Abstract
Background
Gestational diabetes mellitus (GDM) characterized by dysfunction in maintaining glucose homeostasis is recognized as the most common metabolic complication associated with pregnancy leading to adverse clinical outcomes for maternal and fetal health. Although previous analysis of the findings from randomized controlled trials (RCTs) support that regular physical activity reduces the incidence of GDM during pregnancy, less is known about the optimal timing of intervention with respect to trimester stage.
Objectives
To examine the interaction between both the timing and volume of supervised physical activity interventions on reducing the incidence of GDM during pregnancy.
Study design
Electronic databases including CINAHL, Embase, Medline and the Cochrane library were searched for records up to 29 September 2022. Eligibility criteria were RCTs including standard antenatal care + supervised physical activity intervention without dietary modification vs. those receiving standard antenatal care alone in women with no previous diagnosis of GDM, type 1 or type 2 diabetes mellitus.
Results
Of the 3411 records identified, 20 RCTs comprising 6732 participants were included. It was found that supervised physical activity interventions decreased GDM risk when started within the first trimester (RR: 0.57, 95% CI: 0.41–0.79; p = .001) and by accumulating >600 MET·min·wk−1 of exercise (RR: 0.77, 95% CI: 0.60–0.98; p = .03) compared with standard antenatal care alone. Women with a BMI ≤25 kg/m2 experienced the greatest risk reduction in GDM following supervised exercise training (RR: 0.51, 95% CI: 0.34–0.75; p = .001).
Conclusion
Supervised physical activity reduces the incidence of GDM during pregnancy. It is recommended that pregnant individuals achieve a minimum of 600 MET·min·wk−1 of physical activity during the first trimester in order to reduce their odds of developing GDM. Attaining a healthy pre-pregnancy BMI is also an important determinant for the prevention of GDM with exercise.
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance occurring immediately at the start of, or, during pregnancy [Citation1]. The normal physiological response to pregnancy is decreased insulin sensitivity enabling the fetus to receive an adequate glucose supply. This adaptation is usually compensated by an increased insulin production by the pancreas to maintain a normoglycemic pregnancy; however, endogenous insulin secretion is insufficient in some individuals leading to the development of GDM [Citation2].
The clinical significance of GDM is the threat it poses to maternal and fetal morbidity. Individuals with GDM are at greater risk of gestational hypertension, pre-eclampsia, polyhydramnios and cesarean delivery [Citation3]. The complications related to the infant include macrosomia, large for gestational age, pre-term delivery, neonatal hypoglycemia and admission to neonatal intensive care unit [Citation3]. Moreover, infants born by individuals with GDM are more likely to develop obesity, thereby increasing their risk of glucose intolerance and pre-diabetes during adolescence [Citation4]. There are also long-term health implications for the mother, as GDM increases the risk of developing type 2 diabetes mellitus after pregnancy by almost 10 times compared to individuals experiencing a normoglycemic pregnancy [Citation5].
Physical activity improves glucose homeostasis and insulin sensitivity mediated by the translocation of the GLUT-4 glucose transporter protein increasing muscle glucose uptake [Citation6]. Previous meta-analyses of randomized controlled trials (RCTs) have reported that regular exercise decreases the incidence of GDM [Citation7–11] with at least 600 MET·min·wk−1 of moderate-intensity physical activity recommended for women to achieve a clinically meaningful effect [Citation7]. This volume of exercise is consistent with the minimum 150 min·wk−1 advised by The American College of Obstetricians and Gynecologists (ACOG) [Citation12]. However, the timing of exercise with respect to trimester stage was not addressed from these previous reviews. A recent prospective study reported that women persistently active as defined by ACOG guidelines from preconception through second trimester displayed improved glucose metabolism with a “dose–response” relationship (in MET·min·wk−1) during early-to-mid second trimester [Citation13]. These data support the importance of physical activity intervention earlier into pregnancy although there was no significant association on GDM incidence [Citation13]. Therefore, the primary aim of this meta-analysis and systematic review was to examine the interaction between both the timing and volume of supervised exercise training in relation to GDM risk.
Materials and methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and the checklist was completed [Citation14].
Search strategy
Relevant articles were identified through literature searches in the following four electronic databases: the Cochrane Library, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase and Medline. No limits were applied for the publication date, so all available records up until 29 September 2022 were searched. The free-text search term was based on the PICO format (Population/Problem, Intervention, Control, Outcome) [Citation15]. Different combinations of keywords were used in each database search because of the varying search platforms (see the online supplement Figure 1 for general search strategy). Searches were also conducted in OpenGrey and the British Library database to look for relevant grey literature. Additionally, the reference lists of published studies and reviews were manually searched to obtain further eligible trials. Endnote X9.3.3 was used to manage the articles produced by the searches.
Inclusion and exclusion criteria
Studies were eligible for inclusion according to the following criteria [Citation1]: study design – randomized controlled trial [Citation2]; participants – pregnant individuals without contraindication to exercise and previous diagnosis of GDM, type 1 or type 2 diabetes mellitus at commencement [Citation3]; intervention – standard antenatal care + supervised physical activity intervention [Citation4]; control – individuals receiving standard antenatal care alone [Citation5]; outcome measure – GDM incidence measured as a primary or secondary outcome. All studies including a combined dietary intervention were excluded. Interventions were classified as supervised if they included at least one session per week which was supervised by an exercise professional. There were no restrictions imposed on the exercise type, intensity, frequency or duration of the physical activity intervention nor were there exclusions made based on differing GDM diagnostic criteria. We did not apply specific exclusions with respect to weight category to ensure that study participants represented the wider pregnant population. All identified papers were assessed by GB and reviewed by BB; any discrepancies were resolved by NK.
Data extraction
The data extracted from each paper was as follows [Citation1]: the first author’s last name [Citation2]; the publication year [Citation3]; the country in which the study took place [Citation4]; the study population size (n) [Citation5]; the participant characteristics (including age, body mass index and health parameters) [Citation6]; the gestation week that intervention started [Citation7]; the length of intervention [Citation8]; the intervention characteristics including the type, intensity, frequency and duration of sessions [Citation9]; the number of cases of GDM in the intervention and control group [Citation10]; the compliance to intervention, and [Citation11]; the GDM diagnostic criteria. Data extraction tables were created in Microsoft Word. Data was extracted by GB and reviewed by BB; any disputes were resolved by a third reviewer, NK.
Quality assessment
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework was used to assess the quality of evidence initially rated as high for RCTs then downgraded if there were concerns with risk of bias, indirectness, inconsistency, imprecision and publication bias reducing confidence in the observed effects.
All studies were screened for risk of bias following the Cochrane Handbook [Citation16] with potential sources of bias identified as selection bias (inadequate randomization), reporting bias (selective/incomplete outcome reporting), performance bias (compliance to the intervention), detection bias (flawed measurement of outcome) and attrition bias (high loss to follow-up). Performance bias was rated as “high” if women on average attended <70% of supervised exercise sessions throughout the intervention period. Attrition bias was rated as “high” if >10% of women were lost to follow-up and intention-to-treat analysis was not used. Overall risk of bias was considered “serious” if studies displaying “high” risk of bias had a summed weighting which contributed >50% to the pooled estimate.
Indirectness was rated as “non-serious” for all included RCTs given we excluded previous studies investigating the effect of exercise + co-interventions on the odds of GDM. Inconsistency was considered “serious” when heterogeneity was high (i.e. I2 > 50%). Imprecision was considered “serious” when the 95% CI crossed the line of no effect, and was wide, such that interpretation of the data would be different if the true effect were at one end of the CI or the other. Publication bias was investigated through visual inspection of funnel plots and the Classic fail-safe N method. The latter calculates the number of studies required to nullify the significance of the effect size in the meta-analysis, where a low number of studies indicated a “serious” risk of publication bias.
Statistical analysis
Comprehensive Meta-analysis (CMA) software (version 3) was used to perform the statistical analysis. Risk ratios and their 95% confidence interval (CI) were calculated for each RCT with this data combined using a random-effects model to determine the pooled effect size of standard antenatal care + supervised physical activity interventions vs. standard antenatal care alone on GDM incidence. The level for statistical significance was set at p < .05. The presence of heterogeneity was assessed using the I2 statistic, which determines the percentage of total variation across the studies due to heterogeneity rather than chance. It scores heterogeneity between 0% and 100%, with 25%, 50%, and 75% representing low, moderate and high heterogeneity, respectively [Citation17]. This analysis was undertaken separately by identifying a priori subcategories of RCT according to the timing of intervention, weekly exercise training volume, and weight status of study participants. Two categories of RCT were identified based on the timing of intervention (i.e. started within the first trimester vs. started after the first trimester). The first trimester is defined from weeks 1 to 13 of gestation [Citation18]. Most authors provided their start date in the form of a range (e.g. gestational week 6–9) in which cases the median value was used to assign each study a start date commencing either within the first trimester or started after the first trimester. We estimated weekly exercise training volume from details provided by the authors’ on exercise type, intensity, frequency and duration of their intervention. The metabolic equivalents (METs) for each intervention were calculated from the compendium of physical activities [Citation19], based on the type of exercise prescribed. This value was multiplied by the duration (min) and frequency (days·wk−1) of training sessions to estimate total training volume in MET·min·wk−1. Interventions were then grouped into two categories (i.e. <600 MET·min·wk−1 vs. >600 MET·min·wk−1) corresponding to the minimum advised by ACOG of at least 150 min·wk−1 of moderate-intensity physical activity per week [Citation12]. For weight status, included studies were categorized into individuals with low and high pre-pregnancy BMI (i.e. ≤25 kg/m2 vs. >25 kg/m2).
Results
Study selection
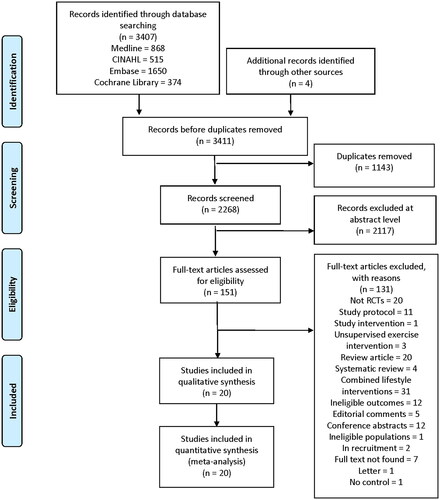
A PRISMA diagram of the study selection process, including reasons for exclusion, is shown in . The initial search identified 3407 records. Additionally, four articles were identified from other sources. Following the removal of duplicates, 2268 articles were screened, and 2117 were excluded based on the title and abstract. The full text of the remaining 151 articles were reviewed for eligibility. Reasons were stated for the exclusion of articles after review of the full text. Finally, 20 articles met the inclusion criteria and were therefore included in the analysis.
Study characteristics
The characteristics of RCTs included in this meta-analysis are summarized in the online supplement Table 1 The physical activity programs varied between studies with respect to exercise type, intensity, frequency and duration with differences in the start date (timing) of the interventions. In the control groups, individuals received standard antenatal care, and they did not participate in any structured physical activity program.
Measurement of study outcomes
All studies included in this meta-analysis recorded the incidence of GDM in their intervention and control group. However, different criteria were used to diagnose GDM in the participants. For example, the WHO 1999 criteria [Citation20], the WHO 2009 criteria [Citation21], and the WHO 2014 criteria [Citation22] were each utilized. Also, Barakat et al. [Citation23] and Daly et al. [Citation24] used the International Association of Diabetes and Pregnancy Study Groups (IADPSG) 2010 criteria, and Pelaez et al. [Citation25] used the Spanish Group of Diabetes and Pregnancy (GEDE) 2006 criteria. The National Diabetes Data Group criteria were used by Cordero et al. [Citation26], and the American Diabetes Association (ADA) criteria were used by Barakat et al. [Citation27] and Price, Amini and Kappeler [Citation28]. The remaining 11 studies did not report the diagnostic criteria they used [Citation29–39].
Study quality assessment
The quality of evidence was rated as “low” across all 20 included RCTs and ranged from “low” to “moderate” from analyzing sub-categories of study (see online supplement Table 2). The most common reason(s) for downgrading the quality of evidence was “serious” risk of bias attributable to high attrition rate and/or relatively low compliance to the intervention. Other reasons for downgrading the quality of evidence included imprecision and inconsistency.
Synthesis of results
From the 20 studies included in this meta-analysis, 3320 individuals received standard antenatal care + supervised physical activity intervention during pregnancy, and 3512 individuals received standard antenatal care alone. Out of those receiving the physical activity intervention, 227 individuals developed GDM, and 346 individuals from the control groups developed GDM. There was a significant 38% decreased GDM risk among individuals randomized to the physical activity interventions (RR = 0.62; 95% CI 0.46–0.82; p = .002; I2 = 62%) with this effect dependent on the subcategory of study analyzed.
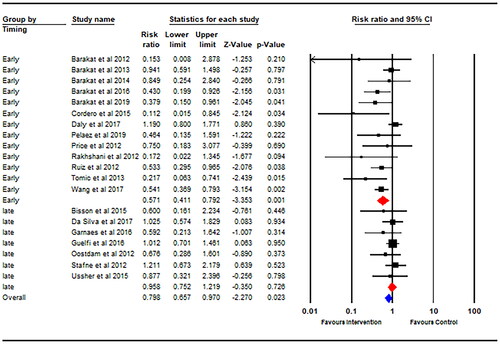
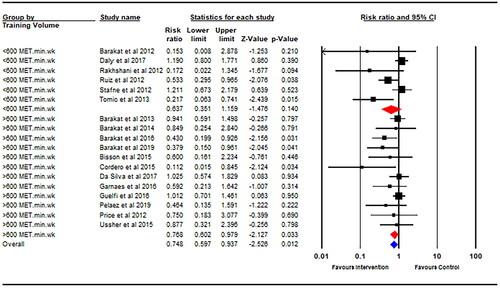
Thirteen studies started their physical activity intervention within the first trimester of pregnancy, and seven studies started their intervention after the first trimester. There was a significantly decreased GDM risk for physical activity interventions started earlier into pregnancy (within the first trimester) (RR = 0.57; 95% CI 0.41–0.79; p = .001; I2 = 50%; ) with no statistically significant risk reduction for physical activity interventions starting later during pregnancy (after the first trimester) (RR = 0.96; 95% CI 0.75–1.22; p = .73; I2 = 0%). Data on weekly exercise training volume (MET·min·wk−1) was available for eighteen studies. There was a significantly decreased GDM risk for interventions with an estimated weekly training volume of >600 MET·min·wk−1 (RR = 0.77; 95% CI 0.60–0.98; p = .03; I2 = 13%; ), whereas an estimated weekly training volume of <600 MET·min·wk−1 did not promote a significant risk reduction (RR = 0.64; 95% CI 0.35–1.16; p = .14; I2 = 66%). There was a significant 49% decreased GDM risk following physical activity intervention in individuals with a mean BMI of ≤25 kg/m2 at baseline (RR = 0.51; 95% CI 0.34–0.75; p = .001; I2 = 33%). There was no significant difference in GDM incidence between the intervention and control group observed in the studies including individuals with a mean BMI >25 kg/m2 (RR = 0.86; 95% CI 0.68–1.08; p = .20; I2 = 29%).
Discussion
The novel, original finding from this meta-analysis and systematic review was that effects from supervised physical activity interventions on decreasing GDM risk were dependent on exercise commencing earlier into pregnancy (i.e. within the first trimester) together with accumulating a minimum amount of exercise (i.e. 600 MET·min·wk−1). These data strengthen support for encouraging healthy women to engage in at least 150 min of moderate-intensity physical activity per week starting between weeks 1 and 13 of gestation. A lower pre-pregnancy BMI was also identified as an additional factor predicting a decreased incidence of GDM following supervised exercise training.
The studies’ pooled estimate found that physical activity was associated with a 38% decreased risk of developing GDM. These results are consistent with previous meta-analyses reporting a decreased GDM risk in pregnant individuals randomized to the physical activity interventions [Citation7,Citation8,Citation10]. Doi et al. [Citation8] found that undertaking a physical activity intervention elicited an overall 31% decreased risk of developing GDM from an analysis of 11 RCTs consisting of 1467 pregnant individuals. This corresponded to 18 participants requiring treatment with physical activity (compared to standard care) to prevent one case of GDM. Russo et al. [Citation10] reported a similar 28% decreased risk of developing GDM in their summary estimate from 10 RCTs. Davenport et al. [Citation7] also reported a 38% reduction in the odds of developing GDM in individuals receiving prenatal exercise following an analysis of 26 RCTs.
The results from this meta-analysis demonstrated that physical activity interventions started earlier in pregnancy augmented the risk reduction for developing GDM, from 38% for the overall effect of the interventions to 43% for the interventions starting during the first trimester. A recent prospective study also reported that the timing of physical activity was important for achieving improved glucose metabolism [Citation13]. It found that women self-reporting higher levels of moderate-to-vigorous physical activity (MVPA) during early-to-mid second trimester displayed significant reductions in maternal glucose concentrations, whereas there was no association in the mid-to-late second trimester [Citation13]. However, this study was unable to find a significant association between maternal MVPA with the risk of GDM. The study design likely contributed to this null finding as the self-reported measures of maternal MVPA were likely higher than what was achieved by the participants. This limitation is overcome in the current meta-analysis by the supervised nature of the interventions. Initiating exercise in the first trimester may be crucial due to the placenta’s function already being predetermined by the end of the first trimester [Citation40]. This could be a contributing factor to why previous RCTs have failed to prevent GDM in their participants. Of the 124 individuals in the exercise intervention, only 16.8% started their exercise program before 14 weeks’ gestation in a RCT conducted by Nobles et al. [Citation41]. The UPBEAT study, a multi-centered RCT conducted in the UK by Poston et al. [Citation42], similarly recruited individuals in their second trimester between 15 and 18 weeks’ gestation. Both studies failed to reduce the incidence of GDM in their intervention groups.
ACOG recommend that pregnant individuals without contraindications to physical activity achieve at least 150 min of moderate-intensity exercise per week [Citation12] with a previous review concluding that 600 MET·min·wk−1 represented a minimum amount of exercise required to achieve a clinically meaningful (i.e. 25%) reduction in GDM risk [Citation7]. Accordingly, this value was used as a cutoff for defining sub-categories of RCT in relation to training volume, with a significant reduction in GDM risk dependent on interventions achieving >600 MET·min·wk−1 of exercise. These findings corroborate those previously reported by Davenport et al. [Citation7] by incorporating 5 new RCTs published after 2018 (2130 additional individuals), thus strengthening the observation.
The subgroup analysis undertaken in the present investigation in which trials were separated by the mean BMI of individuals (i.e. ≤25 kg/m2 or >25 kg/m2) at the commencement of the study, revealed an interesting result. The pooled effect of trials with a mean BMI ≤25 kg/m2 was greater with a significant 49% decreased risk of developing GDM for individuals randomized to the physical activity intervention, compared to a non-significant 14% reduction for those trials including individuals with a mean BMI >25 kg/m2. The tendency for trials including individuals classified as overweight or obese to report an attenuated effect from exercise training on decreasing GDM risk is consistent with the findings from a previous study [Citation7] and may be attributed to the likelihood of these individuals displaying a degree of insulin resistance before pregnancy [Citation43] which was undiagnosed at the commencement of the physical activity intervention. Therefore, this meta-analysis has demonstrated that physical activity alone may not provide a strong enough stimulus for overcoming the negative effects of having a high BMI on metabolic health during pregnancy.
Limitations
A feature of standard antenatal care in many countries is to provide written dietary and exercise advice during pregnancy. Therefore, in the absence of objectively measured physical activity (i.e. accelerometry) data, we cannot discard the possibility that effects from the addition of supervised exercise on the incidence of GDM may have been concealed due to women in the control group exercising throughout the intervention period. Moreover, there may exist disparities between trials in terms of defining “standard antenatal care” given differences in the spending on health care services between countries [Citation44]. These disparities in healthcare provision between trials could limit interpretation of the results as it may not be clear whether a significant finding from a trial is the result of a successful intervention or due to comparison against an ineffective standard of care [Citation45]. Additionally, the requirement for interventions to be supervised reduces ecological validity of these findings, given that supervised interventions are expensive, labor-intensive and require specialist professionals which are unlikely to be available in a clinical setting for the wider population. It should also be highlighted that the quality of evidence was downgraded for many RCTs in which high numbers of women were either lost to follow-up and/or reported low compliance (i.e. serious risk of bias) to interventions spanning 20–30 weeks, thereby potentially weakening the association supervised exercise and GDM risk. The gestational week that physical activity should be commenced was addressed in this review; however, consideration should also be given to the gestation weeks that physical activity spans. For example, the duration of the interventions ranges from 12 to 35 weeks, so some interventions may have started in the first trimester and ended in the second, whereas others continued until term. This information would help strengthen the recommendations made to pregnant individuals by healthcare providers.
Conclusion
In conclusion, women undertaking supervised exercise training started within their first trimester had decreased odds of developing GDM compared to non-active controls. Accumulating 600 MET·min·wk−1 of exercise together with having a healthy pre-pregnancy BMI (i.e. ≤25 kg/m2) represent other additional factors predicting lower GDM incidence following supervised exercise programs. It is recommended that future studies prioritize the development of behavioral and educational support for women in achieving at least 150 min·wk−1 of moderate-intensity physical activity earlier into pregnancy.
Supplemental Material
Download MS Word (29.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27(Supp. 1):S88–S90.
- Plows J, Stanley J, Baker P, et al. The pathophysiology of gestational diabetes mellitus. IJMS. 2018;19(11):3342.
- O’Sullivan EP, Avalos G, O’Reilly M, et al. Atlantic Diabetes in Pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia. 2011;54(7):1670–1675.
- Silverman BL, Rizzo TA, Cho NH, et al. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21(Supp. 2):142–149.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361.
- Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261.
- Davenport MH, Ruchat SM, Poitras VJ, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med. 2018;52(21):1367–1375.
- Doi SAR, Furuya-Kanamori L, Toft E, et al. Physical activity in pregnancy prevents gestational diabetes: a meta-analysis. Diabetes Res Clin Pract. 2020;168:108371.
- Ming WK, Ding W, Zhang CJP, et al. The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):440.
- Russo LM, Nobles C, Ertel KA, et al. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(3):576–582.
- Yu Y, Xie R, Shen C, et al. Effect of exercise during pregnancy to prevent gestational diabetes mellitus: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2018;31(12):1632–1637.
- Physical activity and exercise during pregnancy and the postpartum period: ACOG Committee Opinion, number 804. Obstet Gynecol. 2020;135(4):e178–e88.
- McDonald SM, May LE, Hinkle SN, et al. Maternal moderate-to-Vigorous physical activity before and during pregnancy and maternal glucose tolerance: does timing matter? Med Sci Sports Exerc. 2021;53(12):2520–2527.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
- The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. 2011.
- Ioannidis JPA. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14(5):951–957.
- American College of Obstetricians and Gynecologists. How your fetus grows during pregnancy [updated 2020 August]. Available from: https://www.acog.org/womens-health/faqs/how-your-fetus-grows-during-pregnancy#:∼:text=First%20trimester%20(first%20day%20of,of%20rapid%20growth%20and%20development.
- Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581.
- Stafne SN, Salvesen K, Romundstad PR, et al. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstet Gynecol. 2012;119(1):29–36.
- Garnaes KK, Mørkved S, Salvesen Ø, et al. Exercise training and weight gain in obese pregnant women: a randomized controlled trial (ETIP trial). PLoS Med. 2016;13(7):e1002079.
- Wang C, Wei Y, Zhang X, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol. 2017;216(4):340–351.
- Barakat R, Pelaez M, Lopez C, et al. Exercise during pregnancy and gestational diabetes-related adverse effects: a randomised controlled trial. Br J Sports Med. 2013;47(10):630–636.
- Daly N, Farren M, McKeating A, et al. A medically supervised pregnancy exercise intervention in obese women: a randomized controlled trial. Obstet Gynecol. 2017;130(5):1001–1010.
- Pelaez M, Gonzalez-Cerron S, Montejo R, et al. Protective effect of exercise in pregnant women including those who exceed weight gain recommendations: a randomized controlled trial. Mayo Clin Proc. 2019;94(10):1951–1959.
- Cordero Y, Mottola MF, Vargas J, et al. Exercise is associated with a reduction in gestational diabetes mellitus. Med Sci Sports Exerc. 2015;47(7):1328–1333.
- Barakat R, Cordero Y, Coteron J, et al. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: a randomised controlled trial. Br J Sports Med. 2012;46(9):656–661.
- Price BB, Amini SB, Kappeler K. Exercise in pregnancy: effect on fitness and obstetric outcomes-a randomized trial. Med Sci Sports Exerc. 2012;44(12):2263–2269.
- Barakat R, Refoyo I, Coteron J, et al. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial. Braz J Phys Ther. 2019;23(2):148–155.
- Barakat R, Pelaez M, Cordero Y, et al. Exercise during pregnancy protects against hypertension and macrosomia: randomized clinical trial. Am J Obstet Gynecol. 2016;214(5):649.e1–649.e8.
- Barakat R, Perales M, Bacchi M, et al. A program of exercise throughout pregnancy. Is it safe to mother and newborn? Am J Health Promot. 2014;29(1):2–8.
- Bisson M, Alméras N, Dufresne SS, et al. A 12-week exercise program for pregnant women with obesity to improve physical activity levels: an open randomised preliminary study. PLoS One. 2015;10(9):e0137742.
- da Silva SG, Hallal PC, Domingues MR, et al. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: results from the PAMELA study. Int J Behav Nutr Phys Act. 2017;14(1):175.
- Guelfi KJ, Ming Jing O, Crisp NA, et al. Regular exercise to prevent the recurrence of gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2016;128(4):819–827.
- Tomić V, Sporiš G, Tomić J, et al. The effect of maternal exercise during pregnancy on abnormal fetal growth. Croat Med J. 2013;54(4):362–368.
- Rakhshani A, Nagarathna R, Mhaskar R, et al. The effects of yoga in prevention of pregnancy complications in high-risk pregnancies: a randomized controlled trial. Prev Med. 2012;55(4):333–340.
- Oostdam N, van Poppel MNM, Wouters MGAJ, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG. 2012;119(9):1098–1107.
- Ruiz JR, Perales M, Pelaez M, et al. Supervised exercise-based intervention to prevent excessive gestational weight gain: a randomized controlled trial. Mayo Clin Proc. 2013;88(12):1388–1397.
- Ussher M, Lewis S, Aveyard P, et al. The London Exercise and Pregnant smokers (LEAP) trial: a randomised controlled trial of physical activity for smoking cessation in pregnancy with an economic evaluation. Health Technol Assess. 2015;19(84):1–135. https://www.journalslibrary.nihr.ac.uk/hta/hta19840/#/abstract.
- Catalano P, Demouzon SH. Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int J Obes (Lond). 2015;39(4):642–649.
- Nobles C, Marcus BH, Stanek EJ, et al. Effect of an exercise intervention on gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2015;125(5):1195–1204.
- Poston L, Bell R, Croker H, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(10):767–777.
- Kampmann U, Knorr S, Fuglsang J, et al. Determinants of maternal insulin resistance during pregnancy: an updated overview. J Diabetes Res. 2019;2019:5320156. https://www.hindawi.com/journals/jdr/2019/5320156/.
- Papanicolas I, Mossialos E, Gundersen A, et al. Performance of UK national health service compared with other high income countries: observational study. BMJ. 2019;367:l6326.
- Ayling K, Brierley S, Johnson B, et al. How standard is standard care? Exploring control group outcomes in behaviour change interventions for young people with type 1 diabetes. Psychol Health. 2015;30(1):85–103.