Abstract
Objective
To observe the lacunar-like changes in cesarean scar pregnancy (CSP) ultrasonography in first trimester and to explore its relationship with clinical outcome in early pregnancy termination.
Methods
This is a retrospective case-control study. Patients who were diagnosed as CSP and chose to terminate pregnancy from January 2017 to April 2020 were enrolled. According to occurrence of lacunar-like change in chorion membrane, patients were divided into case and control group. The clinical manifestation, laboratory test, ultrasound data, and outcome were compared.
Results
Fifty-five CSP patients were enrolled with 20 (36.4%) in case group and 35 (63.6%) in control group. As for ultrasound features, the maximum outer diameter of gestational mass (5.6 ± 2.5 cm vs. 3.9 ± 1.5 cm), the maximum thickness of the chorion membrane (median number 1.1 cm vs. 0.7 cm), the longitudinal diameter of the implanting part of gestational mass in uterine lower segment (3.3 ± 1.8 cm vs. 1.2 ± 0.5 cm), uterine lower segment protrusion incidence (12, 60% vs. 2, 5.7%), and the crown-rump length of fetus (median number 1.7 cm vs. 0.7 cm) were bigger or higher in case group than that of the control group; the minimum thickness of the uterine lower segment myometrium (median number 0.08 cm vs. 0.20 cm) was significantly thinner in case group. CDFI grading of case group was different from control group with more cases in higher grades. As for clinical outcome, the patients of case group showed more frequency of CSP lesion resection under open surgery or laparoscopy (7, 35% vs. 1, 2.86%) rather than suction curettage, more blood loss in surgery (median number 35 ml vs. 20 ml) and more hospitalization days (median number 7.5 d vs. 3.5 d) than control group.
Conclusions
Lacunar-like change of chorion can be detected in early gestation and may act as a predictor of complicated and worse clinical outcome.
Introduction
Cesarean scar pregnancy (CSP) is an ectopic pregnancy that occurs at the scar site of a cesarean section in which the placental villi gradually invade the myometrium near the scar [Citation1]. There are differences in the pregnancy outcomes of CSP cases. In some cases, pregnancy can last to the third trimester, with placenta accreta; while in other cases, placenta percreta could occur in the first or second trimester, with uterine rupture and massive bleeding which threaten the lives of the gravidas [Citation2,Citation3]. Studies have indicated that there are correlations between sonographic features and CSP outcomes, including the growth direction of the gestational sac [Citation4] (toward-uterine-serosa vs. toward-uterine-cavity), relationship between the gestational sac and scar (on-the-scar vs. in-the-niche) [Citation5] and ultrasound spectral Doppler parameters (peak systolic velocity (PSV) and resistance index (RI)) [Citation6,Citation7]. Abnormal lacunar-like change in the placenta is an ultrasound sign characterized as presence of numerous lacunae including some that are large and irregular, often containing turbulent flow visible on grayscale imaging [Citation8]. It can also be called “Swiss cheese” sign. Many studies include it as an ultrasound sign of placental accreta in late pregnancy. Only a few studies have addressed it during early pregnancy, which were mainly case reports [Citation9–11]. In our observation, “lacunar-like changes” could be detected in part of CSP cases in early gestation (). As lacunar-like changes represent dilated blood sinuses which is an important placenta accreta sign in late gestation, is it related to massive bleeding from surgical abortions of early pregnancy? This study attempts to compare a case group with this sign to a control group without this sign of CSP cases, with the aim of providing new imaging evidence for risk evaluation and clinical decision making of CSP.
Figure 1. Thirty-two-year-old patient, G2P1, 62 days gestation, no lower abdominal pain or vaginal bleeding. Blood β-HCG 114,384 IU/ml. Ultrasound examination showed an elongated gestational sac of which the lower extremity located at the cesarean section scar (anterior lower segment) with scattered anechoic foci within chorion.
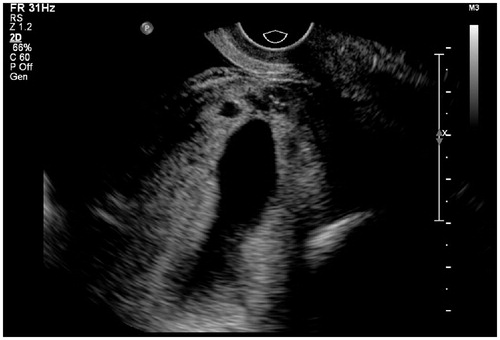
Figure 2. Thirty-seven-year-old patient, G3P1, 52 days gestation. She had suffered from vaginal bleeding for 12 days without lower abdominal pain. Blood β-HCG level 77,198 IU/ml. Ultrasound showed a gestational sac totally located in the lower segment cavity and protruded from uterus contour. Calipers located at the outer edge of gestational sac. CX: cervix; F: uterine cavity fluid.
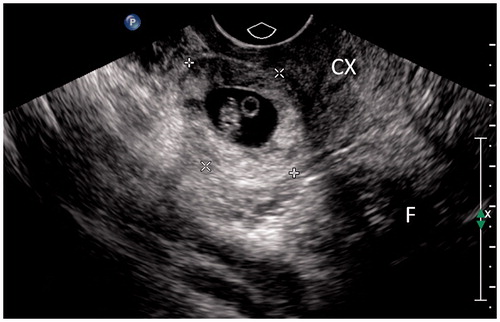
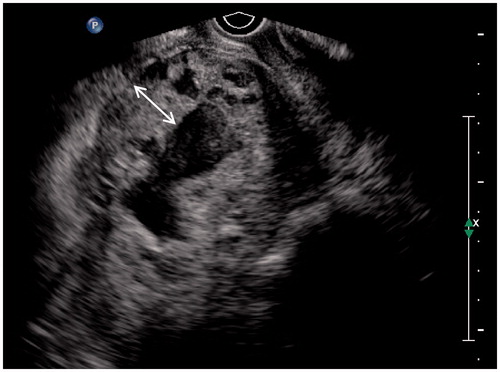
Figure 3. Thirty-nine-year-old patient, G4P1, 75 days gestation. She complained vaginal bleeding for a few days. Blood β-HCG 230 IU/ml. Ultrasound detected a thickened wall gestational mass within lower uterine segment. Multiple anechoic foci of various sizes with hypo-echoic dots could be seen within the thickened wall. The double-head arrow pointed out the thickness measurement of chorion.
Methods
This study received approval from the Institutional Review Board (IRB) of our hospital.
Entry and exclusion criteria
This was a retrospective review of patients who were hospitalized in the gynecological ward of our hospital from January 2017 to April 2020, and were diagnosed with CSP and had chosen termination of pregnancy. The diagnostic criteria for CSP were as follows [Citation12]: (1) emptiness of the uterine cavity and cervical canal; (2) implantation of the gestational sac in the scar of the cesarean section at the lower anterior wall of the uterus; (3) thinning or disappearance of the muscular layer at the scar of the cesarean section at the lower anterior wall of the uterus; and (4) color Doppler ultrasonography showing nourishing blood flow signals at the implantation site of the gestational sac. The exclusion criteria were as follows: the gestational sac was located in the lower part of the uterine cavity, but the mainly blood supplement come from posterior myometrium; inevitable abortion; the patient had received methotrexate (MTX) chemotherapy, suction curettage, or uterine artery embolization (UAE) before our ultrasound examination.
Ultrasound examination and data collection
All cases were examined by transvaginal ultrasound (combined with trans-abdominal ultrasound if necessary) using a diagnostic ultrasound system iU22 (Phillips Healthcare, Bothell, WA) or Voluson E8 Expert (GE Healthcare, Waukesha, WI). The transvaginal ultrasound probe frequency was 5–9 MHz, and the trans-abdominal ultrasound probe frequency was 3.5–5 MHz. Dynamic and static images were saved in computer readable form.
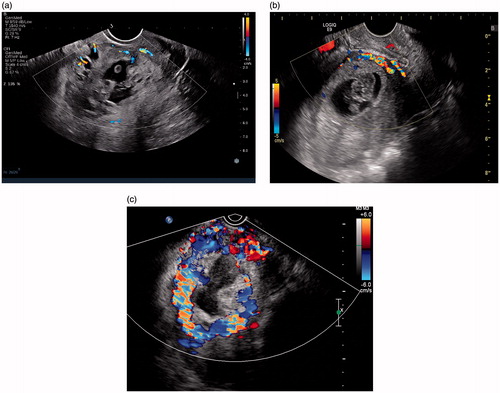
On ultrasound imaging, the maximum outer diameter of the gestational sac (), the maximum thickness of the chorionic membrane (), the length of implanting part of gestational mass in the lower uterine segment (), and the minimum thickness of the myometrium at the lower anterior wall of the uterus () were measured. When measuring the minimum myometrium thickness, we first observed whether the hypoechoic myometrial layer, excluding uterine serosa, disappeared or was indistinguishable. If so, the thickness was recorded as "0"; if the hypoechoic myometrial layer could be detected, then the minimum thickness of this layer was measured. What else, the serosa protrusion of the uterine lower segment was recorded. The detection of the embryo/fetus and the fetal heart beat were recorded; the crown-rump length of the embryo/fetus was measured. The level of blood signal on color Doppler flow imaging (CDFI) of uterine lower segment were divided into three grades (): CDFI grade 1, very little blood signal; CDFI grade 2, moderate blood signal; CDFI grade 3, rich blood signal. PSV and RI value of the trophic vessel in the anterior lower uterus were recorded.
Figure 4. Thirty-six-year-old patient, G4P1, 50 days gestation, vaginal bleeding (+). Blood β-HCG 168,136 IU/ml. Ultrasound showed an elongated gestational sac with lower extremity implanted in the uterine lower segment (between calipers). There was no lacunar-like change of chorion membrane.
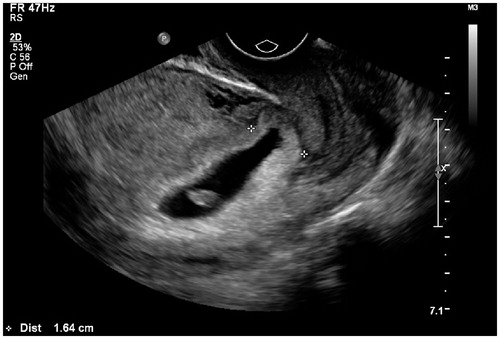
Figure 5. Measurement of minimum thickness of uterine lower segment myometrium: the hypoechoic myometrial layer (double-headed arrow) between the outer edge of the chorion (lower dotted line) and the inner edge of the uterine serosa (upper dotted line) was taken for measurement.
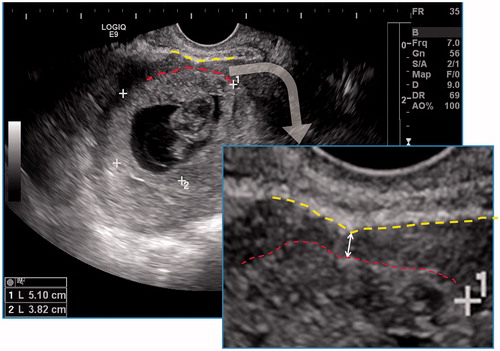
Figure 6. Grading of the blood signal on color Doppler flow imaging (CDFI) of uterine lower segment: (a) CDFI grade 1, very little blood signal; (b) CDFI grade 2, moderate blood signal; (c) CDFI grade 3, rich blood signal (from the same patient of ).
Clinically, the patients’ age, gravid and parity number, cesarean section number, uterine cavity curettage number (including cesarean section and surgical evacuation), gestational days, and clinical symptoms (including lower abdominal pain and vaginal bleeding) were recorded. Blood β-HCG values close to the date of ultrasound examination were recorded. The clinical doctors planed the treatment according to the patients’ clinical features and radiology reports (including ultrasound in all patients and MRI in some patients). Treatment methods mainly included the following three methods: suction curettage under ultrasound guidance or laparoscopic surveillance; scar pregnancy lesion resection by laparoscopy or open surgery; MTX treatment through muscle injection. UAE was an assistant method for reducing surgical bleeding and the usage of it was recorded. The volume of blood loss during surgical treatment, the numbers of hospitalizations, and the total number of hospitalization days were recorded.
Grouping method
Lacunar-like changes were multiple anechoic foci within chorionic membrane, locally in the uterine anterior lower segment part of chorion () or diffusely in the whole circle of chorion ( and ). Sometimes hypo-echoic spots flowing could be observed. Cases were divided into case and control group () according to detection of lacunar-like change or not. All the above clinical and ultrasound parameters were compared between case and control group.
Data analysis and statistics
Statistical analysis was performed using SPSS version 26.0 (SPSS Inc., Chicago, IL). Mainly used statistical modality in this study included independent-samples Kolmogorov–Smirnov test, independent-samples Mann–Whitney’s U test, t-test, and Chi-square (Pearson’s Chi-square and continuity correction Chi-square). Statistical significance was set at p< .05. All p values were two-tailed.
Results
Altogether 55 cases of CSP were enrolled in the study, including 20 (36.4%) and 35 (63.6%) patients in case and control group, respectively. The parameters of clinical manifestation and laboratory test of the two groups are shown in . There was no significant difference between them (p> .05).
Table 1. Comparison of clinical manifestations and laboratory test of cesarean scar pregnancy (CSP) patients in early pregnancy.
The ultrasound features are listed in . The maximum outer diameter of the gestational sac (5.6 ± 2.5 cm vs. 3.9 ± 1.5 cm), the maximum thickness of the chorion (1.05 cm vs. 0.70 cm), the longitudinal diameter of the implanting part of gestational mass in the lower segment (3.3 ± 1.8 cm vs. 1.2 ± 0.5 cm), the lower segment protrusion incidence (12, 60% vs. 2, 5.71%), and the crown-rump length of fetus (1.70 cm vs. 0.70 cm) were bigger in case group than that of the control group; the minimum thickness of the uterine lower myometrium was significantly thinner in case group (0.08 cm vs. 0.20 cm). On CDFI grading, the distribution of case group was different from control group with more cases in higher grades. There was no significant difference between the two groups in other ultrasound parameters.
Table 2. Comparison of sonographic features of cesarean scar pregnancy (CSP) patients in early gestation.
In terms of clinical outcomes listed in , all patients received successful termination of pregnancy in early gestation with fertility preservation. UAE usage ratios were nearly the same. Lesion resection through laparoscopy or open surgery cases were more in case group than control group (7, 35% vs. 1, 2.86%). The surgical bleeding volume were more in case group (35 ml vs. 20 ml). Among the case group, several patients experienced complex medical treatment. Two of them had more surgery bleeding (800 ml and 500 ml, respectively) than usual level of CSP, although UAE were used before surgery. Another patient experienced heavy vaginal bleeding for one month after uterine curettage with UAE and had to receive a second UAE and an open surgery to remove the residual gestational lesion. As for still another patient, the surgical program was converted from uterine curettage to open surgery under emergency circumstance. The total hospitalization days of case group was more than control group (7.5 d vs. 3.5 d).
Table 3. Comparison of treatment methods and hospitalization of cesarean scar pregnancy (CSP) patients in early gestation termination.
Discussion
Ultrasonography is an important imaging method for screening and diagnosing
CSP. Different sonographic features may be correlated with different prognoses [Citation4–7] and also affect clinical decision-making [Citation4,Citation5,Citation7,Citation12–14]. References addressing lacunar-like changes in early pregnancy as an ultrasound feature related to prognosis are limited [Citation9–11], while it has been considered as a sign pointing to worse outcome. We made more observation on this sign. With collecting more cases, we conducted this case-control study. The results showed that there was a serial of differences in the sonographic features and clinical outcomes between the case and control groups. The case group had bigger outer diameter of gestational sac, maximum thickness of the chorionic membrane, the length of lower segment implantation and lower segment protrusion incidence; while the minimum myometrial thickness of uterine lower segment was thinner in case group. In pregnancy termination, the treatment methods of the case group were more complicated with 7 (35%) patients received laparoscopy or surgery lesion resection, compared to 1 (2.86%) patient in control group. The amount of surgical bleeding and the total number of hospitalization days were also higher. It should be taken care of that there was no significant difference in the clinical manifestations and blood β-HCG level between the two groups. These results of this study demonstrated that lacunar-like change of chorion in early gestation is an ultrasound sign pointing to more complicated clinical process and worse outcome of CSP in early gestation termination.
In our conception, lacunar-like change within chorion or placenta tissue is a kind of pathological blood sinus, the formation of which may be related to local ischemia and hypoxia. Previous studies have noted that the blood supply in the scar area of the cesarean section was reduced compared to the normal myometrium and cannot meet the nutritional needs of the villus [Citation15]. This will drive the villus to grow more invasively. On the other side, there are potential gaps in the decidual layer of cesarean scar site, which induce the villus to invade the underlying myometrium to obtain adequate nutrition [Citation16]. The direct contact of villus and myometrium causes the myometrial fibers and capillary damage and blood sinus formation.
In our observation, the lacunar-like change of chorion in early gestation ranges from case to case with various involvement ratio and anechoic foci size. In typical cases, lacunar-like changes showed multiple anechoic foci within the chorion of the gestational sac, accompanied by hypo-echoic spots flowing in these areas. In severe cases, the anechoic lacunae were larger in size and number, involving full- or semi-circle of the gestational sac, the villous tissue appeared fragile and thickened, myometrium disappeared, focal serosa protrusion and obviously increased blood flow signals around the chorion. In lighter cases, anechoic foci were only distributed in the uterine anterior lower segment, smaller in size and number. What should be emphasized is that this sign can grow more significant along with gestational age, so they need careful observation to be picked out in very early gestation (about 5–6 gestational weeks). In this period, thickened chorion with tiny anechoic foci scattered was characteristic for lacunar-like change in CSP which is similar on ultrasound to the gestational sac in tubal pregnancy. In the latter condition, it was also named as “Donut sign” because of the whole-circle thickened chorion. The two pathologies should share the same pathophysiology process.
Calì et al. [Citation17] retrospectively analyzed ultrasound features of abnormally invasive placenta (AIP) in 105 patients with termination of pregnancy in late pregnancy. They reported that at 6–9 weeks of gestation, ultrasound showed only one feature which was the low placental implantation site without any other AIP signs, including abnormal placental lacunae. But the majority of AIP signs appeared on 11–14 weeks and showed a high ratio without statistical differences with that of second and third trimester. In our study, we enrolled the patients of early gestation, covering 5–12 week gestation. We can observe lacunar-like changes within chorion in very early gestation (or early first trimester). The results of Calì et al.’s and ours seem to be different. But it could be speculated that the more early the onset of AIP sign, the worse the clinical outcome. In some extremely severe cases, the gestation could not last to late gestation as high risk of uterus rupture and need to be terminated in first or second trimester to preserve patient’s fertility. Hence, the group of patients in Calì et al.’s study did not show lacunar-like change as observed in our group because the condition was not as serious as ours. In other words, the case with this sign in early pregnancy is more serious, and the risk of severe placental implantation or even uterine rupture in early or middle gestation is even greater, so the pregnancy should be terminated immediately at its diagnosis.
At present, the regression analysis of parameters related to the clinical prognosis of CSP does not include the lacunar-like change proposed in this study [Citation6,Citation7]. It may be that this sign has not attracted the attention of ultrasonographers. In our experience, this sign is more likely to be neglected in very early of the first trimester. Monitoring of extensive chorionic thickening with multiple tiny hypoechoic or anechoic foci in the chorion in lower gain in the ultrasound scan can help in the detection of this sign. On the other side, the over-diagnosis of CSP is another possible reason. Hence, we emphasize the ultrasound differential diagnosis, including other lower pregnancies, cervical pregnancies and inevitable abortions. The diagnosis of scar pregnancy should adhere to strict criteria, and over-diagnosis should be avoided [Citation18,Citation19]. The hemorrhage under the chorionic plate is another pathology need to be differentiated. Under such condition, there were multiple an-echoic foci between the chorion and the myometrium, which is different from the lacunar-like change within the chorion.
The shortcomings of this study include the limited sample size and lack of comparative study between ultrasound and other imaging modality. As a retrospective analysis, the research content is subject to observer bias. Moreover, we observed that although all of the cases with large amount of surgical bleeding and complicated treatment process came from the case group, the prognosis within the case group was quite different. This may be related to the severity of lacunar-like changes mentioned above, but may also be related to a variety of factors including the gestational age at the time of termination of pregnancy, which needs further study.
In conclusion, lacunar-like changes in chorion during early pregnancy are characteristic sonographic sign, which share the same pathophysiology of placenta accreta in second-third gestation. The pregnancy termination of these patients maybe more complicated, with more surgical bleeding and hospitalization days. Therefore, ultrasonographers and clinicians should be aware of this sign and make medical plan more carefully.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Liu DM, Yang M, Wu QQ. Application of ultrasonography in the diagnosis and treatment of cesarean scar pregnancy. Clin Chim Acta. 2018;486:291–297.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Calì G, Timor-Tritsch IE, Palacios-Jaraquemada J, et al. Outcome of cesarean scar pregnancy managed expectantly: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(2):169–175.
- Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6):592–593.
- Kaelin Agten A, Calì G, Monteagudo A, et al. The clinical outcome of cesarean scar pregnancies implanted “on the scar” versus “in the niche”. Am J Obstet Gynecol. 2017;216(5):510.e1–510.e6.
- Gui T, Peng P, Liu X, et al. Clinical and ultrasound parameters in prediction of excessive hemorrhage during management of cesarean scar pregnancy. Ther Clin Risk Manag. 2017;13:807–812.
- Wang Q, Ma H, Peng H, et al. Risk factors for intra-operative hemorrhage and bleeding risk scoring system for cesarean scar pregnancy: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2015;195:141–145.
- Collins SL, Ashcroft A, Braun T, et al. European Working Group on Abnormally Invasive Placenta (EW-AIP). Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47(3):271–275.
- Chen YJ, Wang PH, Liu WM, et al. Placenta accreta diagnosed at 9 weeks' gestation. Ultrasound Obstet Gynecol. 2002;19(6):620–622.
- Sekiguchi A, Okuda N, Kawabata I, et al. Ultrasound detection of lacunae-like image of a cesarean scar pregnancy in the first trimester. J Nippon Med Sch. 2013;80(1):70–73.
- Zosmer N, Fuller J, Shaikh H, et al. Natural history of early first-trimester pregnancies implanted in cesarean scars. Ultrasound Obstet Gynecol. 2015;46(3):367–375.
- Timor-Tritsch IE, Monteagudo A, Santos R, et al. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Chen WL, Jin L. Successful treatment of endogenous cesarean scar pregnancies with transabdominal ultrasound-guided suction curettage alone. Eur J Obstet Gynecol Reprod Biol. 2014;183:20–22.
- Cheng LY, Wang CB, Chu LC, et al. Outcomes of primary surgical evacuation during the first trimester in different types of implantation in women with cesarean scar pregnancy. Fertil Steril. 2014;102(4):1085–1090.e2.
- Khong TY. The pathology of placenta accreta, a worldwide epidemic. J Clin Pathol. 2008;61(12):1243–1246.
- Godin PA, Bassil S, Donnez J. An ectopic pregnancy developing in a previous cesarean section scar. Fertil Steril. 1997;67(2):398–400.
- Calì G, Timor-Trisch IE, Palacios-Jaraquemada J, et al. Changes in ultrasonography indicators of abnormally invasive placenta during pregnancy. Int J Gynecol Obstet. 2018;140(3):319–325.
- Naji O, Wynants L, Smith A, et al. Does the presence of a cesarean section scar affect implantation site and early pregnancy outcome in women attending an early pregnancy assessment unit? Hum Reprod. 2013;28(6):1489–1496.
- Zhenzhen L, Zhimin S, Yao W, et al. The clinical and ultrasound-based comparison between cesarean scar pregnancy and other lower uterine segment pregnancies with a history of cesarean section. J Matern Fetal Neonat Med. 2020. doi:10.1080/14767058.2020.1743669
