Abstract
Objective
The aim of this study is to investigate whether Serpin clade C (SERPINC1), E-selectin, P-selectin, Placental protein 13 (PP13), and Retinol-binding protein-4 (RBP4) levels in maternal serum were associated with the presence of preeclampsia and to compare them with uncomplicated pregnancies.
Methods
This prospective study included 40 women with preeclampsia and 40 healthy pregnant women. An enzyme-linked immunosorbent assay kit was used to measure serum SERPINC1, E-selectin, P-selectin, PP13, and RBP4 levels.
Results
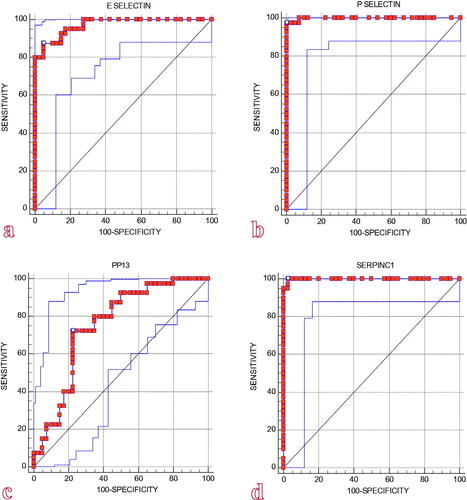
The preeclampsia group had significantly higher E-selectin and P-selectin levels than the control group. PP13 and SERPINC1 levels were also significantly lower than the control group. There was no significant difference in RBP4 levels. The receiver operating characteristic curve revealed the best cutoff values for the following: E-selectin >19.2 ng/mL, with 87.5% sensitivity and 95% specificity; P-selectin >5.1 ng/mL, with 97.5% sensitivity and 100% specificity; PP13 ≤ 107.03 pg/mL, with 72.5% sensitivity and 77.5% specificity; and SERPINC1 ≤ 87.76 ng/mL, with 100% sensitivity and 97.5% specificity.
Conclusion
In this study, the endothelial dysfunction parameters SERPINC1, PP13, E-selectin, and P-selectin were found to be associated with preeclampsia. Endothelial dysfunction biomarkers in maternal non-serum body fluids may differ. More research is needed, especially to determine the relationship between SERPINC1 and preeclampsia.
Introduction
Preeclampsia (PE) is a multisystemic progressive disease characterized by new-onset hypertension and proteinuria or new-onset hypertension and significant end-organ dysfunction with or without proteinuria in the last half of pregnancy or in the postpartum period. It is caused by placental and maternal vascular dysfunction and resolves in a variable amount of time after delivery. In the long term, patients with PE are at risk of developing cardiovascular and renal diseases [Citation1].
Free oxygen radicals enter the maternal circulation in a hypoxic state, which is both the cause and result of poor placentation and may be caused by a variety of unknown factors. The resulting cytokine release is thought to stimulate systemic vascular endothelial dysfunction in the mother and play a role in the pathogenesis of PE. Hypertension is caused by a lack of endothelial regulation of vascular tone, while proteinuria and edema are caused by increased vascular permeability. Eclampsia, visual symptoms, and epigastric pain develop as a result of endothelial dysfunction in the vessels of target organs such as the brain, liver, and kidney. PE and eclampsia are associated with 10%–15% of maternal deaths caused by pregnancy complications worldwide [Citation2].
Stepan et al. [Citation3] defined the pathophysiology of PE as a three-stage problem that has yet to be fully resolved, with each stage causing the next. In the first stage, there is an irregular trophoblast invasion in the first trimester [Citation4]. Free oxygen radicals produced by hypoxia and apoptosis have been implicated. The second stage is pathological uterine perfusion. Placental products released at high rates in maternal blood flow affect trophoblastic cell dynamics [Citation5]. In the third stage, extensive endothelial damage leads to pathophysiological changes. Inappropriate or faulty trophoblastic invasion of the uterine decidua and spiral arteries in early pregnancy has been linked to the development of PE and the restriction of intrauterine growth [Citation6].
Retinol-binding protein-4 (RBP4) is an adipocytokine produced by adipose tissue and has been associated with obesity, insulin resistance, and cardiovascular diseases [Citation7]. High circulating levels of RBP4, an insulin resistance biomarker, are thought to increase the risk of PE [Citation8–10].
Selectins are type I transmembrane glycoproteins expressed in both leukocytes and endothelial cells. P-selectin and E-selectin are involved in implantation as well as maternal immune tolerance and trophoblast migration in spiral arterioles [Citation11]. Because PE is associated with widespread platelet activation and endothelial dysfunction, altered P-selectin and E-selectin expressions are thought to play an important role in the pathophysiology of the disease [Citation12].
Placental protein 13 (PP13), a placental galectin protein, is a homodimeric, phosphorylated protein found in the syncytiotrophoblast. It is an immunoregulatory, vasodilator protein with low lysophospholipase activity and is involved in the placentation process. Low blood PP13 levels have recently emerged as a new biological protein for individualized risk assessment and a target for drug therapy design in the management of PE [Citation13].
Serpin clade C (SERPINC1), encoded by the gene SERPINC1, also commonly known as antithrombin III (ATIII) [Citation14], belongs to the serpin superfamily, is a potent anticoagulant produced in the liver, and commonly found in the blood, kidneys, lungs, heart, brain, and saliva. It is the main inhibitor of serine proteases that are formed during coagulation. This protease exerts both anticoagulant and anti-inflammatory effects. ATIII is the most important inhibitor of the coagulation factor, and even minor aberrations in ATIII cause significant changes in the risk of thromboembolism [Citation15]. Hereditary AT deficiency is a rare autosomal dominant disease caused by mutations in the SERPINC1 gene [Citation16]. AT levels have been shown to vary significantly among women with pregnancy-related hypertension, PE, or eclampsia compared to women with normal pregnancies [Citation17].
Because the onset and clinical course of the disease cannot be predicted, powerful tools are needed for early diagnosis and treatment.
The aim of this study was to examine whether serum SERPINC1, RBP4, E-selectin, P-selectin, and PP13 levels differed between healthy and preeclamptic pregnant women.
Materials and methods
This prospective cross-sectional case-control study included 80 pregnant women, with 40 women diagnosed with, followed up on, and treated for PE in the Department of Obstetrics and Gynecology at the Health Sciences University Umraniye Training and Research Hospital between March and September 2020 and 40 women with normal pregnancy.
The Ethics Committee of our hospital approved the study protocol (date: 19 February, 2020; approval number: B.10.1.TKH.4.34.H.GP.0.01/28). Informed consent was obtained from all participants included in the study.
PE diagnosis was based on the American College of Obstetricians and Gynecologists guideline, which was updated in 2019. Accordingly, women with previously normal blood pressure levels who had recently developed hypertension after gestational week 20 (blood pressure measured at 140/90 mmHg and higher twice at an interval of at least 4 h or at 160/110 mmHg and higher once) in the presence of proteinuria (proteinuria of 300 mg or more and protein-to-creatinine ratio of 0.3 mg/dL in urine sample collected every 24 h or more or protein level of +2 or above in the urine dipstick test) as well as women who did not have proteinuria but whose hypertension was accompanied by signs and symptoms of recent organ damage (such as platelet count less than 100.000; serum creatinine concentrations of over 1.1 mg/dL; doubling of serum creatinine concentration in the absence of other kidney disease; doubling of the blood concentrations of liver transaminases; pulmonary edema; and newly onset headache or visual symptoms that do not respond to medical treatment and cannot be explained by alternative diagnoses) were diagnosed with PE and included in the study [Citation18].
The exclusion criteria were multifetal gestation; premature rupture of the membranes; chorioamnionitis; oligohydramnios; fetal growth restriction; polyhydramnios; fetal anomalies; local or systemic diseases; tobacco use; and medical complications, including autoimmune disorders (antiphospholipid syndrome, systemic lupus erythematosus, haemolytic–uremic syndrome, acute fatty liver of pregnancy, thrombotic thrombocytopenic purpura, and pheochromocytoma); hematologic disease; neurologic disorders; gestational hypertension; hereditary or acquired ATIII deficiency; connective tissue disease; diabetes mellitus; chronic hypertension; inflammation; and chronic renal diseases as well as treatment with aspirin, antihypertensive drugs, non-steroidal antiinflammatory drugs, or antibiotics.
Arterial blood pressure measurements of the patients were taken at the time of diagnosis. Brachial artery blood pressures were measured after 5 min of rest and in the sitting position, with the elbow rest placed at the level of the heart. Complete blood count, urea, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) biochemistry tests as well as complete urinalysis were performed at the time of diagnosis. The gestational weeks at which each patient gave birth were also recorded. The obstetric anamnesis, maternal age, body mass indexes, gestational ages according to the last menstrual period, and crown-rump length of all participating pregnant women were recorded.
After 8 h of fasting following the hospitalization, 5 ml of venous blood samples were collected and placed into gel biochemistry tubes to measure the serum levels of E-selectin, P-selectin, SERPINC1, PP13, and RBP4 in preeclamptic and normotensive pregnant women. None of the patients in labour at the time of the sampling. Serum E-selectin, P-selectin, and SERPINC1 were centrifuged at 3000 rpm for 20 min. Serum PP13 and RBP4 were centrifuged at 1000 × g for 15 min. The serum samples were separated into Eppendorf tubes and labeled with each patient’s name. The samples were stored at −80 °C until the analysis was conducted. The gestational week in which the sample was collected was recorded. The temperatures of the samples stored in the cold chain were brought down to room temperature. The sandwich enzyme-linked immunosorbent assay (ELISA) method was used to measure the biomarker levels.
The ELISA kits used in the study belonged to the company “Bioassay Technology Laboratory.” (1008 Junjiang Inter. Bldg., 228 Ningguo Rd., Yangpu Dist., Shanghai, China) All measurements were taken according to the manufacturer’s instructions. The measuring sensitivity, ranges, intra-assay precision, and inter-assay precision of the kits were 0.56 ng/ml, 3–180 ng/ml, <8% and <10%, respectively, for E-selectin (Cat. No. E0262Hu); 0.22 ng/ml, 0.5–200 ng/ml, <8% and <10%, respectively, for P-selectin (Cat. No. E0201Hu); 2.96 ng/ml, 5–1000 ng/ml, <8% and <10%, respectively, for SERPINC1 (Cat. No. E4267Hu); 0.51 ng/ml, 1–400 ng/ml, <8% and <10%, respectively, for RBP4 (Cat. No. E1206Hu); and 5.52 pg/ml, 10–2000 pg/ml, <8% and <10%, respectively, for PP13 (Cat. No. E4786Hu).
Statistical analysis
The statistical analyses were performed using the Statistical Package for the Social Sciences, version 22 (SPSS, Inc.). The conformity of the parameters to the normal distribution was evaluated by the Kolmogorov–Smirnov and Shapiro–Wilks tests. While evaluating the study data, in addition to descriptive statistical methods (mean, standard deviation, and frequency), the Student’s t-test was used for the comparison of normally distributed parameters between two groups, and the Mann–Whitney U test was used to compare parameters that did not show normal distribution between two groups. The Fisher’s exact test and Continuity (Yates) Correction were used to compare qualitative data. Pearson correlation analysis was used to examine the relationships between the parameters conforming to the normal distribution, and Spearman’s rho correlation analysis was used for the parameters that did not. The most appropriate cutoff point was chosen based on the receiver operating characteristic (ROC) curve analysis. The significance level was set at p < .05.
Results
The study included 80 female subjects aged between 19 and 41 years. The mean age was 28.55 ± 5.44 years. The cases were divided into two groups: the “PE group” and the “control group.” Each group had 40 subjects.
The mean age was significantly lower in the PE group than in the control group (p = .006; p < .05). There was no statistically significant difference between the groups in terms of the mean gestational week and gestational day (p > .05). Gestational week at birth (p = .000; p < .05), mean birth weight (p = .000; p < .05), and Apgar scores at 1 and 5 min were significantly lower in the PE group (p = .001; p = .004; p < .05) than in the control group. There was no statistically significant difference between the groups in terms of the incidence of nulliparity (p > .05). The rate of proteinuria (p = .005; p < .05) and the need for admission to the neonatal intensive care unit (32.5%) were significantly higher in the PE group (p = .029; p < .05) than in the control group. There was no statistically significant difference between the groups in terms of PE history, the incidence of “hemolysis, elevated liver enzymes, and low platelets” (HELLP) syndrome, eclampsia, in utero mort fetalis (IUMF), and the mode of delivery (p > .05). The mean LDH was significantly lower in the PE group than in the control group (p = .047; p < .05). There was no statistically significant difference between the groups in terms of ALT, AST, creatinine, blood urea nitrogen, and platelet levels (p > .05) ().
Table 1. Evaluation of the groups in terms of maternal and perinatal parameters.
E-selectin and P-selectin levels were significantly higher in the PE group than in the control group (p = .000; p < .05). PP13 and SERPINC1 levels were significantly lower in the PE group than in the control group (p = .000; p < .05). There was no statistically significant difference between the groups in terms of RBP4 levels (p > .05) ().
Table 2. Evaluation of the groups in terms of biomarkers.
There was no statistically significant difference between the cases with and without proteinuria in terms of markers (p > .05) ().
Table 3. Evaluation of biomarkers according to the presence of proteinuria in the preeclampsia group.
The area under the curve (AUC) in the ROC analysis for the performance of E-selectin levels in diagnosing PE was 0.971, with a standard error of 0.014 (AUC: 0.971; 95% CI: 0.906–0.995), which was significantly higher (p = .001; p < .05). The cutoff level for E-selectin in the diagnosis of PE was 19.2, with a sensitivity of 87.5% and a specificity of 95%. () The AUC in the ROC analysis for the performance of P-selectin levels in diagnosing PE was 0.998, with a standard error of 0.002 (AUC: 0.998; 95% CI: 0.951–1.000), which was significantly higher (p = .001; p < .05). The cutoff level for P-selectin in the diagnosis of PE was 5.1, with a sensitivity of 97.5% and a specificity of 100%. () The area under the ROC curve for the performance of PP13 levels in the diagnosis of PE was 0.752, with a standard error of 0.056 (AUC: 0.752; 95% CI: 0.643–0.842), which was significantly higher (p = .001; p < .05). The cutoff level for PP13 in the diagnosis of PE was 107.03, with a sensitivity of 72.5% and a specificity of 77.5%. () The area under the ROC curve for the performance of SERPINC1 levels in diagnosing PE was 0.999, with a standard error of 0.002 (AUC: 0.999; 95% CI: 0.952–1.000), which was significantly higher (p = .001; p < .05). The cutoff level for SERPINC1 in the diagnosis of PE was 87.76, with a sensitivity of 100% and a specificity of 97.5%. ().
Discussion
PE is predicted using a large number of biochemical markers [Citation19,Citation20]. There is no single biochemical marker that is sufficient, and the efficacy of a single marker increases when it is used in conjunction with other parameters [Citation21]. For this purpose, we preferred to use more than one biomarker. To the best of our knowledge, our study is the first to compare serum endothelial damage parameters (E-selectin, P-selectin, RBP4, PP13, and SERPINC1) in preeclamptic and healthy pregnant women during the third trimester of pregnancy.
Kovac et al. reported a higher incidence of fetal death, recurrent pregnancy loss, fetal growth restriction, and placental abruption in pregnant women with mutations in the SERPINC1 gene [Citation22]. ATIII deficiency is the main cause of hereditary thrombophilia. A large-scale study reported a significant association between thrombophilia and severe PE [Citation23], but plasma ATIII levels did not differ significantly between the PE patient group and the control group [Citation24]. Besides ATIII deficiency, ATIII encoded by the SERPINC1 gene has been associated with hypertension and kidney disease [Citation16]. Zhou et al. reported that women who developed gestational hypertension before the 20th gestational week had significantly higher SERPINC1 levels than healthy pregnant women [Citation25]. In contrast, in a case report by Tomczykowska et al. researchers reported that SERPINC1 is associated with hypertension, and patients with hypertension have significantly lower ATIII plasma levels than healthy individuals [Citation26]. In addition to its anticoagulant properties, ATIII has been reported to protect against hypertension and proteinuria during pregnancy [Citation27]. Another study reported that while ATIII levels do not change significantly in normal pregnancies, they may decrease significantly in women with pregnancy-induced hypertension, PE, or eclampsia [Citation28]. Similarly, in our study, SERPINC1 levels were significantly lower in the PE group than in the control group. The cutoff level for SERPINC1 in diagnosing PE was 87.76. The sensitivity of this value was found to be 100%, with a specificity of 97.5%. We hypothesize that SERPINC1 levels play an active role in the pathogenesis of PE, considering coagulation disorders, vascular endothelial damage, inflammatory processes, and metabolic imbalances. This study found that serum SERPINC1 levels were significantly lower in patients with PE than in healthy pregnant women.
PP13 serum levels increase gradually during a normal pregnancy. Decreased PP13 levels have been found, particularly in those with early-onset PE [Citation29]. In a study by Luo and Han, significantly elevated PP13 levels were found in the PE group between 24 and 28 weeks of gestation [Citation30]. Romero et al. concluded that maternal serum PP13 levels in the first trimester were a reasonable marker in risk assessment for early PE, but it was found to be a poor marker for severe PE at term and ineffective in diagnosing mild PE at term [Citation29]. Sammar et al. suggest that PP13 acts via nitric oxide and prostaglandin signaling pathways to supply oxygen and nutrients to the growing fetus. They also believed that it could be important, especially in the last trimester, in preventing chronic placental abruption and potential fetal death [Citation31]. Many studies have shown that a decrease in PP13 expression is one of the earliest signs of PE development, demonstrating its predictive performance for PE. It has also been suggested to be a reliable biomarker in the third trimester [Citation13,Citation31–35]. Similarly, in the present study, PP13 levels were significantly lower in the PE group than in the control group. The cutoff point for PP13 in diagnosing PE was 107.03. The sensitivity of this value was 72.5%, with a specificity of 77.5%. The contradictory results regarding PP13 in the 3rd trimester suggest that PP13 may not be sufficient as a single biomarker in the management of PE.
Current reports on the potential role of RBP4 in the pathogenesis of PE are still insufficient and controversial. Changes in circulating RBP4 levels may affect physiological and metabolic adaptations during normal pregnancy and cause various pregnancy complications, including PE [Citation36]. Both maternal and umbilical cord serum RBP4 levels in patients with PE were lower in the third trimester of pregnancy than in normal pregnant women [Citation37].
While some researchers agreed that plasma RBP4 levels differed between patients with severe PE and normal pregnant women [Citation37–39], others disagreed [Citation40]. However, in our study, there was no significant difference between the groups in terms of RBP4 levels. In our opinion, due to the different results obtained, the usability of RBP4 in PE remains limited.
In a study conducted by Acar et al. plasma P-selectin levels were found to be higher in the PE group than in healthy pregnant women, while E-selectin levels did not differ significantly between the two groups, and they believed that high selectin levels were nonspecific consequences of endothelial damage rather than a cause [Citation41]. There was no difference in E-selectin and P-selectin levels between women with a history of severe early-onset PE and women who had an uncomplicated pregnancy approximately 10 years later, implying that angiogenic factors do not contribute to the early detection of women who are at risk for cardiovascular disease in the future [Citation42]. Docheva et al. found that women with PE, patients delivering small for gestational age fetuses, and patients experiencing fetal death had higher levels of E-selectin and P-selectin than normal, healthy pregnant women. They showed that endothelial cell activation, as measured by E-selectin plasma levels, is not specific for PE but is present in pregnancies complicated by small-for-gestational-age fetuses, acute pyelonephritis, and fetal death [Citation43]. Another study showed higher maternal E-selectin levels in the PE group but low fetal E-selectin and P-selectin levels. E-selectin expression increased while placental P-selectin expression decreased in PE. Increased E-selectin levels reflect endothelial dysfunction, which is a feature of PE. Conversely, decreased P-selectin levels in PE may be a protective mechanism to limit endothelial dysfunction [Citation44]. Recent evidence suggests that E-selectin levels in the maternal circulation increase during pregnancy as well as in the presence of certain pregnancy complications such as PE [Citation45,Citation46]. Plasma E-selectin levels were significantly higher in the patient groups than in the control group (p = 0.002). The ROC curve showed that the best cutoff level for E-selectin was 64.3 ng/mL, with a sensitivity of 58% and a specificity of 80.0% [Citation47]. In our study, E-selectin and P-selectin levels were significantly higher in the PE group than in the control group. The cutoff level for E-selectin in diagnosing PE was 19.2. The sensitivity of this value was 87.5%, with a specificity of 95%. The cutoff point determined for P-selectin in diagnosing PE was 5.1. The sensitivity of this value was 97.5%, with a specificity of 100%. We think that selectin levels, especially E-selectin elevation, can be used in the pathophysiology and management of PE.
Screening pregnant women for PE using an effective diagnostic marker can reduce unnecessary procedures and major healthcare costs. In addition, hospitalization of pregnant women with suspected or mild PE and no indication can be prevented. By monitoring the progression of the disease, preterm births can be reduced by planning the ideal delivery time.
Although many biochemical and hematological markers have been studied for the prediction and monitoring of PE, there is no reliable marker for the disease. The presence of hypertension and the degree of proteinuria are poor prognostic factors for maternal and fetal outcomes, and better diagnostic and prognostic markers are needed.
Strengths and limitations
This is possibly the first study on serum SERPINC1, PP13, RBP4, E-selectin, and P-selectin levels in relation to PE. The study has some limitations. Owing to the small number of cases with a history of PE, severe PE, HELLP syndrome, eclampsia, and IUMF, the relationship between the markers and these parameters could not be investigated. Patients with co-morbidities such as diabetes mellitus, fetal growth restriction and congenital or acquired ATIII deficiencies were excluded from the study.
Conclusion
Early identification of pregnant women who are at a high risk of PE, PE management, and improvement of treatment approaches are important obstetric goals. Based on the study data, we believe that high E-selectin and P-selectin levels as well as low SERPINC1 and PP13 values measured in the third trimester can be used to diagnose PE. We believe that the use of E-selectin, P-selectin and PP13, especially in combination with SERPINC1, may have an important role in the pathophysiology and management of PE.
Authors’ contributions
Both authors contributed to the study’s conception and design. Material preparation and data collection and analysis were performed by RM Palalioglu and HI Erbiyik. The first draft of the manuscript was written by RM Palalioglu. commented on previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization: RM Palalioglu; Methodology: RM Palalioglu; Formal analysis and investigation: RM Palalioglu and HI Erbiyik; Writing – original draft preparation: RM. Palalioglu, HI Erbiyik; Writing – review and editing: RM Palalioglu and HI Erbiyik; Resources: RM Palalioglu and HI Erbiyik; Supervision: RM Palalioglu and HI Erbiyik.
Consent for publication
Patients signed informed consent regarding publishing their data.
Availability of data and material
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Acknowledgments
Ethics approval and consent to participate: The Ethics Committee approved the study protocol (Date: February 19, 2020; approval number: B.10.1.TKH.4.34.H.GP.0.01/28). Written informed consent was obtained from all individual participants included in the study.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- American college of obstetricians and gynecologists (ACOG) practice bulletin no. 222: gestational hypertension and preeclampsia. Obstet Gynecol. 2020;135(6):e237–e260.
- O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368–374.
- Stepan H, Faber R, Froster UG, et al. Preeclampsia as a three stage problem: a work shop report. Placenta. 2004;25(6):585–587.
- Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187(5):1416–1423.
- Crocker IP, Cooper S, Ong SC, et al. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol. 2003;162:637–643.
- Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol. 1977;4(3):573–593.
- Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–362.
- Sun Q, Kiernan UA, Shi L, et al. Plasma retinol-binding protein 4 (RBP4) levels and risk of coronary heart disease: a prospective analysis among women in the nurses’ health study. Circulation. 2013;127(19):1938–1947.
- Mol B, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet. 2016;387(10022):999–1011.
- )Scioscia M, Gumaa K, Rademacher TW. Rademacher TW the link between insulin resistance and preeclampsia: new perspectives. J Reprod Immunol. 2009;82(2):100–105.
- Feng Y, Ma X, Deng L. Role of selectins and their ligands in human implantation stage. Glycobiology. 2017;27(5):385–391.
- Holthe MR, Staff AC, Berge LN, et al. Different levels of platelet activation in preeclamptic, normotensive pregnant, and nonpregnant women. Am J Obstetr Gynecol. 2004;190(4):1128–1134.
- Gadde R, Cd D, Sheela SR. Placental protein 13: an important biological protein in preeclampsia. J Circ Biomark. 2018;7:1849454418786159.
- Huntington JA. Shape-shifting serpins–advantages of a mobile mechanism. Trends Biochem Sci. 2006;31(8):427–435.
- Hathcock J. Vascular biology-the role of tissue factor. Semin Hematol. 2004;41(1 Suppl 1):30–34.
- Gatto M, Iaccarino L, Ghirardello A, et al. Serpins, immunity and autoimmunity: old molecules, new functions. Clin Rev Allergy Immunol. 2013;45(2):267–280.
- Weenink GH, Kahlé LH, Lamping RJ, et al. Antithrombin III in oral contraceptive users and during normotensive pregnancy. Acta Obstet Gynecol Scand. 1984;63(1):57–61.
- ACOG. Practice Bulletin No 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):1.
- Ölmez F, Oğlak SC, Gedik Özköse Z. Increased maternal serum aquaporin-9 expression in pregnancies complicated with early-onset preeclampsia. J Obstet Gynaecol Res. 2022;48(3):647–653.
- Behram M, Oğlak SC, Doğan Y. Evaluation of BRD4 levels in patients with early-onset preeclampsia. J Gynecol Obstet Hum Reprod. 2021;50(2):101963.
- Gu Y, Lewis DF, Wang Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2008;93(1):260–266.
- Kovac M, Mitic G, Mikovic Z, et al. Thrombophilia in women with pregnancy-associated complications: fetal loss and pregnancy-related venous thromboembolism. Gynecol Obstet Investig. 2010;69(4):233–238.
- Mello G, Parretti E, Marozio L, et al. Thrombophilia is significantly associated with severe preeclampsia: results of a large-scale, case-controlled study. Hypertension (Dallas, Tex.: 1979). 2005;46(6):1270–1274.
- Dehkordi MA, Soleimani A, Haji-Gholami A, et al. Association of deficiency of coagulation factors (prs, prc, ATIII) and FVL positivity with preeclampsia and/or eclampsia in pregnant women. Int J Hematol Oncol Stem Cell Res. 2014;8(4):5–11.
- Zhou C, Song C, Huang X, et al. Early prediction model of gestational hypertension by multi-biomarkers before 20 weeks gestation. Diabetes Metab Syndr Obes. 2021;14:2441–2451.
- Tomczykowska M, Bielak J, Bodys A. Evaluation of platelet activation, plasma antithrombin III and alpha2-antiplasmin activities in hypertensive patients. Ann Univ Mariae Curie Sklodowska Med. 2003;58(1):15–20.
- Shinyama H, Yamanaga K, Akira T, et al. Antithrombin III prevents blood pressure elevation and proteinuria induced by high salt intake in pregnant stroke-prone spontaneously hypertensive rats. Biol Pharm Bull. 1996;19(6):819–823.
- Weenink GH, Treffers PE, Vijn P, et al. Antithrombin III levels in preeclampsia correlate with maternal and fetal morbidity. Am J Obstet Gynecol. 1984;148(8):1092–1097.
- Daskalakis G, Papapanagiotou A. Serum markers for the prediction of preeclampsia. J Neurol Neurophysiol. 2015;6:264.
- Luo Q, Han X. Second-trimester maternal serum markers in the prediction of preeclampsia. J Perinat Med. 2017;45(7):809–816.
- Sammar M, Drobnjak T, Mandala M, et al. Galectin 13 (PP13) facilitates remodeling and structural stabilization of maternal vessels during pregnancy. Int J Mol Sci. 2019;20(13):3192.
- Sammar M, Dragovic R, Meiri H, et al. Reduced placental protein 13 (PP13) in placental derived syncytiotrophoblast extracellular vesicles in preeclampsia – a novel tool to study the impaired cargo transmission of the placenta to the maternal organs. Placenta. 2018;66:17–25.
- Nasser NB, Al-Habib RM, Risala MJ. Correlation between serum & urinary placental protein (Pp13) in pre-eclamptic women at their third trimester. Indian J. Forensic Med. Toxicol. 2020;14:1814–1818.
- Asiltas B, Surmen-Gur E, Uncu G. Prediction of first-trimester preeclampsia: relevance of the oxidative stress marker MDA in a combination model with PP-13, PAPP-A and beta-HCG. Pathophysiology. 2018;25(2):131–135.
- Wu Y, Liu Y, Ding Y. Predictive performance of placental protein 13 for screening preeclampsia in the first trimester: a systematic review and meta-analysis. Front Med. 2021;8:756383.
- )Mazakitovi S, Romero R, Vaisbuch E, et al. Maternal serum adiponectin multimers in preeclampsia. J Perinatal Med. 2009;37:349.
- Seol HJ, Kim JW, Kim HJ. Retinol-binding protein-4 is decreased in patients with preeclampsia in comparison with normal pregnant women. J Perinat Med. 2011;39(3):287–289.
- Shangguan X, Liu F, Wang H, et al. Alterations in serum adipocyte fatty acid binding protein and retinol binding protein-4 in normal pregnancy and preeclampsia. Clin Chim Acta. 2009;407(1-2):58–61.
- Masuyama H, Inoue S, Hiramatsu Y. Retinol-binding protein 4 and insulin resistance in preeclampsia. Endocr J. 2011;58(1):47–53.
- )Stepan H, Ebert T, Schrey S, et al. Preliminary report: serum levels of retinol-binding protein 4 in preeclampsia. Metab.: clin Exp. 2009;58:275.
- Acar A, Altinbaş A, Oztürk M, et al. Selectins in normal pregnancy, preeclampsia and missed abortus. Haematologia (Budap). 2001;31(1):33–38.
- Gaugler-Senden IP, Tamsma JT, van der Bent C, et al. Angiogenic factors in women ten years after severe very early onset preeclampsia. PLOS One. 2012;7(8):e43637.
- Docheva N, Romero R, Chaemsaithong P, et al. The profiles of soluble adhesion molecules in the “great obstetrical syndromes. J Matern Fetal Neonatal Med. 2019;32(13):2113–2136.
- Mistry HD, Ogalde MVH, Broughton Pipkin F, et al. Maternal, fetal, and placental selectins in women with preeclampsia; association with the renin-angiotensin-system. Front Med. 2020;7:270.
- Krieglstein CF, Granger DN. Adhesion molecules and their role in vascular disease. Am J Hypertens. 2001;14(6 Pt 2):44S–54S.
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594.
- Hassan HE, Azzam H, Othman M, et al. Soluble E-selectin, platelet count and mean platelet volume as biomarkers for preeclampsia. Pregnancy Hypertens. 2019;17:1–4.