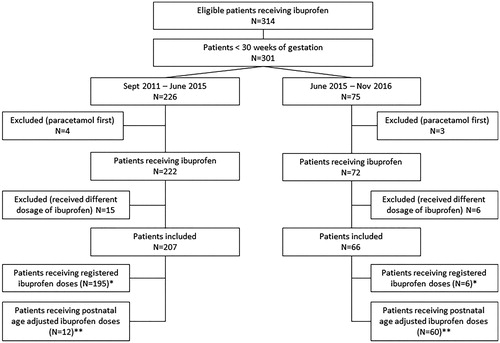
Table 1. Background characteristics of the included patients for group A and B.
Primary outcome
No-hsPDA after treatment was found in 61/184 (33.2%) of patients in group A and in 21/47 (44.7%) in group B (intention-to-treat analysis p = .17; see ). Echocardiography after treatment however, had not been performed and patients were lost to follow-up in 23 (11.1%) and 19 (28.8%) patients in group A and B, respectively (p < .01). No echocardiography was performed because patients deceased (N = 13), had contra-indications for further treatment (N = 20) or echocardiography was not relevant according to clinician (N = 9).
Figure 2. Results of intention to treat analysis after treatment in both groups (left panel) and of per protocol analysis after treatment with standard dosages and postnatal age-adjusted dosages (right panel).
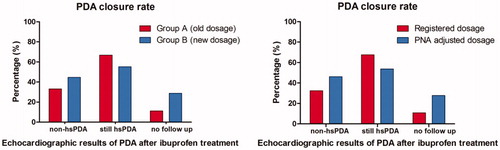
Comparison of different dosage regimen showed that 58/179 (32.4%) of all the patients treated with standard ibuprofen dosage had no-hsPDA compared with 24/52 (46.2%) of the patients treated with postnatal age-adjusted dosages (per-protocol analyses p = .07; see ).
Secondary outcome
Because closure of the ductus arteriosus becomes less problematic with increasing gestational age subanalyses were performed a subgroup analysis in neonates born below 26 and 28 weeks of gestation. The PDA became no-hsPDA in 23/49 (46.9%) of the patients born before 28 weeks receiving the adjusted ibuprofen dosages compared to 48/162 (29.6%) if registered ibuprofen dosages were given (p = .04). For newborns born before 26 weeks of gestation, the ductus became no-hsPDA in 11/25 (44%) versus 25/93 (26.9%) of patients receiving the postnatally adjusted ibuprofen dosage and the registered dosages, respectively (p = .14).
In 68 of the 207 patients in group A (32.9%) side effects were detected. These side effects were mainly gastrointestinal dysfunction (20%), NEC (7.8%), onset or worsening of IVH (4.4%), gastrointestinal perforation (2%), and oliguria (1%). Side effects (N = 27) occurred in 22 of the 66 patients in group B (33.3%), including onset or worsening of IVH (32%), gastrointestinal dysfunction (27%), NEC (18%), oliguria (14%), and gastrointestinal bleeding or perforation (9%). Only oliguria (p = .04) and gastrointestinal dysfunction (p = .04) were significantly higher in the patients who were treated with postnatally adjusted ibuprofen dosages.
Discussion
To the best of our knowledge, this is the first prospective evaluation ibuprofen treatment with dosages adjusted to postnatal maturation of ibuprofen clearance in comparison to the registered ibuprofen dosages. We found that if ibuprofen treatment was started at a median postnatal age of 6 d less than 40% of the PDA became insignificant, even with adjusted dosages for postnatal age.
Currently there is no consensus on the best PDA treatment strategy. Surgical ligation seems less preferable and has been related to impaired neurological outcome [Citation12,Citation13]. PDA in preterm neonates might also reflect a physiological cardiopulmonary state that does not at all benefit from pharmacological or surgical treatment [Citation14]. A conservative strategy might therefore still be considered [Citation15]. Results from randomized controlled trials that compare ibuprofen treatment with no treatment at all are awaited [Citation16].
Regarding pharmacological treatment with NSAIDs the optimal timing (early versus late) and patient selection (based on echocardiographic parameters, on clinical symptoms or patient characteristics) are still being debated [Citation17–19].
Almost all trials on ibuprofen therapy for PDA in preterm neonates during the last two decades addressed the use of prophylactic [Citation5,Citation20] or early ibuprofen therapy for PDA [Citation6]. In those studies, treatment was started almost always within 72 h after birth, often even within 24 h. As the ductus arteriosus often might close spontaneously during the first days of life [Citation21], a postponed treatment strategy would justify therapy for only those neonates in whom spontaneous closure in the first days fails. Our findings suggest, however, that ibuprofen therapy is losing its effectiveness to close the ductus if started too late, even with higher dosing according to maturation of clearance. As ibuprofen clearance increases significantly with postnatal age [Citation10], higher dosages would be needed to reach similar ibuprofen plasma levels in older neonates. The dosages suggested by the pharmacokinetic model of Hirt et al. have yet to be evaluated and validated. Increased efficacy could be reached with a double dose of ibuprofen [Citation22,Citation23]. The ductus arteriosus closed in 63% and 68% of neonates treated with registered ibuprofen dosages compared to 86% and 92% of neonates treated with double ibuprofen dosages. The higher efficacy of ibuprofen in this trial suggests a dose-effect relationship for ibuprofen in PDA treatment. The timing of start of treatment might have played an important role as well. In the above-mentioned trial, ibuprofen was started after mean postnatal ages of 1 and 3 d, respectively, compared to median 6 d in our cohort [Citation22,Citation23].
As this study suggests, ibuprofen treatment at relatively late postnatal ages should be reconsidered even at the postnatal age-adjusted dosage. There is a trend that treatment with postnatal age-adjusted ibuprofen dosage leads to more no-hsPDA compared with the registered ibuprofen dosages. An important impact of the postnatally adjusted ibuprofen dosages is the risk of side effects. We did not find a higher rate of severe side effects like NEC, gastrointestinal perforation or worsening of IVH which might have major impact. Most of the other reported side effects seem are self-limiting such as the significant increase in gastrointestinal dysfunction and oliguria when treated with higher ibuprofen dosages. The long-term safety, including for instance nephrotoxic effects of these higher ibuprofen dosages are yet unknown [Citation24].
There is limited consensus about the echocardiographic definition of a hsPDA [Citation25]. The hemodynamic significance is probably multifactorial. Apart from the ductus diameter and flow through the ductus it also depends on cardiac, pulmonary, and systemic circulatory effects of the ductus arteriosus that may also differ with the infant’s age, weight, and comorbidity [Citation26]. Although surgical ligation itself is a hard outcome measure, the indication for surgical ligation after pharmacological treatment failed is debatable; moreover, there are contradictory reports about the effects of ligation on the long term outcome [Citation12,Citation27].
Our study is strengthened by the fact that all echocardiography data were reanalyzed by one person, thereby limiting the risk of interrater variability on the outcome. The echocardiographic parameters to define significance of the PDA have limitations. No details on the systemic hypoperfusion, such as flow in the arteria mesenterica superior or descending aortae were used [Citation28]. Van Laere et al. also suggest that more echocardiographic parameters should be used for interpreting the hemodynamic significance of a PDA [Citation29]. Unfortunately, not all patients had been evaluated by echocardiography after the end of treatment and loss to follow-up was quite high. As a consequence, the exact effect of ibuprofen therapy could not be evaluated in all patients. Suggesting all ductus became insignificant in the non-evaluated post treatment patients showed that the increased ibuprofen dosages might be more slightly effective, but are related to higher levels of side effects (especially oliguria and gastrointestinal dysfunction).
Although a randomized placebo-controlled trial might have been more suitable, we believe that our cohort study clearly illustrates the limited effect of ibuprofen after the first days of life, even at postnatal age-adjusted dosages. It is unclear why ibuprofen was so less effective in these premature neonates. An important reason for this could be selection bias, because many patients had spontaneous closure of the duct in the first days of live and those patients with a good clinical condition might often not be (re)-evaluated. A case can be made for NSAIDs being more effective in the first days of life by reinforcing the physiological processes at work to close the duct.
In conclusion, we show low effectiveness of ibuprofen to close the ductus arteriosus in preterm neonates if used at older postnatal ages, even at higher dosages. There seems a slight trend toward more effect if increased ibuprofen dosages are used. The optimal window of opportunity for pharmacological treatment of PDA in preterm neonates seems to be in the first days of life.
Disclosure statement
No potential conflict of interest was reported by the authors. N. van Paassen contributed to study design and data collection. I van Beynum was responsible for performing the echocardiography and editing the manuscript. R. Flint edited the manuscript. I. Reiss supervised the design and edited the manuscript. S. Simons supervised the design and execution of the study, performed the final data analyses and contributed to the writing of the manuscript. JCA de Klerk had primary responsibility for the study design, data collection, data analysis, outcome assessment, preliminary data analysis and writing the manuscript.
References
- Clyman RI. The role of patent ductus arteriosus and its treatments in the development of bronchopulmonary dysplasia. Semin Perinatol. 2013;37(2):102–107.
- Laughon M, Bose C, Clark R. Treatment strategies to prevent or close a patent ductus arteriosus in preterm infants and outcomes. J Perinatol. 2007;27(3):164–170.
- Merritt TA, DiSessa TG, Feldman BH, et al. Closure of the patent ductus arteriosus with ligation and indomethacin: a consecutive experience. J Pediatr. 1978;93(4):639–646.
- Varvarigou A, Bardin CL, Beharry K, et al. Early ibuprofen administration to prevent patent ductus arteriosus in premature newborn infants. JAMA. 1996;275(7):539–544.
- Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2011;(7):CD004213.
- Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2013;(4):CD003481.
- Neumann R, Schulzke SM, Bührer C. Oral ibuprofen versus intravenous ibuprofen or intravenous indomethacin for the treatment of patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. Neonatology. 2012;102(1):9–15.
- Lainwala S, Hussain N. Treatment of patent ductus arteriosus with cyclo-oxygenase inhibitors beyond 2 weeks of age in very low birth weight infants. Am J Perinatol. 2016;33(6):584–589.
- Sosenko IR, Fajardo MF, Claure N, et al. Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. J Pediatr. 2012;160(6):929–35.e1.
- Hirt D, Van Overmeire B, Treluyer JM, et al. An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamic study. Br J Clin Pharmacol. 2008;65(5):629–636.
- Roofthooft DW, van Beynum IM, de Klerk JC, et al. Limited effects of intravenous paracetamol on patent ductus arteriosus in very low birth weight infants with contraindications for ibuprofen or after ibuprofen failure. Eur J Pediatr. 2015;174(11):1433–1440.
- Weisz DE, Mirea L, Rosenberg E, et al. Association of patent ductus arteriosus ligation with death or neurodevelopmental impairment among extremely preterm infants. JAMA Pediatr. 2017;171(5):443–449.
- Weisz DE, More K, McNamara PJ, et al. PDA ligation and health outcomes: a meta-analysis. Pediatrics. 2014;133(4):e1024–e1046.
- Sung SI, Chang YS, Chun JY, et al. Mandatory closure versus nonintervention for patent ductus arteriosus in very preterm infants. J Pediatr. 2016;177:66–71.e1.
- Letshwiti JB, Semberova J, Pichova K, et al. A conservative treatment of patent ductus arteriosus in very low birth weight infants. Early Hum Dev. 2017;104:45–49.
- Hundscheid T, Onland W, van Overmeire B, et al. Early treatment versus expectative management of patent ductus arteriosus in preterm infants: a multicentre, randomised, non-inferiority trial in Europe (BeNeDuctus trial). BMC Pediatr. 2018;18(1):262.
- Slaughter JL, Reagan PB, Bapat RV, et al. Nonsteroidal anti-inflammatory administration and patent ductus arteriosus ligation, a survey of practice preferences at US children’s hospitals. Eur J Pediatr. 2016;175(6):775–783.
- Jain A, Shah PS. Diagnosis, evaluation, and management of patent ductus arteriosus in preterm neonates. JAMA Pediatr. 2015;169(9):863–872.
- Hagadorn JI, Brownell EA, Trzaski JM, et al. Trends and variation in management and outcomes of very low-birth-weight infants with patent ductus arteriosus. Pediatr Res. 2016;80(6):785–792.
- Van Overmeire B, Allegaert K, Casaer A, et al. Prophylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9449):1945–1949.
- Rolland A, Shankar-Aguilera S, Diomandé D, et al. Natural evolution of patent ductus arteriosus in the extremely preterm infant. Arch Dis Child Fetal Neonatal Ed. 2015;100(1):F55–F58.
- Dani C, Vangi V, Bertini G, et al. High-dose ibuprofen for patent ductus arteriosus in extremely preterm infants: a randomized controlled study. Clin Pharmacol Ther. 2012;91(4):590–596.
- Meißner U, Chakrabarty R, Topf HG, et al. Improved closure of patent ductus arteriosus with high doses of ibuprofen. Pediatr Cardiol. 2012;33(4):586–590.
- Allegaert K. The impact of ibuprofen or indomethacin on renal drug clearance in neonates. J Matern Fetal Neonatal Med. 2009;22( 3):88–91.
- Zonnenberg I, de Waal K. The definition of a haemodynamic significant duct in randomized controlled trials: a systematic literature review. Acta Paediatr. 2012;101(3):247–251.
- Perez KM, Laughon MM. What is new for patent ductus arteriosus management in premature infants in 2015? Curr Opin Pediatr. 2015;27(2):158–164.
- Bourgoin L, Cipierre C, Hauet Q, et al. Neurodevelopmental outcome at 2 years of age according to patent ductus arteriosus management in very preterm infants. Neonatology. 2016;109(2):139–146.
- de Freitas Martins F, Ibarra Rios D, F Resende MH, et al. Relationship of patent ductus arteriosus size to echocardiographic markers of shunt volume. J Pediatr. 2018;202:50–55.e3.
- van Laere D, van Overmeire B, Gupta S, et al. Application of NPE in the assessment of a patent ductus arteriosus. Pediatr Res. 2018;84(l 1):46–56.
