Abstract
Objective
Antidepressant medications are used by increasing numbers of pregnant women. The evidence on the relationship between antidepressant use during pregnancy and the risk for gestational diabetes mellitus (GDM) is inconsistent. We perform a systematic review and meta-analysis to assess the GDM risk associated with antidepressant exposure during pregnancy.
Methods
We systematically searched the PubMed and EMBASE databases until December 2021. We sought observational studies assessing the association between gestational antidepressant use and GDM.
Results
Five observational studies were included in the analysis. Mothers exposed to antidepressants during pregnancy were at a significantly increased risk for GDM (relative risk [RR] 1.20, 95% confidence interval [CI] 1.11–1.30; p < .001). However, after considering confounding by indication, we observed no significant effect of antidepressant use during pregnancy on the risk of GDM (RR 1.13, 95% CI 1–1.28; p = .054; I2 = 0%). Independent of clinical indication, subgroup analysis based on individual antidepressants suggested that the risk was increased by venlafaxine or amitriptyline use, but not by selective serotonin reuptake inhibitors.
Conclusions
The significant association between antidepressant exposure during pregnancy and GDM may be overestimated due to confounding by indication. However, the evidence remains insufficient, particularly for specific drug classes.
Keywords:
Introduction
Antidepressants are widely used to treat depression, anxiety, obsessive-compulsive disorder, fibromyalgia, headache, and other conditions [Citation1,Citation2]. There has been a notable recent increase in the number of people treated with antidepressants [Citation3,Citation4]. Of particular concern is the use of these drugs by pregnant women, the rate of which has increased to 3% in Europe and 8% in the USA during the past 12 years [Citation5]. In particular, concerns have emerged about reproductive safety.
Gestational diabetes mellitus (GDM) is a common antepartum condition with an estimated global prevalence of 4–16% (varying depending on ethnicity, geographic region, and the diagnostic criteria used) [Citation6]. Pregnancies with GDM are at higher risk of macrosomia, gestational hypertension, pre-eclampsia, and neonatal hypoglycemia [Citation7]. Furthermore, mothers with a history of GDM may be more prone to developing type 2 diabetes and cardiovascular diseases later in life [Citation8]. Preclinical studies have suggested that antidepressants induce hyperglycemia through their ability to decrease insulin secretion and increase β-cell apoptosis [Citation9,Citation10]. A meta-analysis demonstrated an increased risk of type 2 diabetes in non-pregnant women [Citation11]. Antidepressant exposure has been related to weight gain, which increases the GDM risk [Citation12]. It is reasonable to speculate that antidepressant use during pregnancy modifies the risk of GDM. Therefore, several studies [Citation13–17] have investigated the relationship between maternal antidepressant use and GDM. Reis et al. [Citation14] reported that antidepressant use during pregnancy was associated with an increased risk of GDM. In another study [Citation15], maternal antidepressant exposure increased the subsequent risk of GDM, but the results of a subgroup analysis based on antidepressant class and individual antidepressants were inconsistent. However, two latest studies [Citation16,Citation17] observed no increase in the risk of GDM according to antidepressant indications. Thus, the relationship between maternal antidepressant exposure during pregnancy and the risk of GDM remains unclear. Therefore, we conducted a systematic literature review and meta-analysis to assess this association. However, since the various factors associated with antidepressant exposure (i.e. country, antidepressant class, individual antidepressants, and clinical indications) may affect the risk of GDM differently, they must still be analyzed separately.
Methods
Search strategy
The Meta-Analysis for Observational Studies in Epidemiology (MOOSE) checklist was used as a reporting guideline [Citation18] (Table S1). The PubMed and EMBASE databases were searched to identify relevant studies written in English and published before December 2021. The search was performed using the terms: “pregnancy OR mothers OR pregnant OR gestational OR prenatal OR perinatal OR gestation” AND “antidepressant OR selective serotonin reuptake inhibitor OR selective noradrenaline reuptake inhibitor OR SSRI OR SNRI” AND “diabetes OR diabetic OR GDM OR diabetes mellitus.” The reference lists of retrieved articles were manually searched to identify additional eligible articles.
Table 1. Characteristics of included studies.
Inclusion and exclusion criteria
Two authors independently evaluated the eligibility of all articles based on predefined selection criteria. Full texts were retrieved after reading the titles and abstracts. Any discrepancies were resolved by discussion with another author. Articles were considered for inclusion if they were case-control or cohort studies; non-antidepressant users served as the reference groups; the association between antidepressant use and the risk for GDM was investigated; and the data were adequate for extraction of risk estimates. We excluded non-human/animal studies, conference abstracts, series, editorials, correspondence, systematic reviews, and meta-analyses.
Data extraction and quality assessment
Data were independently extracted in a double-blinded manner by two investigators and any discrepancies were resolved by a third author. The extracted data included the first author, year of publication, study design, study location, measurement of antidepressant exposure, diagnostic criteria for GDM, numbers of antidepressant and non-antidepressant users, adjustments, and study quality. The most appropriate adjusted effect size estimate was used when more than one estimate was provided. As recommended by the Cochrane Collaboration, the two authors independently assessed the risk of bias using the Newcastle-Ottawa scale (NOS) [Citation19], which evaluates the quality of observational studies. In brief, the scale features eight criteria and yields scores ranging from 0 (a high risk of bias) to 9 (a low risk). Studies with scores >7 were considered to be of high quality.
Outcome assessment
To assess possible heterogeneity between studies, we performed subgroup analyses based on study region, type of antidepressant, and individual antidepressant. A further analysis was performed to rule out the impact of confounding by indication. We compared outcomes between pregnancies exposed to antidepressants and those for which women with clinical indications were not taking antidepressants.
The types of antidepressant were classified as follows: all antidepressants: selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase A inhibitor (MOAIs), tricyclic antidepressants, and other antidepressants; SSRIs: fluoxetine, paroxetine, fluvoxamine, sertraline, citalopram, and escitalopram; SNRIs: venlafaxine, desvenlafaxine, duloxetine, and milnacipran; serotonin reuptake inhibitors (SRIs): SSRIs and SNRIs; tetracyclic antidepressants (TCAs): imipramine, clomipramine, lofepramine, amitriptyline, nortriptyline, protryptyline, maprotiline; and MOAIs: moclobemide.
Statistical analysis
The statistical analyses were performed using STATA software (ver. 13.0; StataCorp, College Station, TX, USA). The associations between GDM and antidepressant exposure during pregnancy are expressed as risk ratios (RRs) with 95% confidence intervals (95% CIs). Between-study heterogeneity was assessed using the I2 statistic; I2 > 50% was considered to indicate significant heterogeneity [Citation20]. In such instances, a random-effects model was used because it takes account of between-study variability, while the fixed-effects Mantel–Haenszel model was used to evaluate dichotomous outcomes [Citation21]. In forest plots, each study is plotted as a square, the position of which corresponds to the RR (and whose area is proportional to the inverse of the variance of the natural logarithm of the RR). A diamond was used to plot summary RRs, the extremes of which represent the 95% CIs [Citation21]. Publication bias was assessed quantitatively using Egger’s regression test and qualitatively by visual inspection of funnel plots of the logarithm of RR versus standard error [Citation22]. A p-value <.05 was considered statistically significant. All statistical tests were two-sided.
Results
Search results
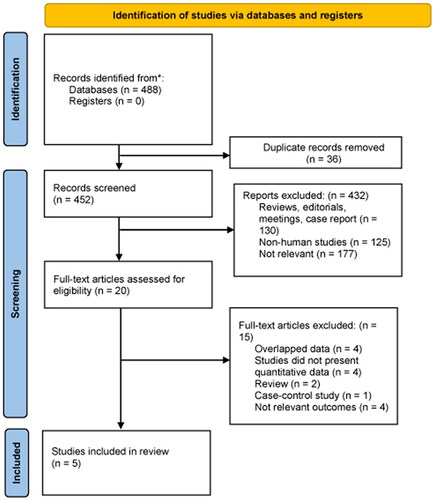
We initially identified 488 potentially eligible articles in the two databases and excluded 36 duplicates. A further 432 articles deemed irrelevant after reviewing the title and abstract were excluded from the analysis. The remaining 20 articles were retrieved for detailed full-text evaluation. Ultimately, five studies [Citation13–17] met our inclusion criteria and were included in the meta-analysis. shows the number of articles excluded at each stage of the eligibility assessment and the reasons for their exclusion.
Characteristics of the included studies
The principal characteristics of the included studies are listed in . The publication year ranged from 2006 [Citation13] to 2022 [Citation17], and the patient cohorts began to be formed as early as 1990 [Citation13]. There were four cohort studies [Citation13,Citation14,Citation16,Citation17]and one nested case-control study [Citation15] involving more than 1,300,000 pregnant mothers. The sample sizes ranged from 2845 to 1,077,011. All studies examined Western populations (three in North America [Citation13,Citation15,Citation16] and two in Europe [Citation14,Citation17]). Antidepressant exposure during pregnancy was assessed using drug prescription databases in all studies. The extent of adjustment for potential clinical risk factors varied considerably across the studies. All studies were of high quality (mean score 8.6). The scores are detailed in Tables S2 and S3.
Table 2. Meta-analysis for studies included in the analysis.
Meta-analysis
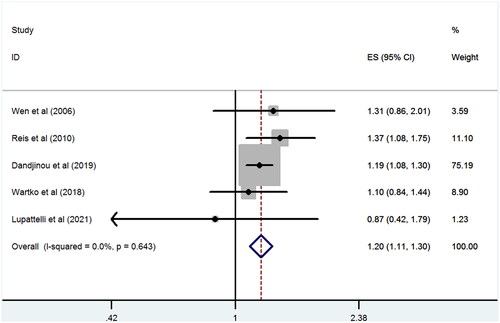
All results are presented in . We found that antidepressant use during pregnancy was associated with an increased risk for GDM (RR 1.20, 95% CI 1.11–1.30; p < .001) (). No between-study heterogeneity was observed (I2 = 0%) and there was no evidence of publication bias (Begg test, p = .46), as shown in Figure S1. Sensitivity analysis revealed no substantial change in the pooled risk estimate upon exclusion of any single study; the pooled RRs for GDM ranged from 1.18 to 1.23.
Figure 2. Antidepressant exposure during pregnancy and risk of GDM vs. unexposed general population.
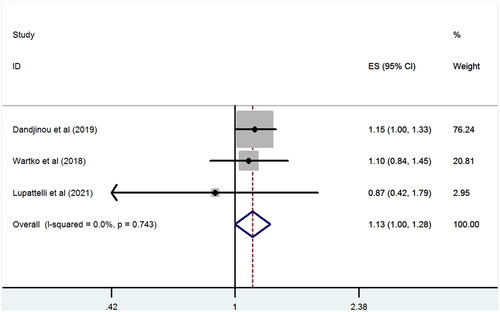
In the subgroup analysis by study region, a significant association was observed in studies conducted in both Europe (RR 1.31, 95% CI 1.04–1.65; p < .001; I2 = 0%) and North America (RR 1.19, 95% CI 1.09–1.29; p = .021; I2 = 0%). When we limited the analysis to studies reporting serotonin reuptake inhibitor (SRI) exposure during pregnancy, a significant positive association between SRI use and the subsequent GDM risk was observed (RR 1.13, 95% CI 1.05–1.23; p = .002; I2 = 0%). Further analysis demonstrated no significant association between maternal SSRI use and the risk of GDM (RR 1.09, 95% CI 0.99–1.19; p = .07; I2 = 0%). When we limited the analysis to studies that accounted for confounding, a lower and non-significant risk of GDM was detected in pregnant women on antidepressants (RR 1.13, 95% CI 1–1.28; p = .054; I2 = 0%) ().
Figure 3. Antidepressant exposure during pregnancy and risk of GDM vs. unexposed with clinical indication.
When we grouped studies by individual antidepressants, a significant association was observed in those using venlafaxine (RR 1.29, 95% CI 1.11–1.50; p = .001; I2 = 0%) or amitriptyline (RR 1.52, 95% CI 1.25–1.84; p < .05), but not sertraline (RR 1.09, 95% CI 0.89–1.32; p = .412; I2 = 22%); fluoxetine (RR 0.92, 95% CI 0.69–1.22; p = .555; I2 = 0%); paroxetine (RR,1.11; 95% CI 0.93–1.32; p = .266; I2 = 0%); or citalopram (RR 1.08; 95% CI 0.93–1.25; p = .316; I2 = 33.3%).
Discussion
We found that maternal exposure to antidepressants during pregnancy may be associated with a higher risk for subsequent GDM. After considering possible confounding by indication, we still observed a lower GDM risk in mothers exposed to antidepressants during pregnancy. However, as we included only a small number of studies, our results should be interpreted with caution.
A possible association between antidepressant use and metabolic disorders has been a controversial topic for decades. In theory, antidepressants can influence blood glucose levels by inhibiting pancreatic insulin secretion, increasing cellular insulin resistance, or indirectly affecting insulin secretion via weight gain [Citation23]. Several animal studies have found that antidepressants induced hyperglycemia [Citation24,Citation25]. Serotonin reuptake inhibitors block the serotonin reuptake transporter, thus affecting serotonin homeostasis [Citation26]. However, serotonin plays a compensatory role when insulin resistance develops naturally during pregnancy [Citation27]. Their affinity to noradrenergic and histamine receptors may contribute to the risk of GDM [Citation28]. In the general population, the effects of TCAs (especially amitriptyline) on glucose dysregulation seem stronger than those of SNRIs and SSRIs.
Previous studies have found that symptoms of anxiety and depression can cause obesity and increased blood lipid and glucose levels, body mass index, and blood pressure [Citation29]. The prevalence of metabolic syndrome was higher in patients with than without psychiatric disorders [Citation30]; however, the current consensus is that metabolic syndrome may lead to type 2 diabetes. Thus, an investigation of the association between antidepressant use during pregnancy and the subsequent risk for GDM should consider maternal psychiatric conditions [Citation31]. In our overall analysis, antidepressant use during pregnancy was associated with GDM when we combined the adjusted data from all five studies; however, the results of the two studies were not adjusted for maternal psychiatric diseases. Thus, the association that we found may be an overestimate. The ideal control for confounding by indication is a reference group of women with psychiatric disorders who did not take antidepressants during pregnancy. Therefore, we further analyzed the three studies that controlled for the effects of maternal psychiatric conditions and observed a lower increase in the risk of GDM. This suggested that confounding by indication largely explains the association observed in our primary analysis.
The risk for GDM may differ among antidepressants that vary in their affinities for the serotoninergic, noradrenergic, and histamine receptors [Citation28,Citation32]. One study found that, because of their high affinities for the serotonin reuptake transporter, SSRIs could trigger hypoglycemia whereas venlafaxine (with high affinity for the 5-HT2c serotonin receptor and the histamine H1-receptor) caused hyperglycemia [Citation30,Citation33]. In our subgroup analysis by individual antidepressant, we found a risk only in mothers on venlafaxine, which implies that antidepressants with different pharmacological properties may have heterogeneous effects on GDM incidence. One included study [Citation15] reported a higher risk of GDM in mothers exposed to amitriptyline, which has high H1 receptor affinity. This finding is consistent with a previous meta-analysis demonstrating that only TCA use was positively associated with new-onset T2DM in the general population [Citation34]. However, our subgroup sample sizes were low; However, our subgroup sample sizes were low; further investigation is required.
This systematic review and meta-analysis are the first to provide an overall estimate of the effects of antidepressants on GDM risk. A strength of our work is the exclusive use of cohort studies, which are less prone to bias in terms of drug exposure assessment during pregnancy than observational studies. For ethical and feasibility reasons, it is impossible to conduct randomized controlled trials to evaluate the reproductive safety of antidepressants. In addition, heterogeneity was low, rendering the pooled results more convincing. Finally, subgroup analysis was used to explore the influence of antidepressant indications on the results.
Several potential limitations should be acknowledged. First, the number of studies included was small, particularly when subgroup analyses were performed, which may compromise the accuracy of our results. Second, residual confounders are always of concern in observational studies. One of the studies included the frequency of mental health care visits as an indicator of the severity of psychiatric symptoms in the inverse probability of treatment weighting calculation. Unfortunately, few of the included studies controlled for these confounders, leading to an overestimation of this association. Further well-designed studies using validated measures of the severity of psychiatric symptoms at baseline are needed to clarify the contribution of maternal psychiatric status to the association between antidepressant use during pregnancy and GDM risk. Third, only one study [Citation15] reported a duration-effect gradient associated with maternal antidepressant use during pregnancy. Therefore, there were insufficient data on the cumulative dose of antidepressant used in the included studies and we could not determine whether this parameter is associated with GDM risk. Moreover, we could not extract enough data to perform subgroup analysis based on exposure duration; further research is thus required. Finally, all studies were performed in Europe and North America (thus none in Asia or Africa); this may affect the generalizability of our findings.
In conclusion, the results of our meta-analysis suggest that the association between maternal antidepressant use during pregnancy and GDM risk may be overestimated because several epidemiological studies failed to control for maternal psychopathology. However, the available information is limited. Larger prospective cohort studies that consider the severity of psychiatric illness during pregnancy are needed to clarify the effects of antidepressant medications on the GDM risk.
Author contributions
X.H.Y. and X.Y.W. searched the library and wrote the manuscript text. X.Y.W. and H.Y.J. extracted data and reviewed all articles. X.Y.W. evaluated the bias. X.H.Y. designed the manuscript. All authors reviewed the manuscript.
Supplemental Material
Download MS Word (3.1 MB)Acknowledgments
X.H.Y. and X.Y.W. conceived the study and revised the manuscript critically for important intellectual content. X.Y.W. and H.Y.J. made substantial contributions to its design, acquisition, analysis, and interpretation of data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Williams T, Stein DJ, Ipser J. A systematic review of network meta-analyses for pharmacological treatment of common mental disorders. Evid Based Ment Health. 2018;21(1):7–11.
- Mercier A, Auger-Aubin I, Lebeau JP, et al. Evidence of prescription of antidepressants for non-psychiatric conditions in primary care: an analysis of guidelines and systematic reviews. BMC Fam Pract. 2013;14:55.
- Bogowicz P, Curtis HJ, Walker AJ, et al. Trends and variation in antidepressant prescribing in English primary care: a retrospective longitudinal study. BJGP Open. 2021;5(4):BJGPO.2021.0020.
- Masarwa R, Lefebvre C, Platt RW, et al. General practitioner prescribing trends among pediatric patients in the United Kingdom: 1998–2018. Pharmacoepidemiol Drug Saf. 2022;31(3):302–313.
- Molenaar NM, Bais B, Lambregtse-van den Berg MP, et al. The international prevalence of antidepressant use before, during, and after pregnancy: a systematic review and meta-analysis of timing, type of prescriptions and geographical variability. J Affect Disord. 2020;264:82–89.
- Saravanan P. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8(9):793–800.
- Bastidas K, Romero XC, Uriel M, et al. Perinatal outcomes associated with the diagnosis of gestational diabetes: systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15(5):102262.
- Bidhendi Yarandi R, Vaismoradi M, Panahi MH, et al. Mild gestational diabetes and adverse pregnancy outcome: a systemic review and meta-analysis. Front Med. 2021;8:699412.
- Liu B, Ruz-Maldonado I, Toczyska K, et al. The selective serotonin reuptake inhibitor fluoxetine has direct effects on beta cells, promoting insulin secretion and increasing beta-cell mass. Diabetes Obes Metab. 2022;24(10):2038–2050.
- Isaac R, Boura-Halfon S, Gurevitch D, et al. Selective serotonin reuptake inhibitors (SSRIs) inhibit insulin secretion and action in pancreatic β cells. J Biol Chem. 2013;288(8):5682–5693.
- Bhattacharjee S, Bhattacharya R, Kelley GA, et al. Antidepressant use and new-onset diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2013;29(4):273–284.
- Gill H, Gill B, El-Halabi S, et al. Antidepressant medications and weight change: a narrative review. Obesity. 2020;28(11):2064–2072.
- Wen SW, Yang Q, Garner P, et al. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol. 2006;194(4):961–966.
- Reis M, Källén B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med. 2010;40(10):1723–1733.
- Dandjinou M, Sheehy O, Berard A. Antidepressant use during pregnancy and the risk of gestational diabetes mellitus: a nested case-control study. BMJ Open. 2019;9(9):e025908.
- Wartko PD, Weiss NS, Enquobahrie DA, et al. Antidepressant continuation in pregnancy and risk of gestational diabetes. Pharmacoepidemiol Drug Saf. 2019;28(9):1194–1203.
- Lupattelli A, Barone-Adesi F, Nordeng H. Association between antidepressant use in pregnancy and gestational diabetes mellitus: results from the Norwegian mother, father and child cohort study. Pharmacoepidemiol Drug Saf. 2022;31(2):247–256.
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012.
- Higgins JP. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration; 2014 [cited 2014 Dec 6]. Available from: www.cochrane-handbook.org
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
- Hennings JM, Schaaf L, Fulda S. Glucose metabolism and antidepressant medication. Curr Pharm Des. 2012;18(36):5900–5919.
- Levkovitz Y, Ben-Shushan G, Hershkovitz A, et al. Antidepressants induce cellular insulin resistance by activation of IRS-1 kinases. Mol Cell Neurosci. 2007;36(3):305–312.
- Carvalho F, Barros D, Silva J, et al. Hyperglycemia induced by acute central fluoxetine administration: role of the Central CRH system and 5-HT3 receptors. Neuropeptides. 2004;38(2-3):98–105.
- Horackova H, Karahoda R, Cerveny L, et al. Effect of selected antidepressants on placental homeostasis of serotonin: maternal and fetal perspectives. Pharmaceutics. 2021;13(8):1306.
- Baeyens L, Hindi S, Sorenson RL, et al. β-Cell adaptation in pregnancy. Diabetes Obes Metab. 2016;18 Suppl 1(Suppl 1):63–70.
- Salvi V, Mencacci C, Barone-Adesi F. H1-histamine receptor affinity predicts weight gain with antidepressants. Eur Neuropsychopharmacol. 2016;26(10):1673–1677.
- Qiu W, Cai X, Zheng C, et al. Update on the relationship between depression and neuroendocrine metabolism. Front Neurosci. 2021;15:728810.
- Khoza S, Barner JC. Glucose dysregulation associated with antidepressant agents: an analysis of 17 published case reports. Int J Clin Pharm. 2011;33(3):484–492.
- Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. J Clin Psychiatry. 2013;74(1):31–37.
- Harvey BH, Bouwer CD. Neuropharmacology of paradoxic weight gain with selective serotonin reuptake inhibitors. Clin Neuropharmacol. 2000;23(2):90–97.
- Coutens B, Yrondi A, Rampon C, et al. Psychopharmacological properties and therapeutic profile of the antidepressant venlafaxine. Psychopharmacology. 2022;239(9):2735–2752.
- Wang Y, Liu D, Li X, et al. Antidepressants use and the risk of type 2 diabetes mellitus: a systematic review and meta-analysis. J Affect Disord. 2021;287:41–53.