Abstract
Introduction
Nonalcoholic fatty liver disease (NAFLD) affects 30% of adults in the United States. Transient elastography (TE) (Fibroscan, Echosens, Paris, France) with controlled attenuation parameter (CAP) is a noninvasive way to evaluate liver steatosis and liver stiffness. The primary objective of this study was to assess prevalence of elevated liver stiffness and steatosis immediately postpartum. Furthermore, we sought to evaluate whether there were differences in rates of metabolic disorders of pregnancy (gestational diabetes mellitus (GDM), gestational hypertension, and preeclampsia) and pre-pregnancy conditions (type 2 diabetes mellitus (DM), chronic hypertension, and obesity) in those with elevated postpartum liver steatosis/liver stiffness.
Methods
IRB approved prospective cross-sectional study in which TE and liver function tests were performed 1–2 days postpartum. CAP ≥300 dB/m was classified as significant steatosis. Increased liver stiffness was defined as ≥7 kPa. Prevalence was determined by proportion of individuals undergoing TE/CAP who met criteria. Chi-square analysis was used to compare differences between groups.
Results
Eighty-nine patients were included: 20 (22%) had GDM, 13 (15%) had gestational hypertension, and 15 (17%) had preeclampsia. Women with kPa ≥7 were more likely to have ALT ≥25, type 2 diabetes, and preeclampsia (p < .05). Pre-gravid BMI, BMI at delivery, and GDM were not associated with increased kPa. Pregravid BMI ≥25 and chronic hypertension were associated with CAP ≥ 300 dB/m (p < .05). GDM, preeclampsia, and gestational hypertension were not associated with CAP ≥300 dB/m.
Conclusions
Patients with preeclampsia, type 2 diabetes, and elevated ALT were more likely to have elevated postpartum liver stiffness. Pregravid BMI ≥25 and ≥30 were associated with increased liver steatosis, although did not impact liver stiffness. GDM was not associated with increased liver stiffness or steatosis. Consideration should be made for screening pregnant patients with preeclampsia, type 2 DM and overweight or obese BMI for liver disease in the postpartum period with potential for lifestyle intervention.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disorder in the United States, affecting approximately 20–30% of the adult population. Of these, 7–30% progress to nonalcoholic steatohepatitis (NASH) [Citation1,Citation2]. Among women, NAFLD is the leading indication for liver transplantation [Citation3]. NAFLD has a bidirectional association with the metabolic syndrome of central adiposity, diabetes mellitus (DM), hypertension, and dyslipidemia. Therefore, the pathogenesis of NAFLD is a result of a complex sequence of events involving excess lipotoxicity in the setting of surplus free fatty acids, peripheral adiposity, and hepatic insulin resistance [Citation4].
Physiologic changes of pregnancy, including rapid weight change and fluctuations in estrogen, may play an important role in the development of NAFLD in both the mother and infant [Citation5]. Adverse perinatal outcomes, including increased risk of preeclampsia, gestational diabetes mellitus (GDM), preterm birth, and low birth weight are associated with NAFLD in patients who are pregnant [Citation6,Citation7]. This highlights the need for postpartum assessments for metabolic syndrome, particularly in patients with a history of pre-pregnancy or prenatal metabolic disease [Citation6,Citation7].
Transient elastography (TE) (Fibroscan, Echosens, Paris, France) with controlled attenuation parameter (CAP) is a noninvasive way to assess for liver steatosis and liver stiffness (which can be a marker of liver inflammation and liver fibrosis). The technology utilizes pulse-echo ultrasonic acquisition, quantifying the speed of mechanically induced shear wave in liver tissue to generate an estimate of the degree of liver fibrosis with a liver stiffness measurement (LSM) in kPa. Hepatic steatosis is quantified by measuring ultrasonic attenuation of the echo wave, known as the CAP, which has been compared with liver histology in prospective studies [Citation8,Citation9].
The technology has been used in the context of pregnancy in limited studies in Europe in patients with preeclampsia [Citation10,Citation11] and in Australia [Citation12] to evaluate patients at risk for GDM. However, the device has not been approved for use during pregnancy in the United States [Citation13] and data in this population, one that is at particularly high risk of the metabolic disease and NAFLD, is scarce. Therefore, the objectives of this study were to assess prevalence of liver stiffness and steatosis in women in the US immediately postpartum, and to assess if patients with metabolic disorders of pregnancy (GDM, gestational hypertension, and preeclampsia), pre pregnancy conditions (type 2 DM, chronic hypertension) as well as overweight (≥25) and obese (≥30 BMI) were more likely to have elevated postpartum liver steatosis/liver stiffness.
Methods
This was an IRB approved cross-sectional study conducted during a pre-defined 6 month period from May to October 2019 in a single academic institution in an urban setting with approximately 5000 deliveries per year. Patients were included if they had delivered a singleton pregnancy, were 1–2 days postpartum, were >18 years of age and were able to provide written informed consent. Patients were approached for participation in the postpartum unit on a weekly basis. Patients were excluded if they had any of the following: cholestasis of pregnancy, preexisting liver disease (primary biliary cirrhosis, Gilbert’s disease or Wilson’s disease, homozygous alpha-1-antitrypsin deficiency or hemochromatosis, hepatitis B, C infection, autoimmune hepatitis, and known bile duct obstruction), acute congestive heart failure, ascites, limited prenatal records, if they were currently pregnant, or consumed a meal within 2 h of the scan. Liver function tests including aspartate transaminase (AST) and alanine aminotransferase (ALT), were obtained on all included patients at time of enrollment. Demographic and clinical data were collected via chart review and patient interview at the time of examination.
VCTE/CAP assessment
TE was performed using the Fibroscan Mini+ 430 by manufacturer certified operators at 1–2 days postpartum bedside in the postpartum unit. The M or XL probe was used according to the manufacturer’s specifications in fasting conditions for no less than two hours. Specifically, the XL probe was used in patients with BMI over 30. The probes were applied to detect the right lobe of the liver in intercostal position according to established protocols [Citation14]. The values obtained represent the median values of at least 10 consecutive measurements together with the corresponding IQR. LSM was considered reliable when it fulfilled the following criteria: ≥10 valid measurements, ≥60% success rate, and interquartile range (IQR) ≤30% [Citation15].
Statistical analysis
Significant steatosis was defined as CAP score ≥300 dB/m. Increased liver stiffness was defined as ≥7 kPa [Citation9,Citation16]. For the primary outcome of interest, prevalence of postpartum elevated steatosis was defined as proportion of individuals with TE/CAP results obtained with CAP ≥300 and prevalence of elevated liver stiffness was defined as proportion of individuals with TE ≥ 7 kPa. For the secondary analysis of interest, we determined differences in baseline and pregnancy-related metabolic characteristics among individuals with and without CAP ≥ 300 and liver stiffness ≥7 kPa. Chi-square analysis and Wilcoxon’s rank sum tests with categorical and continuous variables, respectively, were used to compare differences between groups as appropriate.
Results
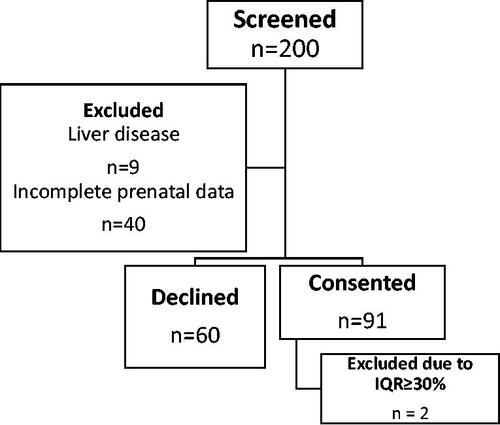
A total of 200 patients were screened for participation. Nine patients were excluded due to preexisting liver disease and 40 patients were excluded due to incomplete prenatal data. Sixty patients declined participation. Overall, 91 patients consented and were enrolled in the study. Of these, 43 patients had diagnosed metabolic disorder(s), while 48 patients did not have any metabolic disorders (). Two patients were excluded from the analysis due to a median IQR of >30%. Baseline characteristics of included participants are shown in .
Table 1. Baseline characteristics of included participants.
Of those with metabolic disorders of pregnancy, 20 (22%) had GDM, 13 (15%) had gestational hypertension, and 15 (17%) had preeclampsia. In regards to pre-pregnancy metabolic conditions, seven (8%) had chronic hypertension and three (3%) had type 2 DM.
Individuals with increased postpartum liver stiffness or kPa ≥7 were more likely to have ALT ≥ 25 IU/L, type 2 DM, and preeclampsia (p < .05) (). Pre-gravid BMI, BMI at delivery, gestational hypertension, and GDM were not associated with increased kPa.
Table 2. Metabolic characteristics in individuals with and without elevated liver stiffness on postpartum transient elastography in kPa, and elevated liver steatosis with controlled attenuation parameter (CAP).
Overweight (≥25) and obese (≥30) pregravid BMI, and chronic hypertension were associated with significant steatosis or CAP ≥300 dB/m (p < .05). GDM, preeclampsia, and gestational hypertension were not associated with CAP ≥300 dB/m.
Discussion
Our study evaluated postpartum TE/CAP results in an obstetric population with high prevalence of metabolic disease (∼12% with pre-pregnancy metabolic disease and ∼50% with metabolic disorders of pregnancy). We demonstrated that postpartum patients with elevated ALT, preeclampsia, and type 2 DM had increased postpartum liver stiffness (which may reflect liver inflammation or fibrosis). Those with increased pregravid BMI and interestingly chronic hypertension demonstrated increased steatosis on TE. Of note, patients with GDM or gestational hypertension did not have evidence of increased liver fibrosis or steatosis on TE.
There are limited data on TE baseline values during normal pregnancy, and none in the United States to our knowledge. A longitudinal cohort study in Sweden [Citation17] performed TE in 24 lean, healthy women at gestational week 18–20, week 26–28 and week 26–38, as well as after 8 weeks postpartum. There were no cases of preeclampsia or GDM in this cohort. Mean liver stiffness increased from 3.8 kPa in the second trimester to 5.9 kPa in the third trimester (p = .002). All except two measurements of liver stiffness (both below <7.9 kPa) were within normal range. Postpartum, liver stiffness decreased to early second trimester levels (5.9–3.8 kPa, p = .002). CAP also increased similarly from second to third trimester (186–215 dB/m, p = .01), and reversed postpartum (215–193 dB/m, p = .03). The authors speculated that the slight elevations observed may have been due to increased liver blood flow, hemodynamic changes, and changes in lipid metabolism in pregnancy [Citation17]. While the increased LSM or CAP we observed may be partly due to physiologic changes of pregnancy, our high risk cohort demonstrated a higher prevalence of patients with elevations than was observed by Ribeiro et al. [Citation17].
Our finding of an association of preeclampsia with elevated liver stiffness has been investigated previously, and was also consistent with findings in our study. The complex pathophysiology of preeclampsia in the liver includes fibrin deposition, hypovolemia, hepatic ischemia, and infarction. In more severe and life-threatening cases, this can lead to subcapsular hematoma and hemorrhage [Citation18]. In a study by Frank Wolf et al. [Citation10] conducted in Israel, 32 patients with preeclampsia underwent TE 1–7 days postpartum. Results were compared to a group of 16 normotensive patients with normal pregnancy outcomes. Increased liver stiffness was seen in patients with preeclampsia compared to normotensive patients, with a positive linear correlation with elevated liver function tests [Citation10]. No differences in steatosis were observed [Citation10]. Similarly, Duvekot et al. [Citation19] demonstrated increased liver stiffness in patients between 24 and 40 weeks’ gestation with acute fatty liver of pregnancy (AFLP) in comparison to those with severe preeclampsia and HELLP syndrome. Normotensive patients had lower LSM values [Citation19]. Ammon et al. [Citation11] also showed that women with preeclampsia had significantly higher liver stiffness as compared to controls. These data are consistent with the findings of our study of increased liver stiffness found in patients with elevated ALT and preeclampsia, while those with gestational hypertension were normal. It also reinforces the concept of preeclampsia and pregnancy-induced hypertensive disease as part of a complex clinical spectrum, with gestational hypertension being less severe and preeclampsia, hemolysis, elevated liver enzymes, low platelet count (HELLP) and subsequently, AFLP being the most severe [Citation18,Citation20]. Association of these entities with elevated liver stiffness postpartum suggests potential ongoing liver injury after delivery and may have implications for future maternal liver health.
The prevalence of NAFLD in pregnancy has nearly tripled in the last decade and is independently associated with hypertensive complications [Citation21]. In our study, patients with chronic hypertension demonstrated increased prevalence of steatosis on TE. This is consistent with the findings of a meta-analysis by Ciardullo et al. [Citation22], which included 11 cohort studies on 390 non-pregnant female and male patients. The study demonstrated that NAFLD diagnosed via blood/biomarkers or varying imaging modalities (ultrasound, computerized tomography, or TE) was associated with approximately 1.6-fold increased risk of developing hypertension over a mean follow-up of 5.7 years. This association did not differ when analysis was stratified based on diagnostic modality [Citation22]. Physiologically, several mechanisms may account for the role of NAFLD as a risk factor for hypertension. Liver steatosis is associated with insulin resistance, which may in turn cause systemic inflammation, endothelial dysfunction, and subsequently vasoconstriction [Citation22,Citation23]. Other pathways linking NAFLD to hypertension may include oxidative stress, and hyperactivity of the sympathetic nervous system and angiotensin aldosterone systems [Citation22,Citation24].
There have also been emerging data suggesting association of GDM with liver disease. Patients with GDM have an increased risk of developing preeclampsia, and later in life type 2 DM, which is also influenced by risk factors such as race, ethnicity, and obesity [Citation25]. Several non-TE studies have demonstrated women with prior GDM to have a higher risk for NAFLD as detected via liver ultrasound [Citation5,Citation7,Citation26]. However, a study by Deng et al. [Citation12] in Australia performed TE on 50 pregnant women prior to 24 weeks and after 30 weeks and found no significant difference in GDM rates between those with NAFLD in early pregnancy and those without. Women that developed GDM had a trend toward a reduction in the CAP score on their second TE after 30 weeks, which also coincided with lower mean maternal gestational weight gain [Citation12]. Similarly, a cross-sectional study by Ciardullo et al. [Citation27] examined 144 women with previous GDM and showed no independent association between NAFLD diagnosed with TE 20–25 years after pregnancy, after adjusting for development of future type 2 DM. Our results were consistent with these data, showing no increase in postpartum liver stiffness or steatosis in patients with GDM antepartum. In our data, patients with higher pregravid BMI had increased CAP scores. Deng et al. [Citation12] postulate that targeted treatments during pregnancy that alter maternal weight gain (glycemic control, diet, and exercise) can influence the CAP score and therefore maternal liver steatosis. This may have a beneficial impact on maternal postpartum liver health.
Limitations of our study include lack of a baseline TE evaluation prior to and during pregnancy, namely due to lack of FDA approval for antepartum use. Our cohort size was relatively small, and larger studies may be needed to validate TE cutoffs for CAP and kPa in pregnancy and postpartum. Furthermore, the number of patients with increased LSM was low overall, limiting our ability to draw conclusions about association of preexisting metabolic conditions. Inadequate fasting time was a technical issue during study enrollment, due to the inherent nature of the postpartum period and caloric requirements for lactation. Strengths of our study include the first known study of TE in a postpartum cohort in the U.S., in a diverse population in an urban academic center, with a high prevalence of metabolic disorders of pregnancy.
In summary, we demonstrated that women with preeclampsia, type 2 DM, and elevated ALT were more likely to have elevated postpartum liver stiffness. Pregravid BMI ≥25 and ≥30 were associated with increased liver steatosis, although did not appear to impact liver stiffness, while GDM was not associated with increased postpartum liver stiffness or steatosis. Consideration should be made for screening women with preeclampsia, type 2 DM and overweight or obese BMI for liver disease during pregnancy with potential for lifestyle intervention. Given our findings and the prior literature, additional studies to determine the magnitude, duration, and potential reversibility of the liver changes seen postpartum in patients with metabolic disorders of pregnancy via TE are warranted.
Ethical approval
Mount Sinai IRB approval ID 19-00078.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–131.
- Loomba R, Adams LA. The 20% rule of NASH progression: the natural history of advanced fibrosis and cirrhosis caused by NASH. Hepatology. 2019;70(6):1885–1888.
- Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253.
- Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–2224.
- Hershman M, Mei R, Kushner T. Implications of nonalcoholic fatty liver disease on pregnancy and maternal and child outcomes. Gastroenterol Hepatol. 2019;15(4):221–228.
- Ajmera VH, Gunderson EP, VanWagner LB, et al. Gestational diabetes mellitus is strongly associated with non-alcoholic fatty liver disease. Am J Gastroenterol. 2016;111(5):658–664.
- Foghsgaard S, Andreasen C, Vedtofte L, et al. Nonalcoholic fatty liver disease is prevalent in women with prior gestational diabetes mellitus and independently associated with insulin resistance and waist circumference. Diabetes Care. 2017;40(1):109–116.
- Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–1730.
- de Ledinghen V, Vergniol J, Capdepont M, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60(5):1026–1031.
- Frank Wolf M, Peleg D, Kariv Silberstein N, et al. Correlation between changes in liver stiffness and preeclampsia as shown by transient elastography. Hypertens Pregnancy. 2016;35(4):536–541.
- Ammon FJ, Kohlhaas A, Elshaarawy O, et al. Liver stiffness reversibly increases during pregnancy and independently predicts preeclampsia. World J Gastroenterol. 2018;24(38):4393–4402.
- Deng D, George J, Pasupathy D, et al. Antenatal FibroScan(R) assessment for metabolic associated fatty liver in pregnant women at risk of gestational diabetes from a multiethnic population – a pilot study. Intern Med J. 2021.
- Fibroscan procedure for users; 2023. Echosens. Available from: https://www.echosens.com/en-us/fibroscanprocedure/
- Mueller S, Seitz HK, Rausch V. Non-invasive diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20(40):14626–14641.
- Boursier J, Zarski JP, de Ledinghen V, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57(3):1182–1191.
- Mikolasevic I, Orlic L, Franjic N, et al. Transient elastography (FibroScan((R))) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease – where do we stand? World J Gastroenterol. 2016;22(32):7236–7251.
- Stenberg Ribeiro M, Hagstrom H, Stal P, et al. Transient liver elastography in normal pregnancy – a longitudinal cohort study. Scand J Gastroenterol. 2019;54(6):761–765.
- Alese MO, Moodley J, Naicker T. Preeclampsia and HELLP syndrome, the role of the liver. J Matern Fetal Neonatal Med. 2021;34(1):117–123.
- Duvekot JV, Neven L, De Man R, et al. Transient elastography of the liver forms a new and discriminating measure to rule out AFLP in hypertensive patients. Pregnancy Hypertens. 2015;5(1):2–3.
- Vidaeff AC, Saade GR, Sibai BM. Preeclampsia: the need for a biological definition and diagnosis. Am J Perinatol. 2021;38(9):976–982.
- Sarkar M, Grab J, Dodge JL, et al. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J Hepatol. 2020;73(3):516–522.
- Ciardullo S, Grassi G, Mancia G, et al. Nonalcoholic fatty liver disease and risk of incident hypertension: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2022;34(4):365–371.
- Ampuero J, Aller R, Gallego-Duran R, et al. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. J Hepatol. 2020;73(1):17–25.
- Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut. 2022;71(1):156–162.
- ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–e64.
- Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116.
- Ciardullo S, Bianconi E, Zerbini F, et al. Current type 2 diabetes, rather than previous gestational diabetes, is associated with liver disease in U.S. women. Diabetes Res Clin Pract. 2021;177:108879.