Abstract
Objective
To compare the fetal thymic thoracic ratio in preeclamptic pregnant women and healthy pregnant women.
Method
Fetal thymic thoracic ratio was evaluated in 240 pregnant women in the third trimester. Patients were examined in two groups. They included 120 preeclamptic pregnant women (study group) women and 120 healthy pregnant women (control group).
Results
The fetal thymic thoracic ratio was found to be statistically significantly lower in preeclamptic cases compared to that in the control group (p = .001). When the fetal thymic thoracic ratio was evaluated between the mild preeclampsia group (0.399 (0.388–0.413)), severe preeclampsia group (0.385 (0.350–0.394)), and the control group (0.43 (0.324–0.462)), it was found statistically significant differences between the groups (p = .001).
Conclusion
The fetal thymic thoracic ratio decreased in preeclamptic pregnant women and this decrease was more pronounced in the severe preeclampsia group. The measurement of fetal thymic thoracic ratio was seen to be beneficial in determining the severity of the disease in preeclamptic pregnant women.
Introductıon
Preeclampsia is a specific pregnancy-related disease characterized by newly onset hypertension and proteinuria after the 20th gestational week [Citation1]. Preeclampsia complicates 2–8% of pregnancies and it is one of the main reasons of maternal and fetal/neonatal morbidity and mortality [Citation1,Citation2]. The exact etiology remains unknown, however, it has been reported that there is a disorder in maternal-fetal immune tolerance and that abnormal placentation is the pathognomonic feature of preeclampsia [Citation3,Citation4].
The thymus is a lymphoepithelial organ originating from the third brachial cleft at the 9th gestational week and descending to the anterior/superior mediastinum at the 12th gestational week [Citation5]. The response of the maternal immune system is of specific importance in limiting the maladaptive immune responses against the semi-allogeneic fetus and placenta with healthy embryo implantation and fetal development [Citation6]. The thymus has a key role in this immune response, and T lymphocytes produced in the thymus, and its subset, the regulatory T cells, have been reported to mediate this response [Citation6,Citation7]. It has been reported that there is deterioration in the maternal-fetal immune tolerance accompanied by decreased regulatory T cells in preeclampsia [Citation8,Citation9], and this case significantly affects both maternal and fetal immune systems [Citation10].
As far as we know, although there are studies in the literature showing that fetal thymus size is reduced in preeclamptic pregnant women [Citation4,Citation11,Citation12], there is no study evaluating fetal thymic thoracic ratio. So we planned this study to compare this ratio in preeclamptic pregnant women and healthy pregnant women with similar gestational weeks.
Materıals and methods
This retrospective study included preeclamptic pregnant women (n:120) who were admitted to Sakarya University Education and Research Hospital, Gynecology and Obstetrics Clinic Perinatology Department between 1 November 2018 and 15 June 2021 and delivered in the same hospital. The control group comprised completely healthy pregnant women (n:120) at a similar gestational week without any pregnancy complications. The data of both groups were obtained from the medical records of the hospital. The fetal thymic thoracic ratio data measured in the third trimester in both groups were obtained from the medical records. Multiple pregnancies, those with gestational or pregestational diabetes, pregnant women with any known medical diseases (chronic hypertension, chronic liver disease, chronic kidney disease, rheumatological diseases), an acute/chronic infectious disease, pregnancies complicated by premature rupture of membranes or chorioamnionitis, patients with fetal structural or chromosomal disorders, patients with evidence of placental insufficiency in the Doppler parameters, pregnant women with sonographic estimated fetal weight <10 percentile and drug use (acetylsalicylic acid, steroids or heparin) were excluded from the study.
Pregnant women with systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg after 20 gestational weeks and with proteinuria (≥300 mg/24-h urine collection, protein to creatinine ratio of ≥0.3 mg/g, or a dipstick reading of 2+ protein) were diagnosed with mild preeclampsia. Cases with gestational hypertension without proteinuria were diagnosed with preeclampsia if they have complications in their heart, lung, liver, kidney, and other vital organs, or the blood system, digestive system, and nervous system. Pregnant women with systolic blood pressure ≥160 mmHg and/or diastolic blood pressure ≥110 mmHg after 20 gestational weeks and with proteinuria (≥300 mg/24-h urine collection, protein to creatinine ratio of ≥0.3 mg/g, or a dipstick reading of 2+ protein) were diagnosed with severe preeclampsia. In the absence of proteinuria, patients with gestational hypertension were diagnosed with severe preeclampsia if they presented any of the following features: new-onset headache, visual disturbances, or abnormalities of the central nervous system; persistent right upper quadrant or epigastric pain; abnormal blood concentrations of liver enzymes mainly manifested by elevated levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST); renal insufficiency: urinary protein quantification ≥2 g/24 h or serum creatinine concentrations greater than 106 μmol0/L; thrombocytopenia: platelet count lower than 100 × 109/L; pulmonary edema [Citation1,Citation13]. The management of the patients was carried out in accordance with current guidelines after they were admitted to the clinic.
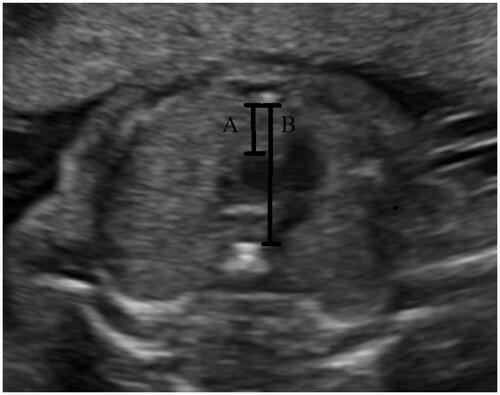
All ultrasound examinations were performed by a single sonographer (Koray Gök) using a Voluson 730 and a Voluson E6 (GE Medical Systems, Milwaukee, WI, USA) ultrasound machine. Thus, the measurements were standardized and bias was limited. All measurements were carried out in the absence of fetal movements. Fetal thymic-thoracic ratio measurement was performed as previously described by Chaoui et al. [Citation14]. Thymus was detected in the three vessels and trachea (3VT) views as a hypoechogenic structure with echogenic dots filling the space between the vessels posteriorly and the anterior chest wall (sternum and ribs) anteriorly. The anteroposterior diameter of the thymus was determined in addition to the midline between the transverse aortic arch border posteriorly, and the posterior chest wall anteriorly. Also, the mediastinal sagittal diameter was determined in addition to the line traced to measure the thymic diameter, as the distance between the anterior edge of the thoracic vertebral body at the level of the transverse arch posteriorly and the internal edge of the sternum anteriorly. Then, the fetal thymic thoracic ratio was calculated as the ratio of the anteroposterior thymic to the intrathoracic mediastinal diameter ().
Figure 1. Thymus and thorax diameters. (A) Thymus diameter (anteroposterior thymus diameter) and (B) thorax diameter (intrathoracic mediastinal diameter), Fetal thymic thoracic ratio: (A)/(B).
The statistical evaluations were carried out using the SPSS 24.0 software (SPSS Inc. and Lead Tech. Inc. Chicago. the USA). The Kolmogorov–Smirnov test was utilized to examine the normality of the distribution of the data. Normally-distributed variables were expressed as mean ± SD whereas skewed variables were reported as the median and interquartile range (IQR). The parametric data were examined by adopting the independent two-sample t-test whereas the non-parametric data were compared using the Mann–Whitney U test. Multiple groups were compared using the Kruskal–Wallis test and the Bonferroni post-hoc correction. Receiver operating characteristic (ROC) analysis was utilized to evaluate the predictive performance of fetal thymic-thoracic ratio for preeclampsia. An alpha <0.05 for Bonferroni correction and a p-value <.05 for other tests were considered to be statistically significant.
Results
The comparison of the properties and fetal thymic thoracic ratio between the preeclamptic cases and the control group are presented in . No statistically significant differences were found between the preeclamptic cases and the control group in terms of age, body mass index (BMI), and the gestational week gestation at which the fetal thymic thoracic ratio was measured. The fetal thymic thoracic ratio was found to be statistically significantly lower in preeclamptic cases compared to that in the control group (p = .001).
Table 1. Comparison of characteristics and the fetal thymic thoracic ratio between preeclamptic cases and the control group.
The fetal thymic thoracic ratio was evaluated utilizing the Kruskal–Wallis test between the mild preeclampsia group, severe preeclampsia group, and the control group, and statistically significant differences were found between the groups (p = .001) (). Evaluating the fetal thymic thoracic ratios using the Mann–Whitney U test within the groups, a statistically significant difference was found between all three groups (p = .001).
Table 2. Kruskal–Wallis test comparing fetal thymic thoracic ratio between the mild preeclampsia group, severe preeclampsia group, and the control group.
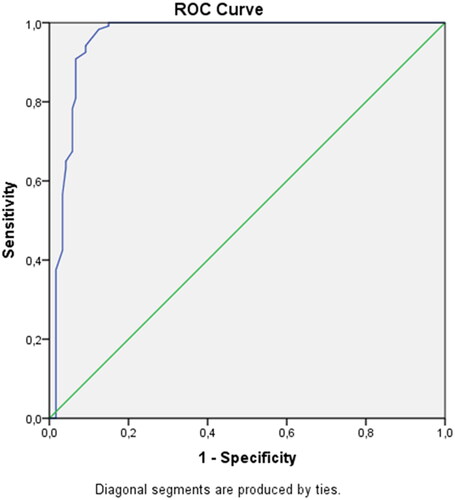
A cutoff value was determined for the fetal thymic thoracic ratio using the ROC curve, and its success in predicting preeclampsia was analyzed. Setting the fetal thymic thoracic ratio cutoff value as 0.409 for the prediction of preeclampsia, sensitivity was found to be 94.2% and specificity to be 90.8% (p = .001) ().
Discussion
The following results were obtained in the present study:
Thymic-thoracic ratio was found to be lower in the preeclamptic group than that in the control group.
Evaluating the preeclamptic pregnant women, it was found that the thymic-thoracic ratio was lower in the severe preeclampsia group.
Setting the fetal thymic thoracic ratio cutoff value as 0.409 to determine preeclampsia, the sensitivity was found to be 94.2% and specificity to be 90.8%.
It has been reported that there was a relationship between the fetal thymus size and various maternal and fetal diseases. Some studies have reported a decrease in fetal thymic transverse diameter in Preterm Prelabor Rupture of Membranes (PPROM) and Intrauterine Growth Restriction (IUGR) [Citation15,Citation16] whereas some have stated that fetal thymus circumference decreased [Citation17,Citation18]. Also, it has been stated that the thymic thoracic ratio decreased in diabetic pregnant women [Citation19,Citation20] and fetuses with congenital heart defects associated with 22q11 microdeletion [Citation14].
In a study on evaluating the fetal thymus size in preeclamptic pregnant women, Mohamed et al. compared 39 preeclamptic pregnant and 70 healthy pregnant women between the 24th and 40th gestational weeks found that both the diameter and the circumference of the fetal thymus were small in the preeclamptic group. The researchers explained this decrease by the fact that cortisol, which is produced as a result of the activation of the hypothalamic-pituitary-adrenal axis due to stress, binds to the cortisol receptor in cortical thymocytes, inducing apoptosis in the fetal thymus [Citation11]. However, it is known that the main problem in preeclampsia is abnormal placentation. Some studies have shown that regulatory T cells decrease in the placenta, cord blood, and maternal blood in preeclamptic pregnant women [Citation8,Citation21–23]. Supporting these data, there are also some studies showing that the fetal thymus size decreases before the development of preeclampsia clinics in preeclamptic pregnant women. One of these studies was conducted between the 11th and 14th gestational weeks [Citation12] whereas another between the 17th and 21st gestational weeks [Citation4]. These studies claimed that the reduction in fetal thymus size may be a valuable result in the prediction of preeclampsia in pregnant women associated with abnormal placentation. The decrease in the fetal thymic thoracic ratio in preeclamptic pregnancies in the present study can also be associated with abnormal placentation in the pathophysiology of these pregnancies. Also, the fetal thymic thoracic ratio was found to be significantly lower in the severe preeclampsia group. This was associated with the fact that, in addition to abnormal placentation, the hypoxic and metabolic stress caused by the disease itself is higher in these pregnant women. Apart from preeclampsia, the decrease in fetal thymus size associated with stress has also been shown in various complicated pregnancies [Citation17,Citation18].
The present study, differing from that conducted by Mohamed et al. [Citation11], adopted a different method including a higher number of patients. Various methods have been reported for ultrasonographic measurement of fetal thymus size [Citation14,Citation24,Citation25]. However, it remains unclear which method is best for detecting maternal and fetal diseases. One of these methods, the measurement of fetal thymic-thoracic ratio is regarded as easy and reliable. Also, the fact that this ratio remains constant throughout the pregnancy, compared to other methods used in the evaluation of fetal thymus size in healthy pregnant women, suggests that this method is more valuable [Citation14].
The retrospective design and the fact that the relationship between fetal thymic thoracic ratio and perinatal outcomes were not investigated can be considered as limitations of this study. However, the present study has advantages including having similar demographic characteristics, having a relatively large sample size, and the fact that measurements were made by a single expert.
Conclusion
It was concluded that fetal thymic thoracic ratio decreased in preeclamptic pregnant women and this decrease was more pronounced in the severe preeclampsia group. The measurement of fetal thymic thoracic ratio was seen to be beneficial in determining the severity of the disease in preeclamptic pregnant women. Prospective, randomized studies are needed to investigate the role of fetal thymic thoracic ratio in prediction of preeclampsia and its relation with perinatal outcomes in the patient group developing preeclampsia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- COG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):1.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–1112.
- Matthiesen L, Berg G, Ernerudh J, et al. Immunology of preeclampsia. Chem Immunol Allergy. 2005;89:49–61.
- Eviston DP, Quinton AE, Benzie RJ, et al. Impaired fetal thymic growth precedes clinical preeclampsia: a case-control study. J Reprod Immunol. 2012;94(2):183–189.
- Tangshewinsirikul C, Panburana P. Sonographic measurement of fetal thymus size in uncomplicated singleton pregnancies. J Clin Ultrasound. 2017;45(3):150–159.
- Collier AY, Smith LA, Karumanchi SA. Review of the immune mechanisms of preeclampsia and the potential of immune modulating therapy. Hum Immunol. 2021;82(5):362–370.
- Moldenhauer LM, Schjenken JE, Hope CM, et al. Thymus-derived regulatory T cells exhibit Foxp3 epigenetic modification and phenotype attenuation after mating in mice. J Immunol. 2019;203(3):647–657.
- Santner-Nanan B, Peek MJ, Khanam R, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183(11):7023–7030.
- Hsu P, Santner-Nanan B, Dahlstrom JE, et al. Altered decidual DC-SIGN + antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am J Pathol. 2012;181(6):2149–2160.
- Santner-Nanan B, Straubinger K, Hsu P, et al. Fetal-maternal alignment of regulatory T cells correlates with IL-10 and Bcl-2 upregulation in pregnancy. J Immunol. 2013;191(1):145–153.
- Mohamed N, Eviston DP, Quinton AE, et al. Smaller fetal thymuses in pre-eclampsia: a prospective cross-sectional study. Ultrasound Obstet Gynecol. 2011;37(4):410–415.
- Basaran OE, Guvendag Guven ES, Guven S. First trimester fetal thymus volume may predict preeclampsia. Pregnancy Hypertens. 2021;26:116–120.
- Brown MA, Magee LA, Kenny LC, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310.
- Chaoui R, Heling KS, Lopez AS, et al. The thymic-thoracic ratio in fetal heart defects: a simple way to identify fetuses at high risk for microdeletion 22q11. Ultrasound Obstet Gynecol. 2011;37(4):397–403.
- Musilova I, Hornychova H, Kostal M, et al. Ultrasound measurement of the transverse diameter of the fetal thymus in pregnancies complicated by the preterm prelabor rupture of membranes. J Clin Ultrasound. 2013;41(5):283–289.
- Ekin A, Gezer C, Taner CE, et al. Prognostic value of fetal thymus size in ıntrauterine growth restriction. J Ultrasound Med. 2016;35(3):511–517.
- Yinon Y, Zalel Y, Weisz B, et al. Fetal thymus size as a predictor of chorioamnionitis in women with preterm premature rupture of membranes. Ultrasound Obstet Gynecol. 2007;29(6):639–643.
- Cromi A, Ghezzi F, Raffaelli R, et al. Ultrasonographic measurement of thymus size in IUGR fetuses: a marker of the fetal immunoendocrine response to malnutrition. Ultrasound Obstet Gynecol. 2009;33(4):421–426.
- Ghalandarpoor-Attar SN, Borna S, Ghalandarpoor-Attar SM, et al. Measuring fetal thymus size: a new method for diabetes screening in pregnancy. J Matern Fetal Neonatal Med. 2020;33(7):1157–1161.
- Dörnemann R, Koch R, Möllmann U, et al. Fetal thymus size in pregnant women with diabetic diseases. J Perinat Med. 2017;45(5):595–601.
- Sasaki Y, Darmochwal-Kolarz D, Suzuki D, et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149(1):139–145.
- Toldi G, Vásárhelyi ZE, Rigó J Jr, et al. Prevalence of regulatory T-cell subtypes in preeclampsia. Am J Reprod Immunol. 2015;74(2):110–115.
- Hu M, Eviston D, Hsu P, et al. Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nat Commun. 2019;10(1):3031.
- Li L, Bahtiyar MO, Buhimschi CS, et al. Assessment of the fetal thymus by two- and three-dimensional ultrasound during normal human gestation and in fetuses with congenital heart defects. Ultrasound Obstet Gynecol. 2011;37(4):404–409.
- De Leon-Luis J, Ruiz Y, Gamez F, et al. Comparison of measurements of the transverse diameter and perimeter of the fetal thymus obtained by magnetic resonance and ultrasound imaging. J Magn Reson Imaging. 2011;33(5):1100–1105.