Abstract
Objective
Perinatal depression (PND) is the most common complication of childbirth and negatively affects the mother. Long noncoding RNA (lncRNA) NONHSAG045500 inhibits the expression of 5-hydroxytryptamine (5-HT) transporter (i.e. serotonin transporter [SERT]) and produces an antidepressant effect. This study aimed to identify a link between the lncRNA NONHSAG045500 and the pathogenesis of PND.
Methods
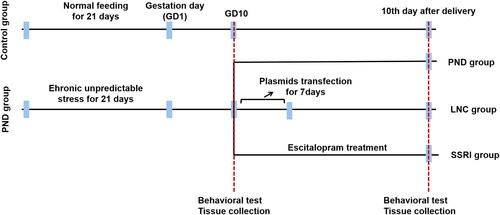
Female C57BL/6 J mice were divided into normal control group (control group, n = 15), chronic unpredictable stress (CUS) model group (PND group, n = 15), lncRNA NONHSAG045500-overexpressed group (LNC group, sublingual intravenous injection of NONHSAG045500 overexpression cells for 7 days, n = 15), and escitalopram treatment group (i.e. the selective serotonin reuptake inhibitor [SSRI] group, with escitalopram administered from the 10th day after pregnancy to the 10th day after delivery, n = 15). Control group mice were conceived normally, whereas, in the other groups, a CUS model was established before mice were conceived. Depressive-like behaviour was assessed via sucrose preference, forced swimming, and open-field tests. The expression levels of 5-HT, SERT, and cAMP–PKA–CREB pathway-related proteins in the prefrontal cortex were detected on the 10th day after delivery.
Results
Mice in the PND group exhibited significant depressive-like behaviours compared with those in the control group, indicating that the PND model was successfully established. The expression of lncRNA NONHSAG045500 was markedly decreased in the PND group compared with that in the control group. After treatment, both LNC and SSRI groups showed a significant improvement in depression-like behaviour, and the expression of 5-HT in the prefrontal cortex was increased in these groups compared with that in the PND group. In addition, the LNC group displayed lower expression of SERT and higher expression of cAMP, PKA, and CREB when in comparison to PND group.
Conclusion
NONHSAG045500 mediates the development of PND mainly by activating the cAMP–PKA–CREB pathway, increasing the level of 5-HT, and decreasing the expression of SERT.
Introduction
Perinatal depression (PND) is a common medical condition in women and is associated with serious consequences [Citation1]. Depression can also occur during pregnancy (prenatally), the year following birth (postpartum), or both [Citation2] and is thought to result from a complex interaction involving genetic, epigenetic, environmental, and social factors [Citation3].
Disturbances in the serotonin system are involved in the pathophysiology and treatment mechanisms of major depressive disorder (MDD) and may be a critical factor in PND susceptibility [Citation4]. 5-hydroxytryptamine (5-HT) reuptake is mediated by its transporter (i.e. serotonin transporter [SERT]). Selective serotonin reuptake inhibitors (SSRIs) downregulate the expression of SERT, thereby increasing serotonergic neurotransmission in the forebrain and neuronal plasticity in the hippocampus [Citation5] and improving mood, and are key treatment options for PND [Citation6]. However, SSRI treatment needs to be administered for an extended period before clinical improvement can be observed. It can cause full remission of depressive symptoms in one-third of patients, with partial or incomplete clinical responses in the remaining patients [Citation7]. Therefore, further exploration of the mechanism underlying SERT modulation is urgently required to develop novel therapeutic strategies for PND.
Long noncoding RNAs (lncRNAs) are important epigenetic regulators [Citation8] that play versatile roles in many aspects of gene regulation [Citation9]. Recent data showed that certain lncRNAs were dysregulated in MDD, indicating their involvement in the pathogenesis of the disease [Citation9,Citation10]. Studies identified 11 lncRNAs that had differential expression in patients with MDD compared with their expression in normal controls, including the lncRNA NONHSAG045500 [Citation11]. Another study indicated that NONHSAG045500 could regulate the expression of SERT [Citation12]. These results suggest that NONHSAG045500 is involved in the occurrence and development of MDD. Depression is caused by reduced concentrations of the second messenger cyclic adenosine monophosphate (cAMP) and reduced levels of protein kinase A (PKA) and cAMP response element-binding protein (CREB) [Citation13–15]. The cAMP–PKA–CREB signaling pathway has been associated with SSRI-induced antidepressant effects [Citation16]. However, whether NONHSAG045500 could potentially be a new diagnostic tool and therapeutic target of PND and how cAMP–PKA–CREB signaling pathway is regulated by NONHSAG045500 remain unknown.
To the best of our knowledge, this is the first study to investigate the effect of upregulating lncRNA NONHSAG045500 expression in depressive-like behaviors in a PND mouse model. In addition, the underlying molecular mechanisms were explored by analyzing the effect of NONHSAG045500 modulation on the expression levels of SERT, 5-HT, and components of the cAMP–PKA–CREB signaling pathway.
Materials and methods
Animals and groups
Wild-type female C57BL/6 J mice weighing 18–20 g were obtained from the SLAC Laboratory Animal Center (Shanghai, China). All animal experimental protocols complied with the relevant guidelines and ordinances issued by the Institutional Animal Care and Use Committee. A total of 60 8-week-old mice were divided into four groups: normal control group (control group, n = 15), chronic unpredictable stress (CUS) model group (PND group, n = 15), lncRNA NONHSAG045500-overexpressed group (LNC group, n = 15), and escitalopram treatment group (positive control group, i.e. SSRI group, n = 15). Control group mice were conceived normally, whereas in the other groups, a CUS model was established before mice were conceived. Following CUS establishment, mice in the LNC group were administered with plasmids to induce the overexpression of lncRNA NONHSAG045500 (sublingual intravenous injection for 7 days), whereas those in the SSRI group were administered escitalopram (from the 10th day after pregnancy to the 10th day after delivery). The study schedules are shown in . Behavioral tests were performed in the following sequence: sucrose preference, open-field, and forced swimming tests [Citation17]. Mice were allowed to acclimatize to their new cages for 1 week before experiments began. All mice were maintained under standard conditions with a 12:12 h light–dark cycle at 22–24 °C and relative humidity of 55% and provided free access to water and food (Supplemental Material 1). All procedures for the animal study were approved by the Ethics Committee of Nanjing Medical University (approval number: 2020(491)). Finally, the mice were euthanized by CO2 inhalation.
NONHSAG045500 overexpression
The NONHSAG045500 gene was synthesized and cloned into pcDNA3.1 () by General Biol Co., Ltd. (Anhui, China). The lncRNA plasmid was transfected into HEK293 cells to induce NONHSAG045500 overexpression. Cells were seeded into 6-well plates for overnight incubation before transfection. lncRNA plasmid was transfected into cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s instructions. NONHSAG045500 overexpression cells (2 × 106) were injected into LNC mice via the tail vein for 7 days.
Escitalopram treatment
Escitalopram was purchased from Selleck Co., Ltd (S4064) and stored at 4 °C. Mice were injected intraperitoneally with escitalopram (10 mg/kg, 50 ml) or saline from the 10th day after pregnancy to the 10th day after delivery in a counterbalanced order.
Sucrose preference test
The sucrose preference test comprised a 2-bottle procedure where mice were allowed to choose between consuming water or 1% sucrose solution. Mice were habituated to drinking water from two bottles, one of which contained only water while the other contained 1% sucrose solution. Water and sucrose consumption was measured the following day (8:00 a.m.). The position of the sucrose bottle was counterbalanced (left versus right) across the different cages to control for potential side preference bias. Preference for sucrose over water (sucrose/[sucrose + water]) was used as a measure of sensitivity to reward.
Open-field test
The open-field test was performed in a closed case (25 × 25 × 30 cm), which was divided into nine equal areas. The subject mouse was first placed in the center area and then allowed to explore the nine areas freely for 10 min. The movement and time spent in each region by the subject mouse were recorded and analyzed using SuperMaze software (Xinruan, Shanghai, China).
Forced swimming test
The forced swimming test is a behavioural procedure where rodents are forced to swim under inescapable conditions. Initially, rodents engage in escape-like behaviors but eventually adopt a posture where they can only make the movements necessary to maintain their head above water. Mice were forced to swim once in a 4-L Pyrex glass beaker containing 3 L of water (25°C ± 1 °C) for 6 min. All beakers were emptied and cleaned after testing each subject mouse. The time (s) to initially adopt a posture of immobility (latency to immobility) as well as the total time (s) spent immobile during the last 4 min of the test were the dependent variables.
Body weight
Body weight was recorded 10 days postpartum.
RT-pcr
Quantitative RT-PCR was used to detect the expression of lncRNA NONHSAG045500. Peripheral blood was collected 10 days postpartum, and the prefrontal cortex was collected after the mice were euthanized. Total RNA was extracted from peripheral blood and 20 mg prefrontal cortex using Trizol reagent (Takara), and 1 μg total RNA was transcribed into cDNA using PrimeScript RT Master Mix (Takara, Dalian, China). Subsequently, SYBR Premix Ex Taq II Kit (Takara) was used to detect the expression of mRNA via RT-qPCR, which was normalized to the expression of GAPDH (endogenous control). Primer sequences were as follows: NONHSAG045500, forward: 5′-ACCTGTTACCCTGGAAGT-3′, reverse: 5′-CACATTAAGGCTGTGAGC-3′; SERT, forward: 5′-CCACACCAGCAGACAAGGCA-3′, reverse: 5′-AGGAAGGCCCCTCCACCATT-3′; GAPDH, forward: 5′-TGTGGATGGCCCCTCTGGAA-3′, reverse: 5′-TGACCTTGCCCACAGCCTTG-3′. Fold changes were calculated using the 2−ΔΔCT method.
Enzyme-linked immunosorbent assay
Prefrontal cortex was collected on the 10th day after delivery. The levels of 5-HT and cAMP in 20 mg prefrontal cortex were determined via Enzyme-linked immunosorbent assay (ELISA), and the experimental procedures were performed according to the kit instructions. ELISA kits were purchased from Sangon Co., Ltd. (Shanghai, China) (5-HT ELISA Kit, D751013-0096; cAMP ELISA Kit, D770001-0096).
Immunohistochemistry
Mouse brains were collected on the 10th day after delivery and removed immediately after euthanasia and then fixed in 4% paraformaldehyde for 24 h before paraffin embedding. The frontal cortex was isolated and cut into 4-μm slices before being mounted on glass slides. Slides were dried at 90 °C for 4 h, dewaxed in xylene, and then rehydrated in graded ethanol solutions. Cooled tissue sections were immersed in 0.3% hydrogen peroxide solution for 15 min to block endogenous peroxidase activity, followed by rinsing with phosphate-buffered saline (PBS) for 5 min and blocking with 3% bovine serum albumin at room temperature for 30 min. After washing with PBS, the sections were incubated with the primary antibodies rabbit anti-mouse PKA or anti-CREB antibody (1:300 dilution; Catalog No. A1531 and A11989, respectively, Abclonal) at 4 °C overnight. Sections were then washed with PBS and incubated with HRP-labeled secondary antibodies at 37 °C for 30 min. Diaminobenzene was used as the chromogen, and hematoxylin was used as the nuclear counterstain. Sections were then dehydrated, washed, mounted, and imaged using a digital camera (CKX5, Olympus Corporation, Tokyo, Japan).
Statistical analyses
All statistical analyses were performed using GraphPad Prism 9.0. Data in all figures are expressed as mean ± SEM. For differences between the two groups, Student’s t-test was used to determine statistical significance. For multiple comparisons, one-way ANOVA followed by post hoc Student’s t-test was used. For all statistical tests, a p-value of <.05 (two-tailed) was considered statistically significant.
Results
CUS-exposed mice showed depressive-like behaviors
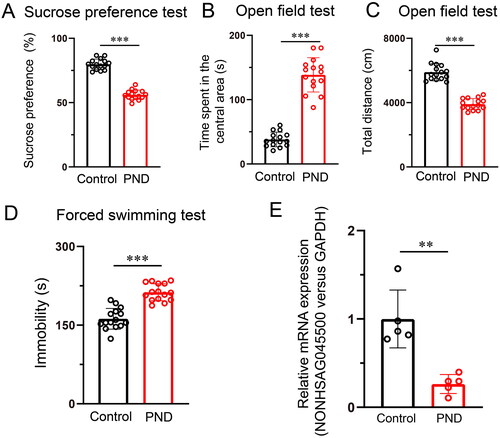
Prenatal exposure to CUS has been shown to increase the risk of PND [Citation18–20]. To observe the effect of CUS exposure in perinatal mice, we examined behavioral changes in the PND model and control groups. The experimental procedures employed are shown in . The behavioral test was performed for the PND and control groups on the 10th day after gestation. The sucrose preference of mice in the control group was relatively stable, whereas there was a marked reduction in the sucrose preference of mice in the PND group (). In the open-field test, mice in the PND group spent less time in the center area than those in the control group (). The total travel distance of mice in the PND group was also decreased compared with that of mice in the control group (). Moreover, in the forced swimming test, the time spent immobile was increased in the PND group (). To determine the potential involvement of lncRNA NONHSAG045500 in the pathogenesis of PND, we detected the expression of NONHSAG045500 in the control and PND groups using RT-PCR. The expression of NONHSAG045500 was significantly downregulated in the peripheral blood of mice in the PND group (). The results suggested that NONHSAG045500 was underexpressed in mice with PND. Collectively, these data indicate that prenatal exposure to CUS can induce depressive-like behaviors and lower the expression of NONHSAG045500 in perinatal mice.
Figure 3. CUS-exposed mice exhibited depressive-like behaviors and lower expression of NONHSAG045500. (A) Sucrose preference test (n = 15 in each group). (B) Time spent in the central area (n = 15 in each group). (C) Total distances travelled (n = 15 in each group). (D) Time spent immobile test (n = 15 in each group). (E) NONHSAG045500 expression in peripheral blood (n = 5 in each group). Differences between the two groups were measured using Student’s t-test. **p < .01, ***p < .001.
Overexpression of lncRNA NONHSAG045500 ameliorated depressive-like behaviors
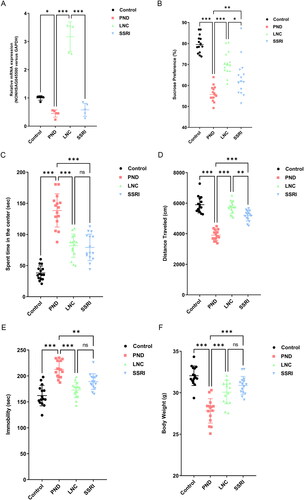
To elucidate the effect of NONHSAG045500 overexpression in the PND mouse model, we constructed an overexpression plasmid containing the coding sequence of lncRNA NONHSAG045500 by cloning it into the pcDNA3.1 vector and then injected this vector into mice in the LNC group as previously described [Citation21]. RT-PCR results verified the efficient overexpression of NONHSAG045500, which was increased in mice in the LNC group compared with that in mice in the PND and SSRI groups (). The behavior of mice with NONHSAG045500 overexpression was compared with that of mice in the PND group as well as with PND mice treated with escitalopram, an effective SSRI agent for depressive disorder, as a positive control [Citation22]. NONHSAG045500 overexpression, as an SSRI treatment, resulted in increased sucrose preference in the PND group (). In the open-field test, the time spent in the central area was higher for LNC- and SSRI-treated mice than for mice in the PND group (). The total distances travelled by mice in the LNC and SSRI groups were increased compared with those of mice in the PND group (). In the forced swimming test, the time spent immobile was also decreased in the LNC and SSRI groups compared with that in the PND group (). Interestingly, the improvement in sucrose preference and total distances travelled in the LNC group were better than those in the SSRI group ( and D)). In addition, antidepressant use is reported to be significantly related to increased body weight [Citation15]. In this study, the body weight of mice in the LNC and SSRI groups was significantly increased compared with that of mice in the PND group (). Overall, these results suggest that the upregulated expression of NONHSAG045500 ameliorated the depressive-like behaviors of mice, which was similar to the effect of escitalopram treatment.
Figure 4. Effect of NONHSAG045500 overexpression on the perinatal depression mouse model. (A) NONHSAG045500 expression (n = 5 in each group). (B) Sucrose preference test (n = 15 in each group). (C) Time spent in the central area (n = 15 in each group). (D) Total distances traveled (n = 15 in each group). (E) Time spent immobile test (n = 15 in each group). (F) Body weight in each group (n = 15 in each group). Differences among the four groups were measured using one-way ANOVA and post hoc Student’s t-test. *p < .05, **p < .01, ***p < .001, ns, not significant.
Overexpressed lncRNA NONHSAG045500 inhibited the expression of SERT and the activation of the cAMP–PKA–CREB signaling pathway in the PND mouse model
The effects of overexpression of NONHSAG045500 on the expression levels of SERT and 5-HT were examined in the PND group. RT-PCR and ELISA were used to assess the expression levels of SERT and 5-HT in the prefrontal cortex of the four groups of mice, respectively. The expression of SERT was lower in the LNC group than in the PND and SSRI groups (), while the expression of 5-HT was significantly increased in the LNC group compared with that in the PND group (). Escitalopram treatment increased the expression of 5-HT but had no effect on the expression of SERT. These findings suggest that the overexpression of lncRNA NONHSAG045500 recovered the disturbances in the serotonin system of PND model mice.
Figure 5. Overexpressed lncRNA NONHSAG045500 inhibited the expression of SERT and the activation of the cAMP–PKA–CREB signaling pathway in the perinatal mouse model. Levels of SERT (A), 5-HT (B), and cAMP (C) in each group (n = 15 in each group). (D)Hematoxylin–eosin staining and expression of PKA and CREB in each group. Bar, 100 μm (black). Differences among the four groups were measured using one-way ANOVA and post hoc Student’s t-test. *p < .05, ns, not significant.
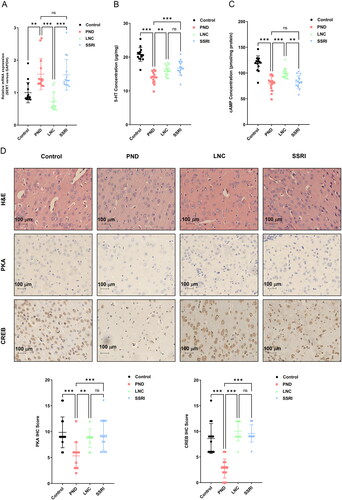
The cAMP–PKA–CREB signaling pathway is closely related to disturbances of the serotonin system and the pathogenesis of depression [Citation15]. Therefore, we speculated that the upregulation of lncRNA NONHSAG045500 influences the depressive-like behavior of PND mice by regulating the cAMP–PKA–CREB signaling pathway. We examined the expression of cAMP, PKA, and CREB in the prefrontal cortex of all mouse groups. ELISA revealed that the expression of cAMP in the PND group was significantly lower than that in the control group (). Compared with the PND group, cAMP levels in the prefrontal cortex of mice in the LNC group were significantly increased (). The pathological results showed that the prefrontal cortex of mice in the PND group had a loose structure, interstitial edema, widened space around cells, vacuolar degeneration, and nerve cell necrosis, which were all improved by lncRNA NONHSAG045500 overexpression and escitalopram treatment (). The level of PKA and CREB proteins, measured via immunohistochemistry, in the PND group were significantly lower than those in control group (). Notably, mice in both the LNC and SSRI groups exhibited a significant increase in PKA and CREB levels in the prefrontal cortex compared with mice in the PND group (). These results suggest that the disturbed serotonin system and inhibited signaling of the cAMP–PKA–CREB signaling was rescued by upregulation of lncRNA NONHSAG045500 expression.
Discussion
In this study, we confirmed that the expression of lncRNA NONHSAG045500 was downregulated in a mouse model of PND. Overexpression of lncRNA NONHSAG045500 alleviated depressive-like behaviors in PND-modeled mice, similar to the effect of escitalopram treatment. The molecular mechanism may be related to the regulation of the serotonin system and cAMP–PKA–CREB signaling pathway. These data provide evidence for a possible link between abnormal lncRNA NONHSAG045500 expression and the onset of PND.
Several animal models of depression have been developed to supplement clinical studies of gestational depression. Typically, these models utilize chronic mild stress or CUS to produce depressive-like behavior in rodents [Citation23]. Most of these models of gestational maternal depression focus on the mid-to-late gestational periods. A review of 31 studies in rodents revealed that only one of them examined the effects of stress in rodents during prepregnancy with depression [Citation23,Citation24]. Most existing studies on maternal depression applied stressors during the latter period of pregnancy and thus missed the most critical and sensitive period of early pregnancy. In the present study, we established a PND model by exposing mice to CUS during the prepregnancy period and found that prepregnancy exposure to CUS led to depressive-like behaviours in mice during the gestational period, and this effect lasted until postpartum. The period of early pregnancy may be a critical and sensitive time window for the onset of PND, which is consistent with our results. Nevertheless, prepregnancy exposure to CUS is considered an acceptable model of PND.
Many studies have explored epigenetic biomarkers for PND and MDD, such as DNA methylation and miRNA. For instance, Numata et al. found significant differences in DNA methylation at 363 CpG sites in the discovery set for patients with MDD and reported that maternal depression was associated with alterations in DNA methylation in maternal T lymphocytes, neonatal cord blood T lymphocytes, and adult offspring hippocampi [Citation25]. In addition, miRNAs have been found to be involved in the development of PND and MDD, and changes in the expression of miRNA were correlated with the improvement of symptoms [Citation26,Citation27]. As an epigenetic regulatory molecule, lncRNAs play important roles in disease pathogenesis, and the use of lncRNAs as novel biomarkers in various cancer types and nervous system diseases has been investigated through clinical research studies [Citation8,Citation28]. lncRNAs may also contribute to the pathophysiology of MDD because of their association with cognitive disorders and synaptic plasticity [Citation11,Citation29]. Recent studies have reported that NONHSAG004550 was significantly differentially expressed in the PND model and that this expression was altered based on the amelioration of depressive symptoms [Citation12,Citation30]. Consistent with the findings of the abovementioned studies, our results showed that the expression of NONHSAG045500 in the PND group was significantly downregulated. Notably, upregulation of NONHSAG045500 ameliorated depressive-like behaviors in PND-modeled mice, along with body weight gain. To the best of our knowledge, our findings provide the first evidence that the lncRNA NONHSAG045500 participates in the pathogenesis of PND and that it could potentially be established as a new therapeutic target of PND. Although lncRNA regulation has increasingly become the new direction of disease treatment, multiple challenges and opportunities exist for the future pharmacological manipulation of lncRNAs.
With several clinical studies suggesting an effect of the serotonin system on PND, SSRIs are the first line of treatment for maternal affective disorders and are prescribed to up to 10% of pregnant women [Citation31]. However, SSRIs administered during the perinatal period can affect the development of the hypothalamic–pituitary–adrenal axis (HPA), and these effects may be linked to PND [Citation32]. The peripartum period is associated with dramatic changes in the HPA. The rapid decline in estradiol levels and abnormal glucocorticoid dynamics during postpartum contribute to risk mechanisms for PND in susceptible women [Citation33]. Disturbances in the serotonin system are involved in the pathophysiology and treatment mechanisms of MDD, and this system is sensitive to hormone fluctuations, which may be a critical factor in PND susceptibility. Regulation of the expression of NONHSAG045500 has been shown to alter the levels of 5-HT and SERT [Citation12]; therefore, we speculated that the overexpression of NONHSAG045500 affects the depressive-like behaviors of PND mice by regulating the serotonin system. We found that the expression level of 5-HT was decreased while that of SERT was increased in the PND mouse model compared with their respective expression levels in control mice. Consistent with previous studies, upregulating the expression of NONHSAG04550 increased the expression level of 5-HT and decreased that of SERT in LNC mice compared with those in PND mice. 5-HT has been linked to various physiological and pathological processes as it is involved in multiple signal transduction pathways. A previous study found that 5-HT is an inhibitory G-protein coupled receptor [Citation34, Citation35]. Agonist binding to 5-HT exchanges GDP for GTP on the subunit of Gi/o and then inhibits adenylyl cyclase, resulting in decreased intracellular cAMP production [Citation36]. In turn, cAMP, as an important second messenger, mediates a number of intracellular signaling cascades, including the PKA–CREB signaling pathway. Several in vivo studies have indicated that the cAMP–PKA–CREB signaling pathway is closely related to depression [Citation14,Citation15,Citation37]. CUS reduced the levels of cAMP, PKA, and CREB in depression model rats [Citation38]. Thus, we speculated that NONHSAG045500 overexpression contributes to improved depressive-like behaviors via the regulation of the cAMP–PKA–CREB signaling pathway. Our results showed that the activity of cAMP–PKA–CREB signaling was downregulated in PND mice and that the overexpression of NONHSAG045500 increased the abnormal inhibition of cAMP–PKA–CREB signaling pathway. In summary, we speculate that overexpression of NONHSAG045500 inhibited the expression of SERT and increased the expression level of 5-HT via cAMP–PKA–CREB signaling to eventually alleviate depressive-like behaviors in PND model mice.
This study has some limitations. First, different types of animal models of depression, such as the reserpine-induced, learned helplessness, CUS, social defeat stress, and corticosterone chronic oral exposure models, are used to explore the molecular mechanism that underlies depression in laboratory experiments [Citation39]. Herein, we only used the CUS model to explore the effect of NONHSAG045500 on PND. Further research will be required to confirm whether our findings are reproducible in other depression models. Second, the LNC group received an injection of 2 × 106 NONHSAG045500 overexpression cells (dose of NONHSAG045500). Our study did not explore the optimal number of cells for injection. In future studies, we need to explore the number of cells injected to determine the optimal injection dose. Third, the overexpression of NONHSAG045500 improved depressive-like behaviors in the CUS model, and whether knockout of NONHSAG045500 will aggravate the depressive-like behaviors requires further research. Fourth, the expression level of NONHSAG045500 may indeed become a potential diagnostic and prognostic marker of PND; however, this needs to be explored and verified in a large population. Moreover, as a potential target of depression, how to intervene NONHSAG045500 expression in patients with depression remains a considerable challenge.
This is the first study to demonstrate the decreased expression of lncRNA NONHSAG045500 in a PND mouse model. Notably, increasing the expression of lncRNA NONHSAG045500 improves depressive-like behaviors in PND model mice, which may be related to the effect of NONHSAG045500 on the regulation of serotonin system. We demonstrated that NONHSAG045500 can regulate SERT expression to ameliorate depressive-like behavior via the cAMP–PKA–CREB signaling pathway. These results suggest that the lncRNA NONHSAG045500 is involved in the pathogenesis of PND and that this lncRNA may be a potential diagnostic and prognostic marker of PND. Further research will be required to confirm these mechanisms at the molecular and gene level and to fully elucidate the antidepressant effects of NONHSAG045500.
Supplemental Material
Download MS Word (17 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Freedman SA, Reshef S, Weiniger CF. Post-traumatic stress disorder and postpartum depression and their reported association with recent labor and delivery: a questionnaire survey cohort. Int J Obstet Anesth. 2020;43:18–24.
- Wenzel ES, Pinna G, Eisenlohr-Moul T, et al. Neuroactive steroids and depression in early pregnancy. Psychoneuroendocrinology. 2021;134:105424.
- Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2008;9(1):11–25.
- Salazar de Pablo G, Solmi M, Vaquerizo-Serrano J, et al. Primary prevention of depression: an umbrella review of controlled interventions. J Affect Disord. 2021;294:957–970.
- Ramsteijn AS, Jasarevic E, Houwing DJ, et al. Antidepressant treatment with fluoxetine during pregnancy and lactation modulates the gut microbiome and metabolome in a rat model relevant to depression. Gut Microbes. 2020;11(4):735–753.
- Unroe KA, Glover ME, Shupe EA, et al. Perinatal SSRI exposure disrupts G protein-coupled receptor BAI3 in developing dentate gyrus and adult emotional behavior: relevance to psychiatric disorders. Neuroscience. 2021;471:32–50.
- Millard SJ, Lum JS, Fernandez F, et al. Perinatal exposure to fluoxetine increases anxiety- and depressive-like behaviours and alters glutamatergic markers in the prefrontal cortex and hippocampus of male adolescent rats: a comparison between Sprague-Dawley rats and the Wistar-Kyoto rat model of depression. J Psychopharmacol. 2019;33(2):230–243.
- Jovcevska I, Videtic Paska A. Neuroepigenetics of psychiatric disorders: focus on lncRNA. Neurochem Int. 2021;149:105140.
- Bella F, Campo S. Long non-coding RNAs and their involvement in bipolar disorders. Gene. 2021;796-797:145803.
- Huan Z, Mei Z, Na H, et al. lncRNA MIR155HG alleviates depression-like behaviors in mice by regulating the miR-155/BDNF axis. Neurochem Res. 2021;46(4):935–944.
- Wang L, Zhang M, Zhu H, et al. Combined identification of lncRNA NONHSAG004550 and NONHSAT125420 as a potential diagnostic biomarker of perinatal depression. J Clin Lab Anal. 2021;35(8):e23890.
- Liu S, Zhou B, Wang L, et al. Therapeutic antidepressant potential of NONHSAG045500 in regulating serotonin transporter in major depressive disorder. Med Sci Monit. 2018;24:4465–4473.
- Wang C, Guo J, Guo R. Effect of XingPiJieYu decoction on spatial learning and memory and cAMP-PKA-CREB-BDNF pathway in rat model of depression through chronic unpredictable stress. BMC Complement Altern Med. 2017;17(1):73.
- Chang B, Liu Y, Hu J, et al. Bupleurum Chinense DC improves CUMS-induced depressive symptoms in rats through upregulation of the cAMP/PKA/CREB signalling pathway. J Ethnopharmacol. 2022;289:115034.
- Li XY, Qi WW, Zhang YX, et al. Helicid ameliorates learning and cognitive ability and activities cAMP/PKA/CREB signaling in chronic unpredictable mild stress rats. Biol Pharm Bull. 2019;42(7):1146–1154.
- Duman CH, Schlesinger L, Kodama M, et al. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61(5):661–670.
- Xiang D, Sun S, Wang G, et al. Effects of CRMP2 DNA methylation in the hippocampus on depressive-like behaviors and cytoskeletal proteins in rats. Front Cell Neurosci. 2021;15:644663.
- Gemmel M, Harmeyer D, Bogi E, et al. Perinatal fluoxetine increases hippocampal neurogenesis and reverses the lasting effects of pre-gestational stress on serum corticosterone, but not on maternal behavior, in the rat dam. Behav Brain Res. 2018;339:222–231.
- Kiryanova V, Meunier SJ, Dyck RH. Behavioural outcomes of adult female offspring following maternal stress and perinatal fluoxetine exposure. Behav Brain Res. 2017;331:84–91.
- Kiryanova V, Meunier SJ, Vecchiarelli HA, et al. Effects of maternal stress and perinatal fluoxetine exposure on behavioral outcomes of adult male offspring. Neuroscience. 2016;320:281–296.
- Xu H, Liu C, Rao S, et al. LncRNA NONRATT021972 siRNA rescued decreased heart rate variability in diabetic rats in superior cervical ganglia. Auton Neurosci. 2016;201:1–7.
- Zepeda-Quiroz N, Luna-Reséndiz R, Soto-Sánchez J. Efficacy of individualized homeopathy in treatment-resistant depression. Cureus. 2021;13(10):e18444.
- Vestring S, Serchov T, Normann C. Animal models of depression – chronic despair model (CDM). J Vis Exp. 2021;(175). DOI:10.3791/62579
- Wang Q, Timberlake MA, Prall K, et al. The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:99–109.
- Nemoda Z, Massart R, Suderman M, et al. Maternal depression is associated with DNA methylation changes in cord blood T lymphocytes and adult hippocampi. Transl Psychiatry. 2015;5(4):e545.
- Wei W, Wang ZY, Ma LN, et al. MicroRNAs in alzheimer’s disease: function and potential applications as diagnostic biomarkers. Front Mol Neurosci. 2020;13:160.
- Wan Y, Liu Y, Wang X, et al. Identification of differential microRNAs in cerebrospinal fluid and serum of patients with major depressive disorder. PLOS One. 2015;10(3):e0121975.
- Meseure D, Drak Alsibai K, Nicolas A, et al. Long noncoding RNAs as new architects in cancer epigenetics, prognostic biomarkers, and potential therapeutic targets. Biomed Res Int. 2015;2015:320214.
- Kaur P, Liu F, Tan JR, et al. Non-coding RNAs as potential neuroprotectants against ischemic brain injury. Brain Sci. 2013;3(1):360–395.
- Cui X, Sun X, Niu W, et al. Long non-coding RNA: potential diagnostic and therapeutic biomarker for major depressive disorder. Med Sci Monit. 2016;22:5240–5248.
- Overgaard A, Lieblich SE, Richardson R, et al. Paroxetine blunts the corticosterone response to swim-induced stress and increases depressive-like behavior in a rat model of postpartum depression. Psychoneuroendocrinology. 2018;89:223–228.
- Seth S, Lewis AJ, Galbally M. Perinatal maternal depression and cortisol function in pregnancy and the postpartum period: a systematic literature review. BMC Pregnancy Childbirth. 2016;16(1):124.
- Guintivano J, Arad M, Gould TD, et al. Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Mol Psychiatry. 2014;19(5):560–567.
- Ślifirski G, Król M, Turło J. 5-HT receptors and the development of new antidepressants. IJMS. 2021;22(16):9015.
- Birnbaumer L. Expansion of signal transduction by G proteins. The second 15 years or so: from 3 to 16 alpha subunits plus betagamma dimers. Biochim Biophys Acta. 2007;1768(4):772–793.
- Kim MH, Leem YH. Chronic exercise improves repeated restraint stress-induced anxiety and depression through 5HT1A receptor and cAMP signaling in hippocampus. J Exerc Nutrition Biochem. 2014;18(1):97–104.
- Zhao L, Guo R, Cao N. An integrative pharmacology-based pattern to uncover the pharmacological mechanism of ginsenoside H dripping pills in the treatment of depression. Front Pharmacol. 2020;11:590457.
- Peng Y, Zhang C, Su Y, et al. Activation of the hippocampal AC-cAMP-PKA-CREB-BDNF signaling pathway using WTKYR in depression model rats. Electrophoresis. 2019;40(8):1245–1250.
- Hao Y, Ge H, Sun M, et al. Selecting an appropriate animal model of depression. Int J Mol Sci. 2019;20(19):4827.