Abstract
Objectives
Increased body mass index (BMI) is associated with several adverse pregnancy outcomes, though the underlying mechanism of this association has not been fully elucidated. A mediating role of low-grade systemic inflammation in these associations is suspected but has been understudied. Our objective was to examine the effect of pre-pregnancy BMI (pBMI) on maternal and neonatal pregnancy outcomes and to explore potential mediation of these effects by C-reactive protein (CRP), a first trimester peripheral marker of inflammation.
Methods
Data from the prospective community-based ABCD-study cohort (n = 3547) was used to assess associations between self-reported continuous and categorized pBMI and outcome measures gestational hypertension (GH) and preeclampsia (PE), preterm birth (PTB) and small for gestational age (SGA) based on national perinatal registration linkage data. High-sensitivity CRP concentrations determined in serum were used to explore potential mediation of these associations by inflammation.
Results
Multivariable logistic regression analyses, adjusted for confounders, showed that pBMI was significantly related to gestational hypertensive disorders (odds ratio (OR) per standard deviation (SD) 1.66, 95% confidence interval (CI) 1.51–1.83) and PTB (OR 1.20, 95% CI 1.05–1.37). Dose–response relationships between categorical pBMI and gestational hypertensive disorders (overweight OR 2.37, 95% CI 1.85–3.03 and obese OR 4.45, 95% CI 2.93–6.72) and PTB (obese OR 2.12, 95% CI 1.16–3.87) were found as well. SGA was only significantly more prevalent in the underweight BMI category (OR 2.06, 95% CI 1.33–3.19). Mediation analyses revealed small but significant indirect effects of pBMI on overall PTB (0.037, bootstrapped 95% CI 0.005–0.065) and spontaneous PTB (0.038, bootstrapped 95% CI 0.002–0.069) through higher CRP. CRP was not a significant mediator of associations between BMI and gestational hypertensive disorders although larger mediation was found for GH than for PE.
Conclusion
Our findings provide additional evidence that high(er) pBMI increases the risk of adverse maternal and neonatal outcomes and that systemic inflammation mediates some of these risks. Further research in large cohorts including (morbidly) obese women is warranted to identify pathways that may be incorporated in future interventions to reduce the risk of adverse pregnancy outcomes due to maternal obesity.
Introduction
The epidemic of maternal obesity world-wide is a major public health threat. Being overweight or obese puts women at risk for adverse pregnancy outcomes such as gestational hypertensive disorders [Citation1,Citation2] and preterm birth (PTB) [Citation3,Citation4]. These outcomes may lead to maternal and infant morbidity and mortality [Citation5,Citation6]. Small for gestational age (SGA) is also more prevalent in obese women [Citation7,Citation8], particularly when assessed with customized instead of population based growth curves [Citation9]. Importantly, SGA neonates born to obese women have higher morbidity and mortality rates than SGA neonates born to normal weight women [Citation10]. Despite the theoretical explication of putative pathophysiological mechanisms involved in the association between body mass index (BMI) and these adverse pregnancy outcomes, there is a lack of robust data-analyses examining the mediating role of systemic inflammation in pregnancy [Citation11].
Markers of systemic inflammation like C-reactive protein (CRP) slightly raise in early gestation indicating adequate placentation. A chronic low-grade systemic inflammatory response with increased proinflammatory cytokine- and CRP levels and endothelial dysfunction is however associated with placental insufficiency leading to adverse outcomes like PE [Citation12,Citation13]. White adipose tissue is an important site for secretion of interleukin-6 (IL-6), a pro-inflammatory cytokine that stimulates hepatocytes to synthesize and secrete CRP [Citation14]. Hence, the number and size of adipocytes in overweight and obese women and pre-pregnancy BMI (pBMI) correlate positively with IL-6 and CRP levels in pregnancy [Citation15,Citation16].
Increased CRP levels have consistently been found associated with gestational hypertensive disorders [Citation17–19], PTB [Citation20,Citation21] and, although less frequently, with fetal growth restriction and SGA [Citation22]. Controlling for BMI however greatly attenuated associations with gestational hypertensive disorders, suggesting an overrepresentation of systemic inflammatory processes among overweight and obese pregnant women [Citation17,Citation19]. Within this context, medically indicated PTB is also more likely [Citation23]. PTB may also occur spontaneously due to localized (e.g. chorioamnionitis) or systemic inflammation, that are both more common in obese pregnancies [Citation15,Citation24]. After excluding localized inflammatory causes, CRP was still associated with spontaneous PTB in higher BMI strata suggesting a separate BMI-related systemic inflammatory pathway to (spontaneous) PTB [Citation21].
Further understanding of mediating inflammatory factors like CRP is essential to mitigate the risk of adverse pregnancy outcomes in overweight or obese populations. To date however only one small-scale case-control study quantified the mediating role of CRP in the effect of pBMI on PE [Citation25]. The objective of this study was to explore the mediating role of CRP in the effect of pBMI on several adverse pregnancy outcomes known to be increased in overweight and obese pregnant by using data of a prospective birth cohort.
Materials and methods
Subjects
The Amsterdam Born Children and their Development (ABCD) study is a prospective population-based mother-child study cohort extensively described elsewhere [Citation26]. Participants were recruited between January 2003 and March 2004 during their first antenatal visit to their general practitioner, midwife, or gynecologist. The ABCD-study has been approved by the Dutch Central Committee on Research Involving Human Subjects, the medical ethics review committees of participating hospitals and the Municipality of Amsterdam and was conducted according to the Helsinki declaration. All participants gave written informed consent. Study data were linked with the Netherlands Perinatal Registry (PRN) database [Citation27].
Procedure
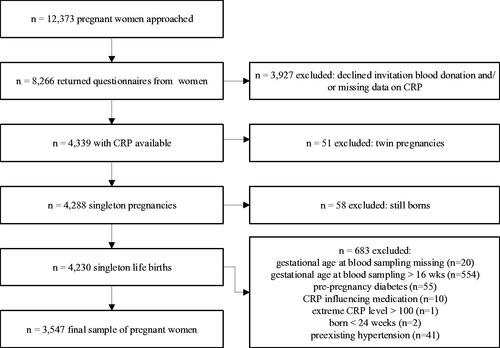
Of the 12,373 women approached, 8266 (67%) completed a pregnancy questionnaire (median of 16 weeks gestation, interquartile range (IQR) 14–16) and 52.5% (n = 4339) donated blood for biomarker analysis during routine blood sampling [Citation26]. Compared to women not participating in blood sampling (n = 3887), included women (n = 3547) were significantly (p < .001) older, more often Dutch, more often nulliparous, had received more years of education, consumed alcohol during pregnancy more often and had a lower pBMI but had similar rates of smoking during pregnancy and chronic hypertension. Exclusion criteria were: multiple gestation, gestational age at blood sampling beyond 16 weeks or missing, preexisting (chronic) hypertension without PE, CRP-levels >100 mg/L, CRP-influencing medication use, diabetes (preexisting or gestational), labor <24 weeks’ gestation and women with still born babies (; Supplemental data).
Variables
Continuous pBMI was based on the self-report questionnaire and calculated by dividing pre-pregnancy weight by the square root of height and categorized in accordance with EU guidelines (i.e. underweight <18.5; normal weight 18.5–24.99; overweight 25–29.99; obese ≥30).
Maternal CRP concentrations were determined in a blood sample taken from non-fasting participants at a median of 13 weeks’ gestation (IQR 12–14 weeks) in a 9-mL Vacuette for the preparation of serum (Greiner BV, Alphen aan den Rijn, The Netherlands) [Citation26]. One-milliliter serum aliquots were processed and prepared by centrifugation (1600×g for 10 min at room temperature) and stored at −80 °C (mean of 22 months; range: 13–29) at the Regional Laboratory of Health Protection Research, Amsterdam. CRP concentration (mg/L) was determined at Medical Laboratories of Dr. Stein and colleagues, Maastricht, The Netherlands with the Dade-Behring high-sensitivity assay. Intra assay coefficient of variation (CV) for CRP was 4.1% for low values and 0.9% for high values. Inter assay CV was 5.2% for low values and 2.6% for high values. Participants with a CRP concentration below the lower detection limit of 0.5 mg/L (n = 445), were included as having a value of 0.25 mg/L. Quartiles of CRP were also calculated for descriptive purposes (Q1 ≤ 1.57; Q2 from 1.58 to 3.24; Q3 from 3.25 to 6.11; and Q4 ≥ 6.12).
Potential confounders maternal age, parity, post-primary education (years), ethnicity, current smoking and alcohol consumption and gestational age (based on PRN ultrasound data or on the last menstrual period in <10%) were selected a priori and based on earlier literature [Citation22,Citation28].
Maternal and neonatal outcomes were obtained from PRN. Gestational hypertension (GH) was defined as diastolic blood pressure ≥ 90 mmHg and/or systolic blood pressure ≥ 140 mmHg from 20 weeks’ gestation onwards. Preeclampsia (PE) was similarly defined but with the addition of proteinuria ≥0.3 g/24 h or dipstick ≥2+ from 20 weeks gestation onwards and also includes PE superimposed on preexisting hypertension (i.e. a diastolic pressure ≥90 mmHg or antihypertensive treatment before pregnancy or before 20 weeks gestation) according to international guidelines [Citation29]. PTB was defined as a delivery between 24.0 and 36.6 weeks gestational age (WHO) and non-PTB as a delivery from 37.0 weeks onwards. Neonates were defined SGA in case of a birthweight < 10th percentile based on gender- and parity-specific national reference curves for birthweight [Citation30].
Statistical analysis
To compare subgroups of maternal characteristics regarding mean pBMI and CRP (mg/L) levels, two-samples t-tests or ANOVA’s were used. Since CRP levels normally increase during gestation, maternal CRP levels were corrected for mean gestational age at blood sampling [Citation31]. Multivariable logistic regression analyses were performed to examine associations of continuous and categorical pBMI with the dichotomous outcomes. pBMI units were divided by their SD, therefore associations of continuous BMI should be interpreted as SD change in the determinant.
Mediation analyses were performed by multivariable linear and logistic regression models through the indirect method of Preacher and Hayes involving bootstrapping approximations [Citation32]. We assessed (i) the effect of pBMI on CRP (the a paths), (ii) the effect of CRP on the four pregnancy outcomes respectively (i.e. the b paths), (iii) the effect of pBMI on the three pregnancy outcomes respectively (gestational hypertensive disorders, PTB, and SGA) (i.e. the direct or c′ paths), and (iv) the effect of pBMI on the three pregnancy outcomes through mediator CRP as shown by the difference in indirect and total effects. Due to dichotomous outcomes, the indirect effect was based on the product of a and b coefficients and the proportion mediated was based on the a*b/(a*b – c′) [Citation33]. Sensitivity analyses were performed for subgroups of outcomes of the mediation analyses.
Missing data on outcomes for hypertensive disorders, PTB, and SGA were respectively 9%, 1.3%, and 1.5%. As missing data were very low for covariates (<1%), imputation strategies were not performed. All analyses were performed on complete case data and adjusted for potential confounders. No additional confounder-adjustment for parity was performed for SGA outcome models because SGA centiles are already based on parity adjusted population-based reference curves. Statistical significance was defined using a two-sided p value at <.05. Mediation models were analyzed in Stata version 14 (College Station, TX). All other analyses were performed in SPSS version 24 (SPSS Inc., Chicago, IL).
Results
Maternal characteristics, BMI, and CRP
Mean pBMI and CRP levels, respectively, were significantly higher in women who were multiparous, overweight, or obese, not consuming alcohol in pregnancy, from non-Dutch ethnic origin and lower educated ().
Table 1. Maternal characteristics, pre-pregnancy BMI and CRP (n = 3547).
BMI and adverse maternal and neonatal outcomes
Of the complete case data per outcome (), 15.2% developed a gestational hypertensive disorder (11.1% GH; 4.1% PE), 5.3% gave birth preterm, and 9.2% of newborns were SGA. Multivariable logistic regression results presented in show that a 1-SD increase in pBMI was related to a 66% higher risk of a hypertensive disorder in pregnancy (aOR 1.66, 95% CI 1.51–1.83). Overweight and obese women had a significantly higher risk of a gestational hypertensive disorder compared to women with normal BMI (aORs 2.37 and 4.45, respectively) indicating a dose–response relationship. The odds ratio of PTB was also significantly higher per SD increase in pBMI (aOR 1.20, 95% CI 1.05–1.37). Obese women were twice as likely to develop PTB compared to normal weight women (aOR 2.12, 95% CI 1.16–3.87). Continuous pBMI was not significantly associated with SGA (aOR 1.08, 95% CI 0.97–1.19). Underweight women were however twice as likely to deliver their child SGA compared to normal weight women (aOR 2.06, 95% CI 1.33–3.19).
Table 2. Multivariable logistic regression of pre-pregnancy BMI with maternal and neonatal outcomes.
Mediation by CRP
Mediation analyses showed direct and indirect effects of pBMI on gestational hypertensive disorders, PTB, and SGA, after controlling for confounders (). In line with findings of multivariable regression analyses (), significant (p < .001) total (c-path) and direct effects (c′-path) for pBMI on gestational hypertensive disorders (GH or PE) were found (). pBMI was positively associated with CRP level (a-path) but the association of CRP with gestational hypertensive disorders (b-path) was not significant (p = .098). The indirect effect of pBMI on gestational hypertensive disorders through an increase of CRP concentration was also not significant (indirect effect = 0.021, bootstrapped 95% CI −0.003 to 0.043). The proportion of the total effect of pBMI on gestational hypertensive disorders mediated by CRP was 4.1%. Sensitivity analyses showed stronger mediation of CRP with GH as outcome than with PE as outcome. Hence, the proportion mediated by CRP of the total effect of pBMI on GH was 4.9% (indirect effect = 0.025, bootstrapped 95% CI −0.0007 to 0.048) while the proportion mediated of the total effect of pBMI on PE was 2.2% (indirect effect = 0.010; bootstrapped 95% CI −0.034 to 0.041).
Figure 2. Mediation of CRP on the effect of pre-pregnancy BMI on, respectively, gestational hypertensive disorders GH and PE (model A), PTB (model B), and SGA (model C). All models are adjusted for confounders for maternal age, parity (except for SGA), gestational age at blood sampling, smoking, alcohol use, and non-Western ethnicity. *p < .05; **p < .01; ***p < .001. Bold indicates significant values.
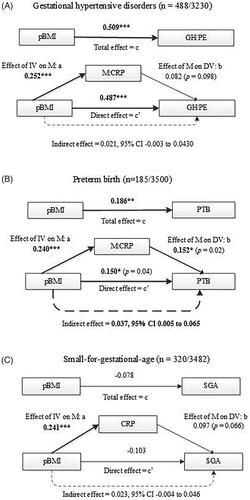
The total effect (c-path) of pBMI on PTB was significant (p < .01) as were both the a-path (pBMI on CRP; p < .001) and b-path (CRP on PTB; p = .02) (). The indirect effect of 0.037 was significant (bootstrapped 95% CI 0.005–0.065) and the proportion mediated by CRP was 19%. The direct effect (c′) was significant (p = .04) after adding CRP to the total effect of pBMI on PTB, indicating partial mediation. Sensitivity analyses excluding medically induced PTB cases (n = 41), showed even stronger mediation of CRP (proportion mediated 24% with indirect effect of 0.038 with bootstrapped 95% CI 0.002–0.069).
For SGA (), the total effect (c-path) of pBMI on SGA was negative and non-significant while the indirect effect of pBMI on SGA through CRP was positive (indirect effect 0.023; 95% CI −0.004 to 0.046). This pattern indicates inconsistent non-significant mediation [Citation34].
Discussion
This study showed that pBMI is associated with gestational hypertensive disorders and PTB (but not SGA) in a continuous and dose–response manner in line with previous findings [Citation1,Citation2,Citation8]. Our study adds to these findings that a significant proportion (i.e. 19%) of the effect of pBMI on PTB was mediated by early pregnancy CRP. CRP did however not significantly mediate the effects of BMI on gestational hypertensive disorders and SGA. The proportion mediated by CRP was larger when restricted to spontaneous PTB cases which is in line with previous findings [Citation21,Citation35]. This suggests that the increased risk for PTB in overweight and obese women originates from systemic inflammation leading to spontaneous labor onset or premature rupture of membranes rather than from vascular problems such as endothelial dysfunction in hypertensive disorders or fetal-growth restriction that often lead to medically induced PTB. Different etiological pathways to spontaneous or to induced PTB in overweight and obese women can thus be assumed [Citation36].
A mediating role of inflammation in the pathogenesis of hypertension in overweight and obese women was also hypothesized [Citation17–19,Citation37]. CRP, however, did not significantly explain the association between pBMI and gestational hypertensive disorders. This is not in line with a small-scale case-control study from the US that previously showed that inflammation (i.e. CRP) was an important partial mediator of the effect of BMI on PE [Citation25]. Sample and design of that study were however inherently different from ours. The ABCD-study had lower rates of pre-pregnancy overweight and obesity and (related) GH or PE in line with other European population-based birth cohorts from early 2000 [Citation38,Citation39]. Perhaps therefore we only observed a trend of CRP as mediator in the association between pBMI and hypertensive disorders in pregnancy. Hence, inflammation may still act as an important mechanism in the development of GH and PE in more severely obese pregnant populations such as in the US [Citation40]. On the other hand, previous studies have also shown that plasma concentrations of multiple systemic inflammatory markers were not consistently elevated in women who later developed PE [Citation41,Citation42]. Moreover, although high CRP levels were more prevalent in overweight and obese women CRP was a stronger predictor of PE in lean women [Citation18]. These findings suggest that multifactorial etiological dysfunctions such as in the renin angiotensin system or increased oxidative stress in play a role in the obesity – PE link as well [Citation43].
Strengths of our study include the prospective design and large community-based cohort with blood sampling in early pregnancy and linkage with national registry data allowing adequately powered longitudinal multivariable and advanced mediation analyses. Moreover, based on the design of data-collection with temporal precedence of pBMI and early pregnancy CRP, respectively, directionality of effects can be assumed for the mediation models. Limitations are that selective participation cannot be ruled out as participating were different from nonparticipating women on some maternal characteristics such as lower educational status and lower pBMI. This might have led to an underestimation of effect sizes but not to spurious associations. Another limitation might be the use of pBMI based on retrospective self-reported maternal weight before pregnancy. However, self-reported BMI has been shown to correctly identify pBMI status [Citation44,Citation45]. Finally, assessing CRP in non-fasting blood samples at various day-time points may be a drawback but diurnal variation in CRP previously not affected vascular risk prediction [Citation46].
For future studies, we suggest to measure visceral adipose tissue thickness by ultrasound to convey more direct risks for PE and PTB beyond BMI [Citation47] and to examine other explanatory factors of the inflammatory pathway from BMI to adverse pregnancy outcomes ideally with prospective cohort-data including more (morbidly) obese women [Citation48]. For example, maternal lipids may cause an overload of oxidative stress that damages endothelial placental cells thereby potentially also inducing hypertensive disorders, particularly in obese women [Citation28,Citation43].
In conclusion, our study shows that pBMI is related to a higher risk of gestational hypertensive disorders and PTB in a dose–response manner. Furthermore, this is the first large-scale prospective cohort that shows CRP, as measure of systemic inflammatory mechanisms, to partly mediate the effect of pBMI on (spontaneous) PTB. Additional research is needed to identify other underlying mediating factors involved in the pathogenesis of PTB and gestational hypertensive disorders in large study samples with overweight and obese pregnant women before clinical recommendations can be made.
Supplement_flow_chart_of_study_participants.docx
Download MS Word (57.6 KB)Acknowledgments
We thank all participating hospitals, obstetric clinics, and general practitioners for their assistance in implementing the ABCD-study. We also gratefully acknowledge all the women who participated in this study for their cooperation. We thank Prof. dr. J.W.R. Twisk for statistical advice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gaillard R, Steegers EA, Hofman A, et al. Associations of maternal obesity with blood pressure and the risks of gestational hypertensive disorders. The Generation R Study. J Hypertens. 2011;29:937–944.
- Savitri AI, Zuithoff P, Browne JL, et al. Does pre-pregnancy BMI determine blood pressure during pregnancy? A prospective cohort study. BMJ Open. 2016;6(8):e011626.
- Nohr EA, Bech BH, Vaeth M, et al. Obesity, gestational weight gain and preterm birth: a study within the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2007;21(1):5–14.
- Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA. 2013;309(22):2362.
- Savitz DA, Danilack VA, Elston B, et al. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol. 2014;180(1):41–44.
- Villamor E, Tedroff K, Peterson M, et al. Association between maternal body mass index in early pregnancy and incidence of cerebral palsy. JAMA. 2017;317(9):925–936.
- Chen YH, Li L, Chen W, et al. Pre-pregnancy underweight and obesity are positively associated with small-for-gestational-age infants in a Chinese population. Sci Rep. 2019;9(1):9.
- Shin D, Song WO. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J Matern Neonatal Med. 2015;28(14):1679–1686.
- Lauring JR, Gupta M, Kunselman AR, et al. The Journal of Maternal-Fetal & Neonatal Medicine Identification of small for gestational age by population-based and customized growth charts in newborns of obese and normal-weight primiparous women Identification of small for gestational age by population-based and customized growth charts in newborns of obese and normal-weight primiparous women. J Matern Fetal Neonatal Med. 2016;29:1–3574.
- Yao R, Park BY, Caughey AB. The effects of maternal obesity on perinatal outcomes among those born small for gestational age. J Matern Neonatal Med. 2017;30(12):1417–1422.
- Denison FC, Roberts KA, Barr SM, et al. Obesity, pregnancy, inflammation, and vascular function. Reproduction. 2010;140(3):373–385.
- Sacks GP, Seyani L, Lavery S, et al. Maternal C-reactive protein levels are raised at 4 weeks gestation. Hum Reprod. 2004;19(4):1025–1030.
- Huda SS, Jordan F, Bray J, et al. Visceral adipose tissue activated macrophage content and inflammatory adipokine secretion is higher in pre-eclampsia than in healthy pregnancy. Clin Sci. 2017;131(13):1529–1540.
- Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–1013.
- Schmatz M, Madan J, Marino T, et al. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol. 2010;30(7):441–446.
- McDade TW, Borja JB, Largado F, et al. Adiposity and chronic inflammation in young women predict inflammation during normal pregnancy in the Philippines. J Nutr. 2016;146(2):353–357.
- de Jonge LL, Steegers EAP, Ernst GDS, et al. C-reactive protein levels, blood pressure and the risks of gestational hypertensive complications. J Hypertens. 2011;29(12):2413–2421.
- Qiu C, Luthy DA, Zhang C, et al. A prospective study of maternal serum C-reactive protein concentrations and risk of preeclampsia. Am J Hypertens. 2004;17(2):154–160.
- Rebelo F, Schlüssel MM, Vaz JS, et al. C-reactive protein and later preeclampsia: systematic review and meta-analysis taking into account the weight status. J Hypertens. 2013;31(1):16–26.
- Lohsoonthorn V, Qiu C, Williams MA. Maternal serum C-reactive protein concentrations in early pregnancy and subsequent risk of preterm delivery. Clin Biochem. 2007;40(5–6):330–335.
- Bullen BL, Jones NM, Holzman CB, et al. C-reactive protein and preterm delivery: clues from placental findings and maternal weight. Reprod Sci. 2013;20(6):715–722.
- Ernst GDS, De Jonge LL, Hofman A, et al. C-reactive protein levels in early pregnancy, fetal growth patterns, and the risk for neonatal complications: the Generation R Study. Am J Obstet Gynecol. 2011;205:132.e1-12.
- Bernardes TP, Zwertbroek EF, Broekhuijsen K, et al. Delivery or expectant management for prevention of adverse maternal and neonatal outcomes in hypertensive disorders of pregnancy: an individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2019;53(4):443–453.
- Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010;116(2, Part 1):393–401.
- Bodnar LM, Ness RB, Harger GF, et al. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol. 2005;162(12):1198–1206.
- Van Eijsden M, Vrijkotte TG, Gemke RJ, et al. Cohort profile: the Amsterdam born children and their development (ABCD) study. Int J Epidemiol. 2011;40(5):1176–1186.
- Méray N, Reitsma JB, Ravelli ACJ, et al. Probabilistic record linkage is a valid and transparent tool to combine databases without a patient identification number. J Clin Epidemiol. 2007;60(9):883–883.e11.
- Vrijkotte TGM, Krukziener N, Hutten BA, et al. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study. J Clin Endocrinol Metab. 2012;97(11):3917–3925.
- Brown MA, Magee LA, Kenny LC, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310.
- Visser GHA, Eilers PHC, Elferink-Stinkens PM, et al. New Dutch reference curves for birthweight by gestational age. Early Hum Dev. 2009;85(12):737–744.
- de Oliveira LC, Franco-Sena AB, Rebelo F, et al. Factors associated with maternal serum C-reactive protein throughout pregnancy: a longitudinal study in women of Rio de Janeiro, Brazil. Nutrition. 2015;31(9):1103–1108.
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731.
- VanderWeele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172(12):1339–1348.
- MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1(4):173–181.
- Catov JM, Bodnar LM, Ness RB, et al. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007;166(11):1312–1319.
- Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet (London, England). 2008;371(9606):75–84.
- Tjoa ML, Van Vugt JMG, Go ATJJ, et al. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol. 2003;59(1):29–37.
- Patel S, Lawlor DA, Callaway M, et al. Association of maternal diabetes/glycosuria and pre-pregnancy body mass index with offspring indicators of non-alcoholic fatty liver disease. BMC Pediatr. 2016;16(1):47.
- Bahadoer S, Gaillard R, Felix JF, et al. Ethnic disparities in maternal obesity and weight gain during pregnancy. The Generation R Study. Eur J Obstet Gynecol Reprod Biol. 2015;193:51–60.
- Fisher SC, Kim SY, Sharma AJ, et al. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med (Baltim). 2013;56(6):372–378.
- Djurovic S, Clausen T, Wergeland R, et al. Absence of enhanced systemic inflammatory response at 18 weeks of gestation in women with subsequent pre-eclampsia. BJOG. 2002;109(7):759–764.
- Taylor BD, Ness RB, Klebanoff MA, et al. First and second trimester immune biomarkers in preeclamptic and normotensive women. Pregnancy Hypertens. 2016;6(4):388–393.
- Zavalza-Gómez AB. Obesity and oxidative stress: a direct link to preeclampsia? Arch Gynecol Obstet. 2011;283(3):415–422.
- Shin D, Chung H, Weatherspoon L, et al. Validity of prepregnancy weight status estimated from self-reported height and weight. Matern Child Health J. 2014;18(7):1667–1674.
- Bannon AL, Waring ME, Leung K, et al. Comparison of self-reported and measured pre-pregnancy weight: implications for gestational weight gain counseling. Matern Child Health J. 2017;21(7):1469–1478.
- Meier-Ewert HK, Ridker PM, Rifai N, et al. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin Chem. 2001;47(3):426–430.
- Ray JG, De Souza LR, Park AL, et al. Preeclampsia and preterm birth associated with visceral adiposity in early pregnancy. J Obstet Gynaecol Can. 2017;39(2):78–81.
- Ferguson KK, Meeker JD, McElrath TF, et al. Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. Am J Obstet Gynecol. 2017;216(5):527.e1–527–e9.