Abstract
Objective
To assess whether oral domperidone compared to placebo increases the rate of exclusive breastfeeding for 6 months among post-lower segment cesarean section (LSCS) mothers.
Methods
This double-blind Randomized Controlled Trial, conducted in a tertiary care teaching hospital in South India, included 366 post-LSCS mothers with delayed initiation of breastfeeding or with subjective feelings of not having enough milk. They were randomized to two groups - Group A: Standard lactation counseling and oral Domperidone and Group B: Standard lactation counseling and a placebo. The primary outcome was an exclusive breastfeeding rate at 6 months. Exclusive breastfeeding rates at 7 days and 3 months and serial weight gain of an infant were assessed in both groups.
Results
Exclusive breastfeeding rate at 7 days was statistically significant in the intervention arm. The exclusive breastfeeding rates at 3 months and 6 months were higher in the domperidone arm compared to placebo but not statistically significant.
Conclusion
Oral Domperidone along with effective breastfeeding counseling showed an increasing trend of exclusive breastfeeding rate at 7 days and at six months. Appropriate breastfeeding counseling and postnatal lactation support are important in enhancing exclusive breastfeeding.
Trial registration
The study was prospectively registered with CTRI – Reg no. CTRI/2020/06/026237
Introduction
World Health Organization defines Exclusive breastfeeding as giving the infant only breast milk for the first six months without adding any additional water or food. Promoting exclusive breastfeeding is an effective intervention for reducing child mortality. However, the prevalence of exclusive breastfeeding remains less than desired [Citation1]. Breastfeeding within the first hour after birth is an important predictor of continued breastfeeding [Citation2]. Studies have shown that infants born by lower segment cesarean section (LSCS) are four times less likely to receive breastfeeding within the first hour of birth than vaginally delivered infants, probably due to associated maternal anxiety, pain, and infant separation [Citation3]. Moreover, with the increasing number of cesarean sections, there is a need to develop effective measures to improve early initiation and enhance exclusive breastfeeding among post-LSCS mothers also.
Though lactation counseling and postnatal lactation support are the key strategies to enhance exclusive breastfeeding, galactagogues can be useful adjuvants. Domperidone and metoclopramide are the common galactagogues used. Domperidone is preferred due to less side effects. It is a peripherally acting dopamine antagonist which increases prolactin and hence improves lactation with minimal drug secretion into breastmilk [Citation4]. There is limited data on domperidone as a galactagogue in the Indian scenario, especially among post-LSCS mothers. Hence this RCT was done with the research question - Among post lower segment cesarean section (LSCS) mothers (P), does oral domperidone (I) compared to placebo (C) increase the rate of exclusive breastfeeding at 6 months (O)?
Materials and methods
This double-blind randomized controlled trial was done at JIPMER, a tertiary care teaching institute in South India from January 2020 to December 2020 after due approval from the Institute Ethics Committee (IEC) (JIP/IEC/2019/486 dated 10/2/2020). The study was prospectively registered with Clinical Trial Registry of India (CTRI/2020/06/026237). Post-LSCS mothers who initiated breastfeeding after 1 h of delivery or with subjective feeling of not having enough milk (within 48 h of delivery) were included in the study. The mothers were interviewed by the first author in the local language Tamil. Perceived insufficiency of milk was considered if mother felt her baby was not satisfied or remained fussy after feeding or if she was in undue stress regarding breastfeeding. Mothers with preexisting cardiac problems, those who underwent LSCS under general anesthesia, mothers or neonates requiring intensive care and mothers whose neonates were delivered before 34 weeks of gestation were excluded from the study. Randomization was done using Computer generated block randomization with variable size of blocks by a faculty not involved in the study. Allocation concealment was done by serially numbered, opaque sealed envelopes (SNOSE) technique.
Sample size was calculated using Open Epi Version 3.01. The exclusive breastfeeding rate at 6 months was 54.9% according to National Family Health Survey (NHFS) 2015–2016 [Citation5]. Assuming an alpha error of 5% and power of 80%, expecting at least 30% increase in the exclusive breastfeeding rates [Citation6], the sample size was calculated as 366, 183 in each arm, considering an attrition rate of 20%.
All mother-infant dyads meeting the inclusion criteria were recruited from the post-LSCS ward within 48 h of delivery. Informed consent was obtained from the participating mothers. All participants included in the study were randomized to two groups - group A and B. All mothers received standard lactation counseling by the first author which included a 5-min video, shown with a handheld tablet, in the local language Tamil. This included the importance of exclusive breastfeeding, early initiation of breastfeeding and the advantages of breast milk for the infant and the mother. The mothers with breast or nipple issues were given appropriate lactation support. The participants in Group A were given oral Domperidone (Mfd. by Torrent Pharmaceuticals Ltd. India)10 mg thrice a day for 3 days. The participants in group B were given a placebo thrice a day for 3 days containing a plain base with calcium, which was similar in taste, appearance and packing of the domperidone tablet used. Good Manufacturing Practice (GMP) guidelines were adhered to in preparing the placebo. On an average post-LSCS mothers stay for 5–7 days in our hospital. Due to COVID pandemic most of them were discharged early in 3–4 days. Hence a duration of 3 days was considered. Compliance was ensured by the daily check of blisters by the staff nurse.
The primary objective was to assess whether oral domperidone compared to placebo increased the rate of exclusive breastfeeding for 6 months among post-LSCS mothers. Our secondary objectives were to compare the exclusive breastfeeding rates at 7 days and 3 months post-LSCS in both groups, to compare the weight gain of the infant at 6 weeks, 10 weeks, 14 weeks and 9 months, and to compare the usage of donor human milk between both the arms, to compare the volume of expressed breastmilk (in stable preterm babies on expressed breastmilk) and to assess the adverse effect of domperidone in mother or infant, if any.
The significant antenatal, natal, and postnatal data were recorded in a pre-designed proforma. The proportion of mothers who had exclusively breastfed at the end of 7 days was calculated. Milk production was measured only in those mothers who expressed breastmilk for their neonates during hospitalization using standard Paladai/syringe. In view of safety issues related to domperidone, women were routinely screened for cardiac issues prior to enrollment. Mothers with known cardiac problems were excluded. Vital parameters of all post-natal mothers were monitored during the first week of delivery by the staff nurses in their respective wards.
The mother-infant dyads were followed up and data regarding exclusive breastfeeding at 3 months and 6 months were collected. The weight gain of the infant was monitored at 6 weeks, 10 weeks, 14 weeks and at 9 months. The above data were collected when they brought their infants to OPD for any trivial illness or during vaccination. For defaulters, data was collected over phone.
Statistical analysis
Age, birth weight, pre-pregnant body mass Index, gestational age (in weeks) were expressed as mean ± SD and analyzed using the Independent student t-test. Socio-economic status (Modified Kuppusamy classification), mother’s education, occupation, perceived adequacy of breast milk supply, medical maternal illness, if any, exclusive breastfeeding rate at 7 days, 3 months, 6 months and requirement of PDHM were expressed as proportion and analyzed using Chi-square test. Weight of the infant on day 7, 6 weeks, 10 weeks, 14 weeks, 9 months and volume of expressed breastmilk between both the groups was expressed as Mean ± SD and analyzed using an independent student t-test. Intention to treat analysis was done and a p value < .05 was considered significant.
Results
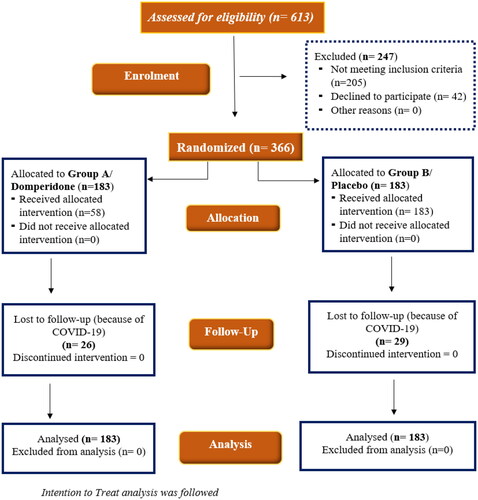
During the study period, a total of 613 mother-infant dyads were assessed for eligibility (). Among them, 366 mother-infant dyads meeting the inclusion criteria were enrolled in the study. The baseline characteristics and clinical parameters were comparable between the groups (). The exclusive breastfeeding rate at 7 days was significantly higher in the domperidone arm compared to the placebo arm (96.17% vs. 85.79%, p = .001) (). The exclusive breastfeeding rate at 3 months (77.05% vs. 71.58%, p = .232) and 6 months (65.03% vs. 58.47%, p = .197) were higher in the domperidone arm compared to the placebo arm. However, the difference was not statistically significant. The weight gain between both groups was higher in the domperidone arm. However, the difference was not statistically significant ().
Table 1. Baseline characteristics and maternal clinical profile.
Table 2. Exclusive breastfeeding rates.
Table 3. Weight gain of infants.
Most of the babies in the study were either term or late preterm. Hence most of them were directly breastfed. However, 20 neonates in domperidone group and 21 in placebo group received expressed breastmilk. The volume of expressed breastmilk (measured only in this small subgroup) was significantly higher in the domperidone arm as compared to placebo arm (312.25 ± 88.83 ml vs. 245.5 ± 85.74, p = .021). Donor Human Milk requirement was lower in the domperidone arm as compared to the placebo arm (12.5% vs. 18.58%, p = .188). No adverse effects were noticed among both mothers and infants during the course of the study.
Discussion
The rate of exclusive breastfeeding is usually lower among LSCS mothers compared to mothers who delivered vaginally [Citation7]. The prevalence of exclusive breastfeeding at hospital discharge was 70.6% among respondents who gave birth by LSCS compared to 79.9% of women who gave birth vaginally [Citation7]. Multiple factors like pain, anxiety could contribute to the lower rate of exclusive breastfeeding in the initial few days among post-LSCS mothers.
In our study, the exclusive breastfeeding rate was higher in the domperidone arm compared to the placebo at 7 days, 3 months and 6 months. During the first seven days of lactation, whether domperidone administered was useful pharmacologically or had placebo effect is a question. Compared to the placebo group, as the exclusive breastfeeding rate was high on day 7 (statistically significant), in the intervention group, the usefulness of domperidone as a galactagogue in the population studied cannot be undermined. It might seem a far-fetched idea that domperidone given for 3 days initially can impact the exclusive breastfeeding rate at 6 months. However, if efforts are made to establish exclusive breastfeeding early in the post-delivery stage, it can help to improve the sustained exclusive breastfeeding rate at 6 months.
At 6 months of age, the exclusive breastfeeding rate was higher in the domperidone arm (65.03%) than the placebo arm (58.47%). However, the difference was not statistically significant. According to NFHS 4 survey, the rate of exclusive breastfeeding at 6 months of age was 54.9% [Citation5]. In our study, the 6-month exclusive breastfeeding rate even in the placebo arm was higher than the national average. This emphasizes the importance of appropriate lactation counseling and postnatal lactation support. Although the exclusive breastfeeding rates were higher at 7 days, 3 months, and 6 months, there was a steady fall in exclusive breastfeeding rates in both groups from 7 days to 6 months. This highlights the importance of repetitive counseling and consistent positive reinforcement to have a sustained increased exclusive breastfeeding rate. After discharge of the mother-infant dyad from the hospital, other family and social factors may be operational and crucial in determining exclusive breastfeeding rates in the community.
Domperidone has a half-life of 7 h[Citation4] but usage of domperidone in addition to lactation support during the initial few days, has shown to have a consistent increased rate of exclusive breastfeeding even at 6 months postpartum. This shows that good lactation establishment during the initial few days has a prolonged effect on the rate of exclusive breastfeeding in the subsequent months. Early initiation of breastfeeding reduces neonatal and early infant mortality through increasing rates of exclusive breastfeeding. Compared with infants initiating breastfeeding within the first hour of life, neonatal mortality was higher in infants initiating at 2–23 h (adjusted relative risk 1·41) and in those initiating at 24–96 h (adjusted relative risk 1·79) [Citation8]. In our study, the rate of exclusive breastfeeding was significantly higher on day 7 of life (96.17 vs 85.79%; p = .001). It was higher at 3 months and at 6 months in the domperidone arm as compared to the placebo arm, however, the data was not statistically significant. Galactagogues, as adjuvants to lactation counseling help in breastfeeding during the initial days, and hence the rate of exclusive breastfeeding at 6 months.
A study by Grzeskowiak et al. showed a steady increase in the use of domperidone as a galactagogue from <0.5% in 2000 to > 5% [Citation9]. Silva O et al. evaluated the efficacy of domperidone in mothers of preterm infants and observed that the increase in the volume of expressed breastmilk among mothers receiving domperidone was 44.5% as compared to 16.6% in the placebo arm [Citation10]. Campbell-Yeo et al. in their study with 46 mothers showed the domperidone arm had higher prolactin levels and a 168% increase in milk secretion (184 to 380 ml) as compared to only 19% in the placebo arm (218 to 250 ml) [Citation11]. In a study by Smolina et al. with 45,518 women who were dispensed domperidone, 21 women were admitted with ventricular arrhythmia and a possible association was considered (adjusted HR = 2.25, 95%C.I 0.84–6.01) [Citation12]. However, in the EMPOWER trial, 90 women enrolled had an ECG at the start of the study and after the 4-week study period, which did not show any evidence of QTc prolongation [Citation13]. A review by Buffery et al. (2016) concluded that there was no QTc prolongation with domperidone in healthy female volunteers [Citation14]. No adverse effects due to domperidone in mothers or neonates were observed in our study.
Conclusion
Use of domperidone significantly increases early exclusive breastfeeding rate at 7 days in post-LSCS mothers with perceived decreased milk secretion. Efforts to establish early lactation with appropriate counseling and oral galactagogues can indirectly improve exclusive breastfeeding at 6 months.
Ethical approval
Obtained from JIPMER Institutional Ethics Committee for interventional studies JIP/IEC/2019/486 dated 10/2/2020.
Authors’ contributions
Arumugom Archana - collected and analyzed the data and drafted the manuscript, Bethou Adhisivam - conceptualized and designed the study, and edited manuscript. Chaturvedula Latha- clinical management of mothers, Sadhana Subramanian – statistical analysis.
Informed consent
Written informed consent was obtained from the participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Roy S, Simalti AK, Nair BT. Prevalence of exclusive breastfeeding and knowledge related to breastfeeding among mothers attending immunization center and well-baby clinic. Acta Medica Int. 2018;5:79.
- Moore ER, Bergman N, Anderson GC, et al. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2016;11(11):CD003519.
- Rowe-Murray HJ, Fisher JRW. Baby friendly hospital practices: cesarean section is a persistent barrier to early initiation of breastfeeding. Birth. 2002;29(2):124–131.
- Wan EW-X, Davey K, Page-Sharp M, et al. Dose-effect study of domperidone as a galactagogue in preterm mothers with insufficient milk supply, and its transfer into milk. Br J Clin Pharmacol. 2008;66(2):283–289.
- India - National Family Health Survey 2015-2016 [Internet]. [cited 2022. Jan 3]. Available from: https://microdata.worldbank.org/index.php/catalog/2949.
- Imdad A, Yakoob MY, Bhutta ZA. Effect of breastfeeding promotion interventions on breastfeeding rates, with special focus on developing countries. BMC Public Health. 2011;11:1–8.
- Kling D, Haile ZT, Francescon J, et al. Association between method of delivery and exclusive breastfeeding at hospital discharge. J Am Osteopath Assoc. 2016;116(7):430–439.
- Timing of initiation, patterns of breastfeeding, and infant survival: prospective analysis of pooled data from three randomised trials. Lancet Glob Health. 2016;4:e266–e275.
- Grzeskowiak LE, Dalton JA, Fielder AL. Factors associated with domperidone use as a galactogogue at an Australian tertiary teaching hospital. J Hum Lact. 2015;31(2):249–253.
- Silva O, Knoppert DC, Angelini MM, et al. Effect of domperidone on milk production in mothers of premature newborns: a randomized, double-blind, placebo-controlled trial. CMAJ Can Med Assoc J. 2001;164:17–21.
- Campbell-Yeo ML, Allen AC, Joseph KS, et al. Effect of domperidone on the composition of preterm human breast milk. Pediatrics. 2010;125(1):e107–e114.
- Smolina K, Mintzes B, Hanley GE, et al. The association between domperidone and ventricular arrhythmia in the postpartum period. Pharmacoepidemiol Drug Saf. 2016;25(10):1210–1214.
- Asztalos EV, Campbell-Yeo M, daSilva OP, et al. Enhancing breast milk production with domperidone in mothers of preterm neonates (EMPOWER trial). BMC Pregnancy Childbirth. 2012;12:87.
- Buffery PJ, Strother RM. Domperidone safety: a mini-review of the science of QT prolongation and clinical implications of recent global regulatory recommendations. N Z Med J. 2015;128:66–74.