Abstract
Objective
The rate of preeclampsia with severe features has increased. Previous studies have shown elevated liver enzymes are an indicator of worsening hypertensive disease of pregnancy and adverse outcomes, therefore leading to their inclusion as a diagnostic criterion for severe features of preeclampsia. Despite this, there are limited data to support an aspartate aminotransferase (AST) or alanine aminotransferase (ALT) concentration ≥ two times the upper limit of normal as the critical point at which maternal harm from ongoing pregnancy exceeds neonatal harm from delivery. The objective of this study is to evaluate the association between elevated liver enzymes and maternal and neonatal outcomes among patients with preeclampsia with severe features.
Methods
Retrospective cohort study among hypertensive patients who delivered ≥23 weeks’ gestation at Oregon Health & Science University (October 2013–September 2018). Those with preeclampsia with severe features (including chronic hypertension with superimposed preeclampsia meeting criteria for severe features) were included after a screening of ICD-9 and ICD-10 codes and chart validation. The primary exposure was elevated liver enzymes prior to delivery, according to the American College of Obstetricians and Gynecologists’ criteria for severe features of preeclampsia: aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥2x the upper limit of normal (above threshold liver function tests [LFTs]). Primary outcomes included adverse maternal and neonatal outcomes. Differences were analyzed by Chi-squared, Fisher’s exact, t-test, and logistic regression, with α = 0.05.
Results
Of 11,825 deliveries, 319 (2.7%) met inclusion criteria and had preeclampsia with severe features. Of these, 44 (13.8%) had above threshold LFTs. Adverse maternal outcomes were no different in those with above threshold LFTs compared to those with below threshold LFTs. The unadjusted odds of an adverse neonatal outcome were 2.08 times greater in patients with above threshold LFTs (95% CI: 1.04–4.14), and 2.43 times greater when adjusting for maternal characteristics (95% CI: 1.17–5.04) compared to those with below threshold LFTs. However, the association between above threshold LFTs and adverse neonatal outcomes became non-significant after adjustment for gestational age at delivery (OR: 1.54, 95% CI: 0.63–3.76).
Conclusion
Among patients with preeclampsia with severe features, above threshold LFTs are not independently associated with an increased risk of adverse maternal or neonatal outcomes. Adverse neonatal outcomes in patients with preeclampsia with severe features and above threshold LFTs are driven by earlier gestational age at delivery. Prospective studies are needed to guide delivery timing in patients with preeclampsia and elevated liver enzymes.
Brief rationale
The criteria for elevated liver function tests (greater than two times the upper limit of normal) are widely accepted among obstetricians to diagnose a severe feature of preeclampsia. However, these criteria are based on expert opinion and extrapolated from data on patients with HELLP syndrome. Since preterm delivery of the neonate is recommended for preeclampsia with severe features, the threshold used to define severe liver enzyme elevation has a direct impact on neonatal outcomes. Therefore, the goal of our study was to determine if patients with preeclampsia with severe features and a pre-delivery AST or ALT level ≥ two times the upper limit of normal have worse maternal and neonatal outcomes compared to those with an AST and ALT below this level.
Introduction
Preeclampsia with severe features contributes disproportionately to maternal morbidity and mortality worldwide [Citation1,Citation2]. The rate of severe preeclampsia has increased more than 3-fold in the past several decades [Citation3]. In the setting of hypertension or preeclampsia and when a severe feature is present, including elevated liver enzymes (greater than or equal to two times the upper limit of normal), the American College of Obstetricians and Gynecologists (ACOG) recommends premature delivery of the fetus due to concern for end-organ injury and maternal harm.
Prior studies have demonstrated that elevated liver enzymes are an indicator of worsening hypertensive disease of pregnancy and adverse outcomes, which has led to their inclusion as a diagnostic criterion for severe features of preeclampsia [Citation4–7]. Elevated liver enzymes have also been reported in hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome, but the liver enzyme values that define HELLP syndrome have varied and are based on expert opinion [Citation8–11]. There are limited data to suggest an aspartate aminotransferase (AST) or alanine aminotransferase (ALT) concentration ≥ two times the upper limit of normal is the critical point at which maternal harm from ongoing pregnancy exceeds neonatal harm from delivery.
Therefore, we sought to evaluate the association between abnormally elevated liver enzymes and maternal and neonatal outcomes among patients with preeclampsia with severe features. We hypothesized that patients with a pre-delivery AST or ALT level ≥ two times the upper limit of normal would have worse maternal and neonatal outcomes compared to those with an AST and ALT below this level.
Materials and methods
A retrospective cohort study was conducted for all deliveries to patients with a hypertensive disorder of pregnancy at Oregon Health & Science University (OHSU) from October 2013 through September 2018. This study cohort was started following publication of the 2013 ACOG Executive Summary on Hypertension in Pregnancy, which updated the diagnostic criteria for preeclampsia [Citation12].
Patients were included in the study database if delivery occurred ≥23 weeks’ gestation and if they were diagnosed with a hypertensive disorder of pregnancy prenatally, which included gestational hypertension, preeclampsia without severe features, preeclampsia with severe features, chronic hypertension, chronic hypertension with superimposed preeclampsia, and HELLP syndrome. Patients were initially screened for a hypertensive disorder of pregnancy via ICD-9 and ICD-10 hospital discharge codes following delivery (Supplementary Appendix A). Diagnoses were then confirmed by individual chart review by two physicians, with disagreements adjudicated by a third physician (RMB). Therefore, misclassification bias in regard to the diagnosis of severe preeclampsia was likely very low after individual chart review and validation. Data abstracted from patients’ charts included demographic information, past obstetrical and medical history, laboratory findings during admission for delivery, management of the hypertensive disorder of pregnancy, and maternal and neonatal outcomes. Data were abstracted and maintained utilizing REDCap (Research Electronic Data Capture). Information bias was minimized by training the researchers prior to data abstraction and random chart audits.
In this analysis, only those with preeclampsia with severe features (including HELLP syndrome) or chronic hypertension with superimposed preeclampsia (who met criteria for preeclampsia with severe features) were included.
Patients were then stratified into two groups based on the primary study exposure of elevated liver enzymes greater than or equal to two times the upper limit of normal, in accordance with ACOG’s most updated diagnostic criteria for severe features of preeclampsia [Citation4]. Liver enzyme tests were drawn as part of routine hospital care prior to delivery. Based on the reference range for normal AST and ALT concentrations at our institution (OHSU), an AST level ≥82 U/L and an ALT level ≥120 U/L were considered greater than or equal to two times the upper limit of normal (referred to as “above threshold liver function tests [LFTs]”). Those with an AST level <82 U/L and ALT level <120 U/L were referred to as the “below threshold LFT” group. Although some patients who were admitted to the hospital long-term had multiple labs drawn before delivery, only the peak AST and peak ALT values prior to delivery were included in the study database. No AST or ALT values prior to admission for delivery were included. Patients with cholestasis of pregnancy, multifetal pregnancy, hepatitis C or B, chronic kidney disease, or missing pre-delivery AST and ALT laboratory data were excluded from the analysis. Information about other liver diseases was not specifically extracted as part of the data analysis. Selection bias was assessed by comparing baseline maternal characteristics of patients in the final analytic sample to those with missing LFT laboratory data pre-delivery (Supplementary Appendix B).
The primary outcomes included a composite adverse maternal and composite adverse neonatal outcome. The composite adverse maternal outcome included: blood transfusion, eclampsia, intensive care unit (ICU) admission, liver infarction/rupture, stroke, oliguria (<500 ml urine in 24 h), vision loss (i.e. complete loss of vision in one or both eyes), cardiomyopathy or placental abruption. Of note, imaging studies to evaluate for liver infarction/rupture were performed only in subjects with high clinical suspicion for this adverse outcome. The composite adverse neonatal outcome included: neonatal intensive care unit (NICU) admission, neonatal intubation, respiratory distress syndrome, very low birth weight less than 1500 g, 5-min Apgar score <7, arterial cord pH <7, stillbirth, neonatal demise, neonatal seizure, bronchopulmonary dysplasia, necrotizing enterocolitis, intraventricular hemorrhage, hypoxic-ischemic encephalopathy, retinopathy of prematurity, and neonatal sepsis.
Demographic data included maternal age, pre-pregnancy body mass index (BMI at first prenatal visit), nulliparity, diabetes mellitus (Type 1 or Type 2), gestational diabetes mellitus, race (non-Hispanic white, Hispanic, non-Hispanic Black, Asian or other [American Indian/Alaska Native, Native Hawaiian or other Pacific Islander, more than one race or other]), insurance type (private [individual or group] vs. public [Medicaid or Medicare]), substance use (tobacco, alcohol, marijuana, opioids, and amphetamines individually categorized as current or quit during pregnancy vs. quit prior to or for pregnancy, or never used), and other severe features of preeclampsia (severe range systolic or diastolic blood pressure [≥160/110 mmHg], platelets <100,000 × 109/L, serum creatinine >1.1 mg/dL, severe right upper quadrant pain, pulmonary edema, severe headache, or visual disturbances pre-delivery). The definition of severe headache or visual disturbances was based on the ACOG definition of “new-onset cerebral or visual disturbances” described in the Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy [Citation12]. Given all data were abstracted via individual chart review, severe features of preeclampsia were assigned as was documented by the clinician in the medical record. If a subject was not documented as having right upper quadrant pain, a headache, or visual disturbances, then the subject was registered as “no” for this severe feature.
Differences in clinical variables and laboratory values between groups were assessed by t-test for comparison of means and Chi-squared or Fisher’s exact test for categorical data. We utilized univariable and multivariable logistic regression to further evaluate the association between liver enzymes and adverse maternal and neonatal outcomes. During multivariable logistic regression, we adjusted for confounders determined a priori or that were found to be different during univariable analyses (p < .05). Results were reported as an unadjusted odds ratio (OR) or adjusted odds ratio (aOR), with a 95% confidence interval (CI). Significance was determined by α = 0.05 for the primary outcomes. Our study was powered (α = 0.05, β = 0.80) to detect a 100% relative increase in adverse maternal outcomes and 50% relative increase in adverse neonatal outcomes, assuming baseline rates of 20% and 50% for adverse maternal and neonatal outcomes, respectively. All analyses were performed using Stata/IC 15.1 software. OHSU Institutional Review Board approval was received prior to study initiation and informed consent was waived.
Results
Of the 11,825 deliveries from October 2013 through September 2018 at our institution, 1616 (13.7%) were to patients diagnosed with a hypertensive disorder of pregnancy. Among this cohort, 434 (26.9%) were diagnosed with a severe form of preeclampsia prenatally, either preeclampsia with severe features (n = 283) or chronic hypertension with superimposed preeclampsia meeting criteria for preeclampsia with severe features (n = 151). Of this sample, 18 (4.1%) were diagnosed with cholestasis of pregnancy, 24 (5.5%) had a multifetal pregnancy, 5 (1.2%) had hepatitis C, none had hepatitis B, 14 (3.2%) had chronic kidney disease, and 53 (12.2%) were missing pre-delivery AST and ALT laboratory data and were excluded, with a remaining final analytic sample of 319 patients (2.7% of all deliveries; ). Among the final sample, 206 (64.6%) had a diagnosis of preeclampsia with severe features and 113 (35.4%) had a diagnosis of chronic hypertension with superimposed preeclampsia with severe features.
Figure 1. Flow diagram for study inclusion.
LFTs, liver function tests.
aExcluded patients with chronic hypertension with superimposed preeclampsia who did not meet criteria for preeclampsia with severe features.
bBased on OHSU lab values, where an AST ≥82 U/L and an ALT ≥120 U/L are considered two times the upper limit of normal or above threshold LFTs.
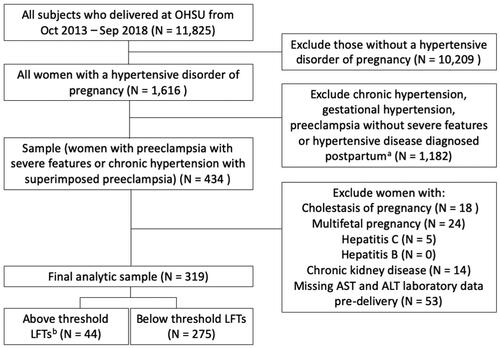
Within the final cohort, 44 (13.8%) of patients had an AST and/or ALT level ≥ two times the upper limit of normal (above threshold LFTs). Less than 2.5% of patients were missing data for maternal characteristics except for 21.9% with missing data for gestational diabetes mellitus, 6.0% for alcohol, 17.9% for marijuana, 18.5% for opioid, and 18.2% for amphetamine use. There were no missing data for the outcome variables.
Maternal characteristics are presented in Supplementary Appendix C, stratified by liver enzymes pre-delivery. Baseline characteristics were similar between the two groups except for mean gestational age at delivery, which was significantly lower for the above threshold LFT group (34 vs. 36 weeks, p = .032). The median gestational age at delivery for the above threshold LFT group was 34 weeks (range: 24–40 weeks) compared to 36 weeks for the below threshold LFT group (range 24–41 weeks). presents all univariable analyses for both adverse maternal and neonatal outcomes. There was no statistically significant association between above threshold LFTs and the maternal composite of adverse outcomes (20% vs. 15%, p = .38). Conversely, the neonatal composite of adverse outcomes was more common in the above threshold LFT group compared to the below threshold LFT group (70% vs. 53%, p = .035). An analysis of pre-delivery severe features between the above threshold LFT and the below threshold LFT groups found severe systolic blood pressure was less common among the above threshold LFT group compared to the below threshold group (Supplementary Appendix D; 64% vs. 79%, p = .030). Conversely, severe right upper quadrant pain was more common among the above threshold LFT group compared to the below threshold group (41% vs. 12%, p < .001). Given right upper quadrant pain is associated with abnormal LFTs and likely in the causal pathway between elevated LFTs and adverse outcomes, we did not adjust for this in subsequent multivariable regression analyses. On the other hand, we did adjust for severe systolic blood pressure.
Table 1. Adverse maternal and neonatal outcomes among patients with preeclampsia with severe featuresa stratified by elevated liver enzymes pre-delivery.
Multivariable logistic regression demonstrated the composite adverse maternal outcome was also not significantly associated with above threshold LFTs for both the unadjusted and adjusted models (Supplementary Appendix E). The odds of the composite adverse neonatal outcome was significantly associated with the above threshold LFTs group for the unadjusted model (OR 2.08, 95% CI: 1.04–4.14), and when adjusting for maternal age, pre-pregnancy BMI, race (non-Hispanic white vs. other), nulliparity, and severe systolic blood pressure pre-delivery (OR 2.43, 95% CI: 1.17–5.04). However, after additional adjustment for gestational age at delivery, the association between above threshold LFTs and adverse neonatal outcomes was no longer significant (OR 1.54, 95% CI: 0.63–3.76).
We also compared pre-delivery severe features of preeclampsia between patients with preeclampsia with severe features and those with chronic hypertension with superimposed preeclampsia and severe features (Supplementary Appendix F). Using an α = 0.05, these two groups were similar, except patients with chronic hypertension with superimposed preeclampsia were more likely to experience severe right upper quadrant pain compared to those with preeclampsia with severe features (19% vs. 11%, p = .042).
Discussion
Among patients with preeclampsia with severe features, we found that above threshold LFTs (AST or ALT ≥ two times the upper limit of normal) were not independently associated with adverse maternal or neonatal outcomes compared to below threshold LFTs. While above threshold LFTs were associated with adverse neonatal outcomes in unadjusted analyses, the association was non-significant in multivariable analyses, once adjusted for gestational age at delivery. These findings suggest that subjects diagnosed with preeclampsia with severe features by LFTs are more likely to undergo earlier iatrogenic preterm birth compared to those diagnosed with preeclampsia with severe features by other diagnostic criteria. Therefore, preterm delivery appears to be the primary driver of neonatal morbidity among patients with preeclampsia with severe features, rather than LFT values specifically.
While elevated liver enzymes are broadly associated with adverse maternal and neonatal outcomes in the setting of preeclampsia, such data have been generated by comparing patients with preeclampsia and liver enzyme elevation to all other patients with preeclampsia, without controlling for disease severity. Kozic et al. found that patients with preeclampsia and abnormal liver function tests were more likely to have a preterm birth, low birth weight neonate, experience stillbirth or have an adverse maternal outcome [Citation7]. However, the study did not distinguish between individual hypertensive disorders of pregnancy and utilized a lower LFT threshold.
Martin et al. assessed pre-delivery laboratory data to predict adverse maternal outcomes in severe preeclampsia with or without HELLP syndrome [Citation8]. They found that those with an AST >150 U/L, but not ALT >100 U/L, were at high risk for significant maternal morbidity (37% chance). Demir et al. similarly found an association between abnormally elevated liver enzymes and adverse maternal outcomes, although laboratory data were likely drawn early in pregnancy and specific LFT thresholds were not provided [Citation5]. Our study was unique and distinct from these studies because we compared adverse maternal and neonatal outcomes in patients with preeclampsia with severe features and above threshold LFTs to patients with preeclampsia with severe features and below threshold LFTs, allowing us to isolate the effect of LFT elevation.
Our findings support ACOG’s latest guidelines on diagnosis and management of severe features of preeclampsia as it relates to abnormally elevated liver enzymes. Adverse maternal outcomes were not increased in patients with severe preeclampsia and above threshold LFTs, suggesting that healthcare providers may be intervening appropriately in such cases to minimize maternal harm. Although adverse neonatal outcomes were increased among patients with above threshold LFTs, this association dissipated once adjusting for the earlier deliveries among this group. This suggests that healthcare providers may be delivering patients sooner for elevated liver enzymes compared to other severe features of preeclampsia, leading to neonates being born at an earlier gestational age.
Current liver enzyme thresholds for delivery in preeclampsia are based on expert opinion but appear to be adequate to minimize maternal harm. However, iatrogenic preterm delivery remains a major source of neonatal morbidity in preeclampsia. As premature delivery by 34 weeks is recommended for patients with preeclampsia and elevated liver enzymes, additional studies are needed to determine the liver enzyme value beyond which maternal harm from ongoing pregnancy exceeds neonatal harm from early delivery. Prospective studies may also be considered to better guide delivery timing in patients with preeclampsia and elevated liver enzymes.
The strengths of this study include its contemporaneous design. We included only those patients diagnosed with preeclampsia following ACOG’s updated guidelines on hypertension in pregnancy in 2013 [Citation12], and we strictly adhered to LFT thresholds for preeclampsia with severe features [Citation4]. Finally, all data were chart validated, allowing us to confirm the clinical diagnoses and abstract pre-delivery laboratory data thereby minimizing misclassification bias.
This study has several limitations. Data were collected from a single institution with a large non-Hispanic white population, which may lower generalizability. Due to the small number of patients with above threshold LFTs, our study was underpowered to rule out modest differences in adverse maternal and neonatal outcomes between groups. Additionally, a larger study would be needed to assess differences in individual adverse outcomes. While not the purpose of our study, our design did not allow us to assess lower LFT thresholds, and we lacked the power to evaluate the association between higher LFT thresholds and adverse outcomes. Finally, we included patients with chronic hypertension with superimposed preeclampsia (and severe features), and this group may differ from severe preeclampsia without chronic hypertension. It is possible grouping these patients together may have introduced selection bias into the study, however, we found that the two groups of patients were similar except for severe right upper quadrant pain (Supplementary Appendix F).
In conclusion, hypertensive disorders of pregnancy are common and associated with significant adverse pregnancy outcomes. Among our study population, for patients with preeclampsia with severe features, abnormally elevated liver enzymes ≥2x the upper limit of normal were not associated with worse maternal and neonatal outcomes compared to those with normal liver enzymes. This is an important area of research given its clinical implications for timing of delivery among this high-risk population.
Supplemental Material
Download MS Word (35 KB)Acknowledgement
Presented as a poster at the Pregnancy Meeting, 41st Annual Meeting, Society for Maternal Fetal Medicine, 25–30th January 2021.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Abalos E, Cuesta C, Carroli G, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121 Suppl 1:14–24.
- Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–1074.
- Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
- ACOG Practice Bulletin No 202: Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):e1–e25.
- Demir SC, Evruke C, Ozgunen FT, et al. Factors that influence morbidity and mortality in severe preeclampsia, eclampsia and hemolysis, elevated liver enzymes, and low platelet count syndrome. Saudi Med J. 2006;27(7):1015–1018.
- Hauth JC, Ewell MG, Levine RJ, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for preeclampsia prevention study group. Obstet Gynecol. 2000;95(1):24–28.
- Kozic JR, Benton SJ, Hutcheon JA, et al. Abnormal liver function tests as predictors of adverse maternal outcomes in women with preeclampsia. J Obstet Gynaecol Can. 2011;33(10):995–1004.
- Martin JN, Jr., May WL, Magann EF, et al. Early risk assessment of severe preeclampsia: admission battery of symptoms and laboratory tests to predict likelihood of subsequent significant maternal morbidity. Am J Obstet Gynecol. 1999;180(6 Pt 1):1407–1414.
- Sibai BM, Ramadan MK, Usta I, et al. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169(4):1000–1006.
- Sibai BM, Taslimi MM, el-Nazer A, et al. Maternal-perinatal outcome associated with the syndrome of hemolysis, elevated liver enzymes, and low platelets in severe preeclampsia-eclampsia. Am J Obstet Gynecol. 1986;155(3):501–509.
- Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142(2):159–167.
- American College of Obstetricians and Gynecologists. Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
