Abstract
Objective
To explore the association between inter-pregnancy intervals and placenta previa and placenta accreta spectrum among women who had prior cesarean deliveries with respect to maternal age at first cesarean delivery.
Methods
This retrospective study included clinical data from 9981 singleton pregnant women with a history of cesarean delivery at 11 public tertiary hospitals in seven provinces of China between January 2017 and December 2017. The study population was divided into four groups (<2, 2–5, 5–10, ≥10 years of the interval) according to the inter-pregnancy interval. The rate of placenta previa and placenta accreta spectrum among the four groups was compared, and multivariate logistic regression was used to analyze the relationship between inter-pregnancy interval and placenta previa and placenta accreta spectrum with respect to maternal age at first cesarean delivery.
Results
Compared to women aged 30–34 years old at first cesarean delivery, the risk of placenta previa (aRR, 1.48; 95% CI, 1.16–1.88) and placenta accreta spectrum (aRR, 1.74; 95% CI, 1.28–2.35) were higher among women aged 18–24. Multivariate regression results showed that women at 18–24 with <2 years intervals exhibited a 5.05-fold increased risk for placenta previa compared with those with 2–5-year intervals (aRR, 5.05; 95% CI, 1.13–22.51). In addition, women aged 18–24 with less than 2 years intervals had an 8.44 times greater risk of developing PAS than women aged 30-34 with 2 to 5 years intervals (aRR, 8.44; 95% CI, 1.82–39.26).
Conclusions
The findings of this study suggested that short inter-pregnancy intervals were associated with increased risks for placenta previa, and placenta accreta spectrum for women under 25 years at first cesarean delivery, which may be partly attributed to obstetrical outcomes.
Introduction
Placenta previa (PP) is associated with high risk or high incidence of maternal morbidity and mortality [Citation1–5], and its incidence is approximately 1 in 200 pregnancies [Citation5,Citation6]. The rates of placenta previa continue to increase, particularly because of rising rates of cesarean delivery (CD) [Citation2,Citation7] and increased maternal age [Citation8–10]. Due to the abnormal location and the invasion of the placenta, PP puts women at high risk for postpartum hemorrhage (PPH), severe PPH, and the need for blood transfusion-especially emergency transfusion [Citation11–14]. And the risk for a blood transfusion was approximately 12 times more likely with cesarean section (CS) for PP than with CS for other indications [Citation15]. Placenta accrete spectrum (PAS) is the general term applied to abnormal adherence of the placental trophoblast to the uterine myometrium; it is also referred to as morbidly adherent placenta, including placenta accreta, placenta increta, placenta precreta [Citation16]. PAS is one of the most dangerous conditions associated with pregnancy because bleeding can lead to multisystem organ failure, disseminated intravascular coagulation, the need for intensive care unit admission, hysterectomy, and even death [Citation17–20].
The cesarean section rate in China has consistently remained high over the past few decades [Citation21,Citation22]. Paralleling the adjustment to China’s childbirth policy (two-child and three-child policies), the demand for childbearing again among women with a history of CD has increased [Citation23–25].
Short inter-pregnancy interval (IPI, the interval between a previous delivery and subsequent conception) appears to be associated with increased risks of adverse pregnancy outcomes for women, including placenta previa, placenta accreta spectrum, small for gestational age (SGA) birth, spontaneous preterm delivery, stillbirth, and infant and maternal mortality [Citation26–30]. Further, advanced maternal age was one of the independent risk factors for PP [Citation31,Citation32]. Interestingly, a previous study reported that the “J”-shaped trend of placenta previa was observed with age in multipara with the lowest point at 27 to 28 years [Citation33]. Our previous studies also indicated that maternal age under 25 years at first CD increased the risk of PP in subsequent pregnancy [Citation34]. However, the risk for placenta previa and placenta accreta spectrum in the subsequent pregnancy among women who had prior cesarean deliveries stratified by maternal age at first cesarean delivery and the inter-pregnancy interval was not previously evaluated. We, therefore, retrospectively analyzed the clinical data from 9981 singleton pregnant women with a history of CD at 11 public tertiary hospitals in seven provinces of China from January 2017 to December 2017.
Materials and methods
The survey and sampling design
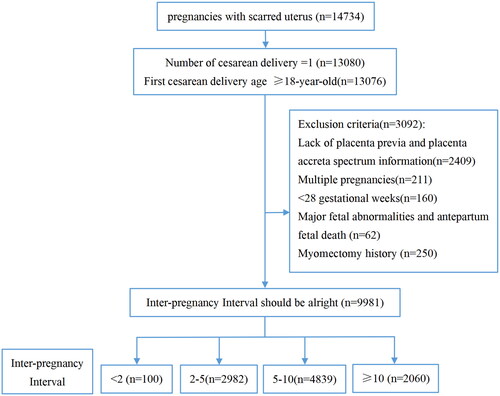
This was a multicenter, cross-sectional cohort study conducted at 11 public tertiary hospitals in seven provinces of China (Beijing, Chongqing, Guangdong, Henan, Hubei, Shanxi, and Xinjiang). The cohort included 14,734 women with a history of a CD who delivered again between January 2017 and December 2017. The inclusion criteria required women with one CD history and an age greater than 18 years at the time of CD. Lack of information with respect to placenta previa or placenta accreta spectrum, history of multiple pregnancies, being under 28 weeks of gestation at delivery, with major fetal abnormalities or antepartum fetal death, and exhibiting a history of myomectomy were excluded. According to the length of the IPI, the cases were divided into four groups: less than two years (100 cases), 2–5 years (2982 cases), 5–10 years (4839 cases), and >10 years (2060 cases). illustrates a flow diagram of the women’s inclusion and exclusion processes. A summary of missing data is portrayed in Supplementary Table 1.
Table 1. The general characteristics of the study population according to interpregnancy interval.
Outcome and explanatory variables
In addition to maternal age at first CD, the clinical data at subsequent delivery were analyzed, including age at delivery, gestational weight gain, body mass index before pregnancy (BMI), gravidity, parity, use of assisted reproductive technology (ART), previous medical history (previous history of miscarriage or placenta previa [HPP] or placenta accreta spectrum [HPAS]), PP, PAS, number of gestational weeks, amount of bleeding at delivery, and history of preterm birth. The maternal diseases were classified and grouped according to WHO’s Classification of Diseases (ICD)-10.
Statistical analysis
We performed all the analyses using Empower (R) (www. empowerstats. com, X& Y Solutions, Inc. Boston MA) and R (http://www.R-project.org). Mean ± standard deviation (SD) was calculated for numerical and normally distributed data and numbers and percentages were provided for categorical data. Medians (with 25% and 75% quantiles) are presented for non-normally distributed numerical data. Independent-sample t-test or the non-parametric Kruskal-Walli’s rank-sum test was performed for comparisons of quantitative data. For comparisons of qualitative data, the chi-squared test or Fisher’s exact-probability method was used; with the latter used when the theoretical frequency was <5. First, we used multivariate logistic regression to analyze the relationship between PP, PAS and first-CD age. Subsequently, we further analyzed the relationship between PP, PAS and IPI according to the first-CD age category by using the same statistical method. Since the incidence of PP and PAS was lowest in the group aged 30–34 for the first cesarean section, this group was used as the control group (Supplementary Figure 1). Results are presented as odds ratios with 95% confidence intervals (95% CI) and p values. Differences with p values of <.05 (two-sided) were statistically significant.
Results
The present study encompassed 9,981 women with a history of CD. According to their inter-pregnancy intervals, all cases were divided into four groups: under two years, 2–5 years, 5–10 years, and >10 years; and these numbered 100, 2982, 4839, and 2060 cases, respectively. depicts the general characteristics of all our studied cases based on IPI groups. We observed significant differences in gestational weight gain, BMI, gravidity, ART, and previous history of miscarriage (p < .05) among the four groups ().
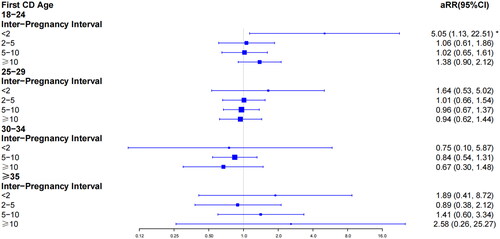
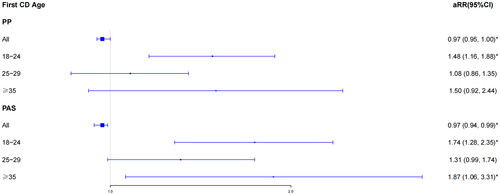
First, we analyzed the pregnant outcomes of the study population according to interpregnancy interval category in 9981 pregnancies (Supplementary Table 2). We found significant differences in the number of PP (p < .05) and PA (p < .05) in the group of 18–24-year-old (Supplementary Table 2). We then assessed the relationship between PP, PAS and first CD age by adjusting for BMI, gravity, previous history of miscarriage, and ART (). In the overall study population without grouping, the risk of PP (aRR, 0.97; 95% CI, 0.95–1.00) and PAS (aRR, 0.97; 95% CI, 0.94–0.99) declined with increasing age at first cesarean delivery (as a continuous variable). However, we found that the overall trend was not consistent with the individual trends for different age groups. Compared to the women at 30–34, the risk of PP (aRR, 1.48; 95% CI, 1.16–1.88) and PA (aRR, 1.74; 95% CI, 1.28–2.35) in the group of 18–24-year-old increased significantly. Moreover, women with advanced age also had rising risk of PA (aRR, 1.87; 95% CI, 1.06–3.31).
Figure 2. Multivariate logistic regression analysis of the relationship between placenta previa and first CD age category in 9981 pregnancies. *p < .05.
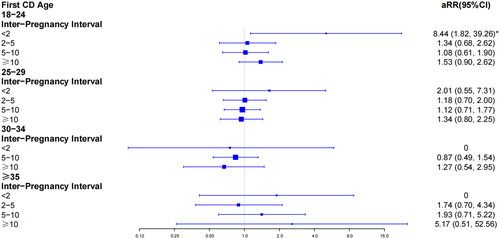
We further evaluated the effect of inter-pregnancy interval on PP and PAS stratifying by maternal age at first CD (, ). The women at 18–24 with inter-pregnancy intervals shorter than two years had an approximately 5.05-fold increased risk for PP compared with women at 30–34 displaying 2–5-year intervals (aRR, 5.05; 95% CI, 1.13–22.51). Likewise, women aged 18–24 with less than 2 years intervals had an 8.44 times greater risk of developing PAS in a second pregnancy than women aged 30–34 with 2 to 5 years intervals (aRR, 8.44; 95% CI, 1.82–39.26).
Discussion
We ascertained that inter-pregnancy interval and maternal age at first CD were associated with placenta previa and placenta accreta spectrum in women with a subsequent pregnancy. Compared with the reference group (with an interval of 2–5 years), an interval of less than two years increased the risk of PP and PAS for women at a maternal age of between 18 and 24 years at their first CD. We, therefore, recommend that these women decide whether to appropriately extend the inter-pregnancy intervals based on the actual situation. Additionally, our study can be used to counsel patients between 18 and 24 years at their first CD regarding the risk of placenta previa and placenta accreta spectrum after stratification of inter-pregnancy intervals with respect to a subsequent pregnancy. We noted in our study that, intriguingly, women at a maternal age between 18 and 24 years at first CD and showing less than a two-year interval increased their risk for PP and PA in the second pregnancy.
Some clinical and public health recommendations suggest a minimal inter-pregnancy interval of 18 months [Citation35,Citation36]. However, we emphasize that short inter-pregnancy intervals (<2 years) for women between 18 and 24 years of age at first CD increase the risks for PP and PAS. Investigators have recently found that short inter-pregnancy intervals were associated with severe maternal morbidities such as PP and PAS [Citation26, Citation37–39]. However, Schummers found that risks of maternal mortality or severe morbidity were increased at short inter-pregnancy intervals among women 35 years or older, although there was no significant difference [Citation26]. We speculate that the difference in the study population may have caused the discrepancy in results. We selected pregnant women with a history of CD as our research cohort, and with the premise of having had a CS, advanced age during a subsequent pregnancy exerted little effect on the risk for placenta previa and placenta accreta spectrum. We posit that our study can be used for counseling patients of all ages regarding the risk of placenta previa and placenta accreta spectrum after stratifying them by IPI with respect to a subsequent pregnancy.
The mechanism by which IPI and maternal age at first CD might increase the risk of PP is uncertain. According to the WHO, ∼11% of total births worldwide are by women under 25 years of age, and about 95% of these births occur in less-developed countries [Citation40] and are usually among the most disadvantaged young adults [Citation41,Citation42]. In the US, the highest rate of unintended pregnancy was noted among young adult women aged 18–24 years [Citation41]; and 55% of all unintended pregnancies in the US. were to women in their twenties, while less than 20% were to adolescent women [Citation43,Citation44]. Young adult women are likely to be unmarried, cohabiting prematurely, or participating in the labor force without completing their higher education [Citation45,Citation46]. It was also reported that almost one-fifth of young adult pregnant women lived in socioeconomically disadvantaged neighborhoods [Citation47]. In addition, use of tobacco, marijuana, and alcohol was more common during pregnancy among young adult women aged 18–24 years than among other mothers; this was approximately triple the rate for pregnant Canadian women who smoked during pregnancy (13%) [Citation48,Citation49], and far exceeded the smoking rate for non-pregnant teenagers (5.9%) [Citation50]. These adverse indices were also independent risk factors for PP [Citation51]. Several studies have, in addition, confirmed that placenta previa is an independent risk factor for placenta accreta spectrum [Citation52,Citation53].
Strengths and limitations
This study possessed several strengths. We included clinical data from 9981 mothers undergoing singleton pregnancies at 11 public tertiary hospitals in seven provinces of China between January 2017 and December 2017, and thus significantly avoided the bias of single-center and small-sample studies. Second, this was the first study of its kind to address the association between short inter-pregnancy interval and placenta previa with respect to maternal age at first CD. However, there were also several limitations. This was a historical cohort study, and data from some centers were not complete. Moreover, the number of women at their first CD aged 35 years or older or with an inter-pregnancy interval less than two years was relatively small. Thus, additional and confirmatory studies are still necessary.
Conclusions
In this paper, we presented robust evidence to guide clinicians who counsel women considering short inter-pregnancy intervals and at an age of first CD between 18 and 24 years. In conclusion, this multi-center, historical, cross-sectional cohort study of singleton pregnancies showed that women at a maternal age between 18 and 24 years at first CD and with an inter-pregnancy interval less than two years were at increased risk for PP and PAS with the subsequent pregnancy. The mechanisms underlying these relationships, however, remain unclear; and further studies are needed to confirm that maternal age between 18 and 24 years at first CD is a risk factor for PP and PAS in the subsequent pregnancy.
Ethical approval and consent to participate
This study was approved by the Medical Ethics Committee of Guangzhou Medical University with Medical Research No. 2016 (0406) approved on 6 April 2016. All the methods were performed in accordance with the relevant guidelines and regulations, and informed consent was obtained from all participants.
Consent for publication
Not applicable.
Author contributions
YL: propose ideas, data analysis, writing, review and editing. LZ: data analysis, review and editing. LH, YL, JC: propose original questions, review and editing. SB, MH, HT, SL, JL and SG: investigation, data collection, and visualization. JJ, SW, ZW, YC, SW, XX, LF, XZ, YZ, QZ, HQ, LZ and HL: investigation, and resources. LD, and DC: supervision, project administration, and funding acquisition.
Supplemental Material
Download MS Word (17.9 KB)Acknowledgments
We would like to thank LetPub for its linguistic assistance to edit and proofread this manuscript.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and/or analyzed during the current study are available in the Population Health Data Archive (PHDA) (https://www.ncmi.cn/). Data are also available from the authors upon reasonable request.
Additional information
Funding
References
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33(4):244–251.
- Solheim KN, Esakoff TF, Little SE, et al. The effect of cesarean delivery rates on the future incidence of placenta previa, placenta accreta, and maternal mortality. J Matern Fetal Neonatal Med. 2011;24(11):1341–1346.
- Bowman ZS, Eller AG, Bardsley TR, et al. Risk factors for placenta accreta: a large prospective cohort. Am J Perinatol. 2014;31(9):799–804.
- Vintzileos AM, Ananth CV, Smulian JC. Using ultrasound in the clinical management of placental implantation abnormalities. Am J Obstet Gynecol. 2015;213(4 Suppl):S70–S7.
- Silver RM. Abnormal placentation: placenta previa, vasa previa, and placenta accreta. Obstet Gynecol. 2015;126(3):654–668.
- Vahanian SA, Lavery JA, Ananth CV, et al. Placental implantation abnormalities and risk of preterm delivery: a systematic review and metaanalysis. Am J Obstet Gynecol. 2015;213(4 Suppl):S78–S90.
- Downes KL, Hinkle SN, Sjaarda LA, et al. Previous prelabor or intrapartum cesarean delivery and risk of placenta previa. Am J Obstet Gynecol. 2015;212(5):669.e1-6–669.e6.
- Bose DA, Assel BG, Hill JB, et al. Maintenance tocolytics for preterm symptomatic placenta previa: a review. Am J Perinatol. 2011;28(1):45–50.
- Hasegawa J, Nakamura M, Hamada S, et al. Prediction of hemorrhage in placenta previa. Taiwan J Obstet Gynecol. 2012;51(1):3–6.
- Taki M, Sato Y, Kakui K, et al. Management of fetal death with placenta previa. J Matern Fetal Neonatal Med. 2012;25(2):196–199.
- Frederiksen MC, Glassenberg R, Stika CS. Placenta previa: a 22-year analysis. Am J Obstet Gynecol. 1999;180(6 Pt 1):1432–1437.
- O'Brien JM. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol. 2007;109(1):203–204; (author reply 204).
- Dahlke JD, Mendez-Figueroa H, Maggio L, et al. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol. 2015;213(1):76. e1–76.e10.
- Gibbins KJ, Einerson BD, Varner MW, et al. Placenta previa and maternal hemorrhagic morbidity. J Matern Fetal Neonatal Med. 2018;31(4):494–499.
- Royal College of Obstetricians and Gynaecologists. Blood transfusions in obstetrics. Green-top guideline no. 47. London: RCOG; 2015.
- Silver RM, Branch DW. Placenta accreta spectrum. N Engl J Med. 2018;378(16):1529–1536.
- Carcopino X, d‘Ercole C, Bretelle F. Optimal management strategies for placenta accreta. BJOG. 2009;116(11)1538; (author reply :1538; author reply 1538–1538; author reply 1539).
- Warshak CR, Ramos GA, Eskander R, et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet Gynecol. 2010;115(1):65–69.
- Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107(6):1226–1232.
- Bailit JL, Grobman WA, Rice MM, et al. Morbidly adherent placenta treatments and outcomes. Obstet Gynecol. 2015;125(3):683–689.
- Lumbiganon P, Laopaiboon M, Gülmezoglu AM, et al. World health organization global survey on maternal and perinatal health research group. Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007–08. Lancet. 2010;375(9713):490–499.
- Yan J, Wang L, Yang Y, et al. The trend of caesarean birth rate changes in China after 'universal two-child policy’ era: a population-based study in 2013-2018. BMC Med. 2020;18(1):249.
- 21.Liang J, Mu Y, Li X, et al. Relaxation of the one child policy and trends in caesarean section rates and birth outcomes in China between 2012 and 2016: observational study of nearly seven million health facility births. BMJ. 2018;360:k817.
- Zhang H-X, Zhao Y-Y, Wang Y-Q. Analysis of the characteristics of pregnancy and delivery before and after implementation of the two- child policy. Chin Med J. 2018;131(1):37–42.
- Zeng C, Yang M, Ding Y, et al. Placenta accreta spectrum disorder trends in the context of the universal two-child policy in China and the risk of hysterectomy. Int J Gynaecol Obstet. 2018;140(3):312–318.
- Schummers L, Hutcheon JA, Hernandez-Diaz S, et al. Association of short interpregnancy interval with pregnancy outcomes according to maternal age. JAMA Intern Med. 2018;178(12):1661–1670.
- Zhang L, Shen S, He J, et al. Effect of interpregnancy interval on adverse perinatal outcomes in Southern China: a retrospective cohort study, 2000-2015. Paediatr Perinat Epidemiol. 2018;32(2):131–140.
- McKinney D, House M, Chen A, et al. The influence of interpregnancy interval on infant mortality. Am J Obstet Gynecol. 2017;216(3):316.e1–316.e9.
- Marinovich ML, Regan AK, Gissler M, et al. Associations between interpregnancy interval and preterm birth by previous preterm birth status in four high-income countries: a cohort study. BJOG. 2021;128(7):1134–1143.
- Tessema GA, Pereira G. Challenging the assumption that interpregnancy interval causes stillbirth in low-income and middle-income countries. Lancet Glob Health. 2020;8(1):e16–e17.
- Kollmann M, Gaulhofer J, Lang U, et al. Placenta praevia: incidence, risk factors and outcome. J Matern Fetal Neonatal Med. 2016;29(9):1395–1398.
- Saleh Gargari S, Seify Z, Haghighi L, et al. Risk factors and consequent outcomes of placenta previa: report from a referral center. Acta Med Iran. 2016;54(11):713–717.
- Zhang X, Xu H, Hu R, et al. Changing trends of adverse pregnancy outcomes with maternal age in primipara with singleton birth: a join point analysis of a multicenter historical cohort study in China in 2011–2012. Acta Obstet Gynecol Scand. 2019;98(8):997–1003.
- Bi S, Zhang L, Chen J, et al. Maternal age at first cesarean delivery related to adverse pregnancy outcomes in a second cesarean delivery: a multicenter, historical, cross-sectional cohort study. BMC Pregnancy Childbirth. 2021;21(1):126.
- ACOG Committee Opinion No 736: optimizing postpartum care. Obstet Gynecol. 2018;131(5):e140–e150.
- Office of Disease Prevention and Health Promotion. Healthy People.gov. US Healthy people 2020 family planning objective 5. Healthy People 2020 Web site. https://www.healthypeople.gov/2020/topics-objectives/topic/family-planning/objectives. Updated September 7, 2018. Accessed March 3,2018.
- Garg B, Darney B, Pilliod RA, et al. Long and short interpregnancy intervals increase severe maternal morbidity. Am J Obstet Gynecol. 2021;225(3):331.e1–331.e8.
- Liu C, Snowden JM, Lyell DJ, et al. Interpregnancy interval and subsequent severe maternal morbidity: a 16-year population-based study from California. Am J Epidemiol. 2021;190(6):1034–1046.
- Martimucci K, Bilinski R, Perez AM, et al. Interpregnancy interval and abnormally invasive placentation. Acta Obstet Gynecol Scand. 2019;98(2):183–187.
- WHO guidelines on preventing early pregnancy and poor reproductive health outcomes among adolescents in developing countries. Geneva: World Health Organization; 2011.
- United Nations Population Fund. Girlhood, not motherhood: preventing adolescent pregnancy. New York: United Nations Population Fund; 2015.
- United Nations Population Fund. State of world population 2016. 10: how our future de - pends on a girl at this decisive age. New York: United Nations Population Fund; 2016.
- The National Campaign to Prevent Teen and Unplanned Pregnancy. Briefly: unplanned pregnancy among unmarried young women. 2012.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84(5):478–485.
- Kornides ML, Kitsantas P, Lindley LL, et al. Factors associated with young adults’ pregnancy likelihood. J Midwifery Womens Health. 2015;60(2):158–168.
- Norton M, Chandra-Mouli V, Lane C. Interventions for preventing unintended, rapid repeat pregnancy among adolescents: a review of the evidence and lessons from High-Quality evaluations. Glob Health Sci Pract. 2017;5(4):547–570.
- Wong SPW, Twynstra J, Gilliland JA, et al. Risk factors and birth outcomes associated with teenage pregnancy: a Canadian sample. J Pediatr Adolesc Gynecol. 2020; Apr33(2):153–159.
- Cook JL, Green CR, de la Ronde S, et al. Epidemiology and effects of substance use in pregnancy. J Obstet Gynaecol Can. 2017;39(10):906–915.
- Irvine B, Dzakpasu S, León JA. Perinatal health indicators 2013: a surveillance report by the public health agency of Canada’s perinatal surveillance system. Health Promot Chronic Dis Prev Can. 2015;35(1):23–24.
- East KA, Reid JL, Rynard VL, et al. Trends and patterns of tobacco and nicotine product use among youth in Canada, England, and the United States from 2017 to 2019. J Adolesc Health. 2021;69(3):447–456.
- Jauniaux E, Chantraine F, Silver RM, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: epidemiology. Int J Gynaecol Obstet. 2018;140(3):265–273.
- Jung EJ, Cho HJ, Byun JM, et al. Placental pathologic changes and perinatal outcomes in placenta previa. Placenta. 2018;63:15–20.
- Chen X, Man GCW, Liu Y, et al. Physiological and pathological angiogenesis in endometrium at the time of embryo implantation. Am J Reprod Immunol. 2017;78(2):e12693.