Abstract
Objective
To compare maternal and perinatal outcomes between randomized trials and observational studies in which conservative management was performed for more than 48 h in patients with early-onset severe preeclampsia.
Methodology
We searched PubMed, LILACS, Cochrane and Google Scholar. The studies were divided in two groups: randomized and observational studies, from 1990 to 2018 that included patients with severe preeclampsia before 34 weeks of gestation with pregnancy prolongation ≥48 h but that did not include fetal growth restriction or HELLP syndrome at the beginning. The main variables recorded were maternal and perinatal complications.
Main Results
Forty-four studies met the inclusion criteria, and 5 of these were randomized. The average pregnancy prolongation was 9 days, with no difference between groups. Maternal complications were significantly more common in observational studies, RR = 0.71, 95% CI (0.54–0.93), p = .009. Perinatal complications were also significantly more common in observational studies (RR = 0.89, 95% CI (0.80–0.98), p = .01) at the expense of stillbirth and neonatal deaths. The percentages of cesarean sections were significantly higher in randomized studies, RR = 1.54, 95% CI (1.46–1.64). There were 2 maternal deaths, both in observational studies.
Conclusion
Observational studies in which conservative management of early-onset preeclampsia is performed and do not include patients with fetal growth restriction or patients with HELLP syndrome and where at least 2 days of pregnancy prolongation is achieved are associated with significantly more maternal and perinatal complications.
Introduction
Preeclampsia is a condition that only occurs during pregnancy; it is characterized by hypertension occurring for the first time accompanied by proteinuria after 20 weeks of pregnancy and is estimated to affect between 2 and 5% of pregnant women [Citation1]. Preeclampsia can be subdivided into early-onset preeclampsia (birth <34 weeks of gestation) and late-onset preeclampsia (birth ≥34 weeks of gestation) [Citation1]. Recently, in a study with more than half a million pregnant women during a period of 10 years in Norway [Citation2], it was found that the incidence of early-onset preeclampsia is one in every 200 pregnant women (0.5%), representing 13% of all preeclampsia cases. However, the percentage of early-onset severe preeclampsia is unknown.
The treatment or cure for preeclampsia is termination of pregnancy [Citation3], particularly if the patient meets the criteria for severe preeclampsia [Citation4]. If a pregnant woman has early-onset severe preeclampsia, management becomes a dilemma for the clinician because of the possibility of maternal complications such as placental abruption, hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome, disseminated intravascular coagulation, eclampsia, renal failure, hepatic hematoma/rupture, pulmonary edema and maternal death [Citation5] and fetal or neonatal complications such as growth restriction, respiratory distress syndrome, intraventricular hemorrhage, neurological damage, and intrauterine and neonatal death [Citation6] with or without the termination of pregnancy. Three decades ago, the first randomized study was published suggesting a neonatal benefit without harming the mother when conservatively managing patients with severe preeclampsia before 34 weeks of gestation [Citation7]. However, a similar study [Citation8] with more patients was recently published and showed no perinatal benefits with conservative management compared with aggressive management; on the contrary, conservative management was associated with increased fetal growth restriction and increased placental abruption [Citation8].
The most recent Cochrane systematic review [Citation6] on the management of early-onset severe preeclampsia concludes that with the few existing randomized studies, expectant management is associated with a decrease in neonatal morbidity. We do not know if observational studies lead to the same results.
The objective of this historical review is to compare maternal and perinatal outcomes between randomized controlled trials (RCTs) and observational studies where conservative management was performed for more than 48 h in patients with early-onset severe preeclampsia (≤ 34 weeks) without growth restriction and without HELLP syndrome and compare results.
Materials and methods
This research is a systematic review with the following inclusion criteria: all RCTs and observational studies or case series from 1990 to 2018 that included patients with severe preeclampsia at 34 or fewer weeks of gestation where pregnancy was prolonged by 48 or more hours, in which patients with fetal growth restriction or HELLP syndrome were not included at the beginning of the study. If fetal growth was used to definition of severe preeclampsia the study was excluded.
We searched the online databases PubMed, LILACS, Cochrane and Google Scholar for studies that included the established inclusion criteria. We used the following search strategy (in all fields): (“gestational hypertensive disorder” OR “pregnancy-induced hypertension” OR (“pre-eclampsia” or “preeclampsia”) OR “hypertension” AND “pregnancy” AND “early intervention,” “early birth,” “interventionist,” “conservative,” “active management,” “conservative management,” “expectant management,” “aggressive management,” “conservative treatment”) with the search limits “human.” In addition, references from, review articles, and clinical guidelines were reviewed looking for possible studies. Complete studies published in English, Portuguese and Spanish were reviewed, in addition to the English abstract of studies published in other languages, which were translated into English if included in the review.
Main results: Each study selected after meeting the inclusion criteria had the following information added to a database: type of study (randomized or observational), year of publication, country where it was performed, whether the country was industrialized, total patients, gestational age, duration of pregnancy prolongation, most frequently reported maternal complications (placental abruption, HELLP syndrome, renal failure, intravascular coagulation, acute pulmonary edema, eclampsia, maternal death), most frequently reported perinatal complications (fetal growth restriction, intrauterine death), perinatal death (intrauterine death plus neonatal death), intraventricular hemorrhage, respiratory distress syndrome, whether the cause for the interruption was maternal or fetal, and how the pregnancy was terminated. Of the RCTs, only the group that received expectant management was analyzed. Some women and some neonates had more than one complication, and the total number of complications was recorded.
We report in accordance with the PRISMA-IPD statement.
Statistical analysis: The statistical analysis was performed using EPI Info version 7 (Centers for Disease Control and Prevention, Atlanta, GA). For each variable, the results of all studies were compared between RCTs and observational studies. In addition, the pregnancy outcomes were compared from 28 to 34 weeks, and results were compared between countries according to per capita income. For the analyses, Fisher’s exact test or the chi-squared test was used as appropriate, and the risk ratio (RR) was calculated with the 95% confidence interval (CI). p values less than .05 were considered significant.
Results
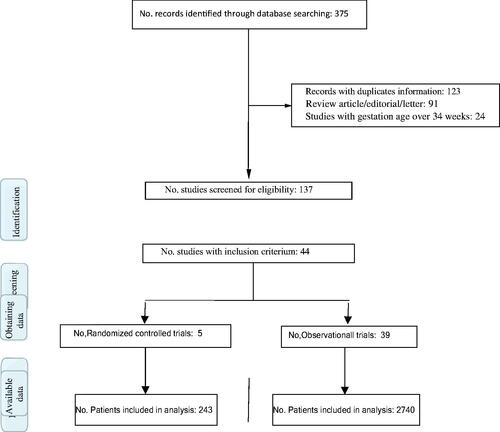
For the 29-year period from 1990 through 2018, 44 studies met the inclusion criteria, of which 5 were RCTs [Citation7–11] ( and ). Of the 5 RCTs, 2 were conducted in countries with a high per capita income, and of the 39 observational studies [Citation5,Citation12–49], 21 were in countries with a medium or low per capita income. The countries with the highest per capita income and more publications were the USA, Japan and the Netherlands, and those with a low and medium per capita income and more publications were South Africa, India, and Mexico. The study with the most patients (340) was performed in South Africa [Citation16], and that with the fewest patients (10) was conducted in Thailand [Citation33]. The total number of patients for RCTs and observational studies was 243 and 2740, respectively (). The average duration of pregnancy prolongation was 9 days and was very similar between randomized trials and observational studies.
Table 1. Studies included in the review.
Table 2. General information of randomized controlled trials and observational studies with conservative management of early-onset severe preeclampsia.
Maternal complications were significantly more common in observational studies, RR = 0.71, 95% CI (0.54–0.93), p = .009; severe complications were predominant, such as renal failure, pulmonary edema and eclampsia (). This difference is maintained when we analyze studies conducted in high per capita income countries, but it is not observed if we only analyze studies conducted in medium and low per capita income countries. Furthermore, no significant differences were observed with maternal complications when comparing RCTs with observational studies that only included patients between 28 and 34 weeks of gestation ().
Table 3. Main maternal and perinatal outcomes of randomized controlled trials and observational studies with conservative management of early-onset severe preeclampsia.
Table 4. Maternal and perinatal outcomes of randomized controlled trials and observational studies with conservative management of early-onset severe preeclampsia.
Perinatal complications were also significantly more common in observational studies (RR = 0.89, 95% CI (0.80–0.98), p = .01), at the expense of fetal deaths (intrauterine) and postnatal deaths (). When we analyzed intrauterine deaths and perinatal deaths (intrauterine plus postnatal) between both types of studies for gestational ages from 28 to 34 weeks, in countries with high per capita income and in countries with medium and low per capita income, there were significantly more deaths in observational studies ().
The percentages of cesarean sections were significantly higher in RCTs, RR = 1.54, 95% CI (1.46–1.64). This difference was always maintained in favor of RCTs if we only analyzed studies that investigated gestational ages between 28 and 34 weeks, if we only analyzed studies from high per capita income countries or if we only analyzed studies from low- and middle-income countries.
In the RCTs, the main cause of pregnancy interruption was maternal in 59.2% of cases. In observational studies, the main cause of pregnancy interruption was also maternal but only in 27% of cases, p = .0001.
In this review, there were 2 maternal deaths, both in observational studies of nonindustrialized countries.
Discussion
This historical review of RCTs and observational studies with conservative management of early-onset severe preeclampsia shows that maternal and perinatal complications are statistically more common in nonrandomized studies. Perinatal mortality and, especially, intrauterine (fetal) mortality are more common in observational studies, regardless of level of development of the country where the study was conducted. The frequency of cesarean sections in RCTs was 90%, approximately 30% higher than that in observational studies.
The best clinical practice is based on the results obtained from RCTs. The first RCT [Citation7] on the conservative management of early-onset severe preeclampsia was published in December 1990, and the second was published in 1994 [Citation9]. These 2 studies led to the conservative management of early-onset severe preeclampsia in clinical practice, which was confirmed through the publication of 30 observational studies [Citation12–41] until the end of 2013, when the RCT [Citation8] with the largest number of patients was published. The results of that RCT were not encouraging for the conservative management of early-onset severe preeclampsia.
To our knowledge and certainly of any clinician in the world, the results of observational studies (cohorts, historical cohorts, case-controls, case series) should find similar results than do RCTs. There are great differences when comparing observational studies with RCTs, but if only are results that are easy to measure, are universally defined in the exact same way and are reported in all randomized and observational studies are analyze, we can achieve relevant clinical conclusions. However, the observational studies analyzed in this review show great heterogeneity and therefore the results should be interpreted with great caution.
The sum of maternal complications in RCTs are not different when comparing conservative versus aggressive management [Citation6]; however, maternal complications are much more common in observational studies; therefore, they equate to higher percentages, with significant differences, than those in RCTs with groups undergoing expectant or aggressive management. This difference, of more maternal complications in observational studies, is up to 4 times higher in studies from high-income countries. Our review found 2 maternal deaths, both in observational studies, and we consider this finding to be unacceptable and unjustifiable.
Interestingly, the percentages of cesarean sections are very high, with a significant difference favoring RCTs [Citation7–11]. Possibly due to the nature of these studies, there is a much more aggressive behavior at the time of pregnancy termination; it is likely that this aggressive behavior and strict surveillance explains the few fetal and neonatal deaths between the expectant and interventionist groups in RCTs [Citation7–11] and probably also explains the existence of fewer maternal complications in RCTs than in observational studies, and it is possible a Hawthorne effect.
One of the most important variables to report in studies with conservative management of severe preeclampsia far from term is perinatal mortality (stillbirth and neonatal death). Our analysis allows us to conclude that 2.4 times more fetuses and neonates die in observational studies than in randomized studies, regardless of gestational age and level of development of the country where the study was conducted. Recently, in Norway [Citation2], a study reported a relative risk of fetal death by preeclampsia ranging from 11.6 at 26 weeks to 1.1 at 34 weeks for every 1000 women, with a risk of fetal death of approximately 3.0 for every 1000 women with preeclampsia at ≤ 34 weeks of gestation. Our review finds that the risks of fetal death in RCTs and observational studies is 8.2 for every 1000 women and 113 for every 1000 women, respectively.
However, our study did not stratify by gestational age and, as is obvious, there are enormous differences in the two types of studies.
Unfortunately, the majority of observational studies [Citation12,Citation13,Citation15–32,Citation39,Citation46–48] suggest conservative management of early-onset preeclampsia; others question that option [Citation14,Citation38,Citation43–45,Citation49], particularly after the MEXPRE Latin study [Citation8].
The practice of medicine today depends largely on the results of studies with better evidence; the expectant management of early-onset severe preeclampsia has been based on low or very low evidence [Citation3,Citation6]. Interestingly, for each randomized study, there are approximately 8 observational studies, and the vast majority of these studies suggest expectant management. This means that it is possible that the majority of hospitals around the world, based on the few randomized studies and on many observational studies, perform expectant management as routine management protocols. Unfortunately, this review demonstrates that the maternal and perinatal outcomes of observational studies are worse than those of randomized studies.
A strength of this review is the number of studies (44) and patients analyzed (2983), representing 3 decades of research and reports from different countries around the world. Another strength is that the findings with differences show high statistical value and are maintained despite different gestational age groups or levels of country development. Yet another strength is the comparison of strict follow-up studies (RCTs) with studies reporting daily life or actual practice observational studies.
The limitations of this research include the nature of the review because results from randomized studies are compared with those from observational studies, which is questionable. Another limitation is the possibility that some studies were not found by the search engines used. The span of the study (29 years) may introduce biases, not only in the definition of severe preeclampsia used, but also in the improvements in neonatal care.
In summary, observational studies in which conservative management of early-onset preeclampsia is performed and do not include patients with fetal growth restriction nor patients with HELLP syndrome and in which pregnancy is prolonged for at least 2 days are associated with a significantly greater number of maternal complications and perinatal deaths (fetal and neonatal). The results of this study should be interpreted with great caution since there are great differences in the methodologies of observational and randomized studies. Large and very rigorous randomized studies are necessary to define the best management of patients with severe pre-eclampsia far from the term.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Poon LC, Shennan A, Hyett JA, et al. The international federation of gynecology and obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynecol Obstet. 2019;145(S1):1–33.
- Harmon QE, Huang L, Umbach DM, et al. Risk of fetal death with preeclampsia. Obstet Gynecol. 2015;125(3):628–635.
- WHO. WHO recommendations: policy of interventionist versus expectant management of severe pre-eclampsia before term. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO.
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Washington, DC: American College of Obstetricians and Gynecologists; 2013. Available from http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Preg-nancy. Retrieved January 24, 2018.
- McKinney D, Boyd H, Langager A, et al. The impact of fetal growth restriction on latency in the setting of expectant management of preeclampsia. Am J Obstet Gynecol. 2016;214(3):395.e1–395–e7.
- Churchill D, Duley L, Thornton JG, et al. Interventionist versus expectant care for severe pre-eclampsia between 24 and 34 weeks’ gestation. Cochrane Database Syst Rev. 2018;10(10):CD003106.
- Odendaal HJ, Pattinson RC, Bam R, et al. Aggressive or expectant management for patients with severe preeclampsia between 28 and 34 weeks’ gestation: a randomized controlled trial. Obstet Gynecol. 1990; 76(6):1070–1075.
- Vigil-De Gracia P, Reyes Tejada O, Calle Miñaca A, et al. Expectant management of severe preeclampsia remote from term: the MEXPRE Latin Study, a randomized, multicenter clinical trial. Am J Obstet Gynecol. 2013;209(5):425.e1–425.e8.
- Sibai BM, Mercer BM, Schiff E, et al. Aggressive versus expectant management of severe preeclampsia at 28 to 32 weeks’ gestation: a randomized controlled trial. Am J Obstet Gynecol. 1994;171(3):818–822.
- Mesbah EMM. Severe preterm preeclampsia: aggressive or expectant management? Med J Cairo Univ. 2003;71(1):175–182.
- Duvekot J, Bax C, Bloemenkamp K, et al. Temporizing management versus termination of pregnancy in women with severe preeclampsia at 28–34 weeks (TOTEM-Trial). Am J Obstet Gynecol. 2015;212(1):S246.
- Sibai BM, Akl S, Fairlie F, et al. A proto-col for managing severe preeclampsia in the second trimester. Am J Obstet Gynecol. 1990;163(3):733–738.
- Moodley J, Koranteng SA, Rout C. Expect-ant management of early onset of severe pre-eclampsia in Durban. S Afr Med J. 1993; 83:584–587.
- Olah KS, Redman WG, Gee H. Management of severe, early pre-eclampsia: is conservative management justified? Eur J Obstet Gynecol Reprod Biol. 1993;51(3):175–180.
- Chammas MF, Nguyen TM, Li MA, et al. Expectant management of severe preterm preeclampsia: is intrauterine growth restriction an indication for immediate delivery? Am J Obstet Gynecol. 2000;183(4):853–858.
- Hall DR, Odendaal HJ, Steyn DW, et al. Expectant management of early onset, severe preeclampsia; maternal outcome. Int J Gynaecol Obstet. 2000;107(10):1252–1257.
- Romero Arauz JF, Lara Gonzalez AL, Izquierdo Puente C. Severe preeclampsia ans its conservative management. Ginec Obst Mex. 2000;68:51–54.
- Murphy DJ, Stirrat GM. Mortality and morbidity associated with early-onset preeclampsia. Hypertens Pregnancy. 2000;19(2):221–231.
- Blackwell SC, Redman ME, Tomlinson M, et al. Severe preeclampsia remote from term: what to expect of expectant management. J Matern Fetal Neonatal Med. 2002;11(5):321–324.
- Kobayashi T, Terao T, Ikenoue T, et al. Treatment of severe preeclampsia with antithrombin concentrate: results of a prospective feasibility study. Semin Thromb Hemost. 2003;29(6):645–652.
- Vigil-De Gracia P, Montufar-Rueda C, Ruiz J. Expectant management of severe pre-eclampsia between 24 and 34 weeks’ gesta-tion. Eur J Obstet Gynecol Reprod Biol. 2003;107(1):24–27.
- Haddad B, Deis S, Goffinet F, et al. Maternal and perinatal outcomes during expectant management of 239 severe preeclamptic women between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2004;190(6):1590–1595.
- Oettle C, Hall D, Roux A, et al. Early onset severe pre-eclampsia: expectant management at a secondary hospital in close association with a tertiary institution. BJOG. 2005;112(1):84–88.
- Shear RM, Rinfret D, Leduc L. Should we offer expectant management in cases of severe preterm preeclampsia with fetal growth restriction? Am J Obstet Gynecol. 2005;192(4):1119–1125.
- Budden A, Wilkinson L, Buksh MJ, et al. Pregnancy outcome in women pre-senting with pre-eclampsia at less than 25 weeks’ gestation. Aust N Z J Obstet Gynaecol. 2006;46(5):407–412.
- Hall DR, Grove D, Carstens E. Early-pre-eclampsia: what proportion of women qualify for expectant management and if not, why not? Eur J Obstet Gynecol Reprod Biol. 2006;128(1–2):169–174.
- Gaugler-Senden IPM, Huijssoon AG, Visser W, Steegers EAP, et al. Maternal and perinatal outcome of preeclampsia with an onset before 24 weeks’ gestation. Audit in a tertiary referral center. Eur J Obstet Gynecol Reprod Biol. 2006;128(1–2):216–221.
- Porras-Poma R. Manejo expectante de preeclampsia severa en el embarazo pretérmino en el hospital nacional docente madre niño. Universidad Nal San Marcos. 2006;1:3–29.
- Ganzevoort W, Rep A, Bonsel G, et al. Dynamics and incidence patterns of maternal complications in early-onset hypertension of pregnancy. BJOG. 2007;114(6):741–750.
- Sezik M, Ozkaya O, Sezik HT, et al. Expectant management of severe pre-eclampsia presenting before 25 weeks of gestation. Med Sci Monit. 2007;13:523–527.
- Bombrys AE, Barton JR, Nowacki EA, et al. Expectant management of severe preeclampsia at less than 27 weeks’ gestation: maternal and perinatal outcomes according to gestational age by weeks at onset of expectant management. Am J Obstet Gynecol. 2008;199(3):247.e1–e6.
- Sarsam DS, Shamden M, Al Wazan R. Expectant versus aggressive management in severe preeclampsia remote from term. Singapore Med J. 2008;49(9):698–703.
- Jantasing S, Tanawattanacharoen S. Perinatal outcome in severe preeclamptic women between 24–33(+6) weeks gestation. J Med Assoc Thai. 2008;91(1):25–30.
- Bombrys AE, Barton JR, Habli M, et al. Expectant management of severe preeclampsia at 270/7-336/7 weeks’ gestation: maternal and perinatal outcomes according to gestational age by weeks at onset of expectant management. Amer J Perinatol. 2009;26(06):441–446.
- Abdel-Hady e-S, Fawzy M, El-Negeri M, et al. Is expectant management of early-onset severe preeclampsia worthwhile in low-resource settings? Arch Gynecol Obstet. 2010;282(1):23–27.
- Mogollón-Saker SP, Salcedo-Ramos F, Ramos-Clason EC. Maternal and perinatal results of preeclampsia before 31 weeks of gestation at clínica maternidad Rafael Calvo. Rev Cienc Biomed. 2011; 2(2):262–269.
- Belghiti J, Kayem G, Tsatsaris V, et al. Benefits and risks of expectant management of severe preeclampsia at less than 26 weeks gestation: the impact of gestational age and severe fetal growth restriction. Am J Obstet Gynecol. 2011;205(5):465.e1-6–465.e6.
- Kumar M, Meena J, Gupta U, et al. Management of early onset severe preeclampsia in a tertiary hospital in India: does expectant management alter perinatal outcome? Indian J Med Sci. 2011;65(12):535–542.
- Swamy MK, Patil K, Nageshu S. Maternal and perinatal outcome during expectant management of severe pre-eclampsia between 24 and 34 weeks of gestation. J Obstet Gynecol India. 2012;62(4):413–418.
- Astudillo R, Suy A, Alijotas-Reig J, et al. Expectant management in pregnant women with early and severe preeclampsia and concomitant risk factors. Pregnancy Hypertension: Int J Women’s Cardiovascular Health. 2013; 3(4):235–241.
- Castellón-Pasos RM, Hernández-Pacheco JA, Estrada-Altamirano A, et al. Criterios de inducción del nacimiento en mujeres con pree-clampsia severa en tratamiento expectante. Ginecol Obstet Mex. 2013;81:92–98.
- Chen Q, Shen F, Gao YF, et al. An analysis of expectant management in women with early-onset preeclampsia in China. J Hum Hypertens. 2015;29(6):379–384.
- Suzuki S, Shimada M, Shibata-Hiraizumi Y. Clinical trial of expectant management of severe preeclampsia that develops at <32 weeks’ gestation at a Japanese perinatal center. J Matern‐Fetal Neonatal Med. 2014;27(15):1568–1571.
- Ertekin AA, Kapudere B, Eken MK, et al. Does aggressive and expectant management of severe preeclampsia affect the neurologic development of the infant?. Int J Clin Exp Med. 2015;8(10):19325–19331.
- Rendón-Becerra CA, Ortiz-Martínez RA. Comparación de dos protocolos de manejo en preeclampsia severa lejos del término, y resultados maternos y neonatales: una cohorte histórica hospital universitario San José, Popayán (Colombia). Rev Colomb Obstet Ginecol. 2016;67(1):26–35.
- Ernawati Gumilar E, Kuntoro Soeroso J, Dekker G. Expectant management of preterm preeclampsia in Indonesia and the role of steroids. J Matern‐Fetal Neonatal Med. 2016;29(11):1736–1740.
- Ueda A, Kondoh E, Kawasaki K, et al. Magnesium sulphate can prolong pregnancy in patients with severe early-onset preeclampsia. J Matern Fetal Neonatal Med. 2016;29(19):3115–3120.
- Deepak AV, Reena RP, Deepa A. Fetal and maternal outcome following expectant management of severe pre-eclampsia remote from term. Int J Reprod Contracept Obstet Gynecol. 2017;6(12):5420–5424.
- Vázquez-Rodríguez JG, Barboza-Alatorre DY. Maternal and perinatal outcomes of expectant treatment of severe preeclampsia. Rev Med Inst Mex Seguro Soc. 2018;56(4):379–386.