Abstract
Objective
This study aimed to evaluate the effects of the home quarantine on pregnancy outcomes of gestational diabetes mellitus (GDM) patients during the COVID-19 outbreak.
Methods
The complete electronic medical records of patients with GDM with home quarantine history were collected and classified into the home quarantine group from 24 February 2020 to 24 November 2020. The same period of patients with GDM without home quarantine history were included in the control group from 2018 to 2019. The pregnant outcomes of the home quarantine and control groups were systematically compared, such as neonatal weight, head circumference, body length, one-minute Apgar score, fetal macrosomia, and pre-term delivery.
Results
A total of 1358 patients with GDM were included in the analysis, including 484 in 2018, 468 in 2019, and 406 in 2020. Patients with GDM with home quarantine in 2020 had higher glycemic levels and adverse pregnancy outcomes than in 2018 and 2019, including higher cesarean section rates, lower Apgar scores, and higher incidence of macrosomia and umbilical cord around the neck. More importantly, the second trimester of home quarantine had brought a broader impact on pregnant women and fetuses.
Conclusion
Home quarantine has aggravated the condition of GDM pregnant women and brought more adverse pregnancy outcomes during the COVID-19 outbreak. Therefore, we suggested governments and hospitals strengthen lifestyle guidance, glucose management, and antenatal care for patients with GDM with home quarantine during public health emergencies.
Introduction
A new coronavirus that caused severe acute respiratory syndrome (SARS-CoV-2, COVID-19) emerged in late 2019, which has caused over 270 million infections and more than 5,300,000 deaths (https://covid19.who.int). COVID-19 can cause severe complications, including cough, fevers, myalgia, pharyngitis, dyspnea, pneumonia, acute respiratory distress syndrome (ARDS), and multisystem organ failure [Citation1,Citation2]. The rapid spread of the epidemic around the world brought suffering and life threats to the infected individuals and their families and seriously affected the economy and health of uninfected people. Varying degrees and types of lockdowns are effectively used to prevent the widespread of COVID-19, which has significantly changed people’s daily lifestyle, including decreased sports activities, increased sleep time, increased intake of high-calorie food, and so on [Citation3,Citation4]. Lockdown made them more vulnerable to over-eating and lifestyle sedentary, leading to further weight gain and increased cardiovascular risk [Citation5,Citation6]. These changes have indirectly threatened uninfected people, such as worsening sleep quality, increased risk of adolescent psychiatric disorder, impaired immune function, and increased risk of infections and autoimmunity [Citation7–10].
Gestational diabetes mellitus (GDM) is a typical metabolic disorder closely associated with inappropriate diet and exercise [Citation11]. Considerable evidence shows that active exercise benefits weight control and glycemic control, reducing the risk of large gestational age (LGA) and postpartum depression in patients with GDM [Citation12,Citation13]. In contrast, patients with GDM with high fat, high carbohydrate, high energy, and low fibrous diet will lead impaired gut microbiota which can be transmitted to the offspring and further increase the risk of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) in patients with GDM and their offspring [Citation14–17]. Retrospective studies of patients with GDM during the COVID-19 pandemic lockdown also showed that reduced physical activity, modified dietary habits, and anxiety exacerbated poor glycemic control [Citation18,Citation19]. Although these studies showed that home quarantine has changed the lifestyle of patients with GDM and further affected their health, it is unclear whether home quarantine impacts pregnancy outcomes.
During the outbreak of COVID-19 in China, Chongqing was subject to strict home quarantine from 24 January 2020 to 20 April 2020. Only essential activities were allowed during the lockdown, and most people’s mobility was limited to acquiring food and medicines; living at home was compulsory. Therefore, a retrospective cohort study was conducted on women diagnosed with GDM during the lockdown to assess the impacts of home quarantine on maternal and fetal outcomes in patients with GDM. This study aimed to provide information to guide antenatal care and clinical decision-making for patients with GDM during public health emergencies.
Materials and methods
Study design and participants
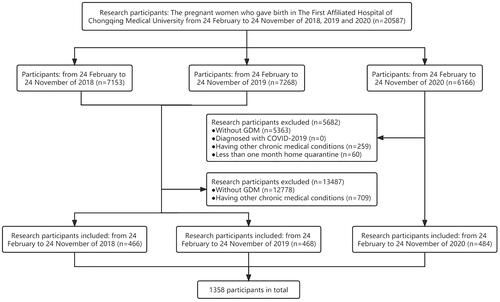
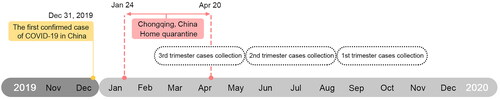
This was a single-center retrospective cohort study; information about the subjects was collected in The First Affiliated Hospital of Chongqing Medical University, a Grade III and Grade A hospital with an average of 10,000 births per year. The time of home quarantine of the patients with GDM was inferred from their gestational weeks and delivery date, and those with at least one month of home quarantine in 2020 were included in the analysis. As the strict epidemic lockdown was from 24 January 2020 to 20 April 2020 in Chongqing, China, the women with GDM who delivered on 24 February 2020 were the earliest participants enrolled in our study, while women with GDM who delivered on 24 November 2020 were the latest participants in our cohort. Subsequently, complete clinical information of patients from 24 February 2020 to 24 November 2020 was collected and classified into the home quarantine group, and data from the same period in 2018 and 2019 were also collected and classified into the control group (). The length of COVID quarantine in the case group was about three months. Patients were further divided into three groups according to different periods of pregnancy during home quarantine to explore the effects of home quarantine in different periods on pregnancy outcomes (). Subjects were excluded if they had chronic medical conditions, such as hypertension, gestational hypertension, preeclampsia, type 2 diabetes mellitus, and heart or kidney diseases. In addition, all patients tested negative for SARS-CoV-2 by RT-qPCR. Women with GDM in 2018 and 2019 have taken routine antenatal checkups and antenatal care (ANC), but patients in 2020 experienced a COVID-19 lockdown, resulting in varying degrees of reduction and lack of antenatal care and necessary antenatal care. This study was approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University (ID: 20200501).
Figure 1. The timeline of home quarantine was used to collect the clinical information of patients with GDM during the COVID-19 outbreak. The patients with GDM were further divided into three groups according to different periods of pregnancy during home quarantine, including first (from 24 February to 24 May), second (from 25 May to 24 August), and third (from 25 August to 24 November) trimester. The non-home quarantine groups were collected from the same period of 2018 and 2019.
Definition
All participants were offered a 75 g Oral Glucose Tolerance Test (OGTT) during the routine antenatal checkups at 24–28 weeks after overnight fasting for 8–10 h in early pregnancy, and venous blood glucose was analyzed. Then, all participants were clinically diagnosed with GDM according to the OGTT biochemical criterion that at least one glucose value must be equaled or exceeded to define the patient as having gestational diabetes. With GDM diagnosed from venous samples according to the IADPSG/WHO 2010 criteria (fasting plasma glucose ≥ 5.1 mmol/L, 1 h plasma glucose ≥ 10.0 mmol/L or 2 h plasma glucose ≥ 8.5 mmol/L) (IADPSG), which was the clinical guideline in use during the study [Citation20]. Macrosomia is typically defined as birth weight ≥ 4000 g, and preterm birth is defined as babies born at <37 weeks of gestational age [Citation21]. Placenta accreta spectrum (PAS) is a disease that damages and destroys myometrium, referring to all 3 forms of pathologically adherent placentae including accreta, increta, and percreta [Citation22]. Oligohydramnios is defined as decreased amniotic fluid volume (AFV) for gestational age, and polyhydramnios is defined as increased amniotic fluid volume, which is evaluated by ultrasound of AFV [Citation23,Citation24]. Polyhydramnios has been defined as an AFV of greater than 2000 ml [Citation25]. Oligohydramnios has been defined as an AFV that is less than 200 ml or 500 ml [Citation26,Citation27].
Data collection
All data were collected from hospital electronic medical records, including maternal characteristics, maternal outcomes, fetal outcomes, and obstetric complications. Maternal characteristics covered age, gestational age, gestational weight gain, parity, gravidity, fetal sex, fetal number, and pre-pregnancy BMI. Vaginal delivery, pregnancy BMI after, fasting glycemia, 60 min glycemia, 120 min glycemia, polyhydramnios, and oligohydramnios were collected to the maternal outcomes. Fetal outcomes include neonatal weight, abdominal circumference, head circumference, body length, placental weight, placental thickness, length, width, umbilical cord length, amniotic fluid volume, and Apgar Scores. Concerning obstetric complications, placental accreta spectrum, umbilical cord around the neck, pre-term delivery, and fetal macrosomia was collected. It is routine for all placentas to be measured and all the work done by the pathologist. Additionally, the ultrasonic wave was used to measure the fluid volumes by imaging doctors within a week before delivery. To protect the privacy of patients, the personal identification information of all cases was deleted in the process of data collection and analysis.
Statistical analyses
All statistical analyses were performed using the SPSS software program, version 22.0 (IBM, Armonk, NY, USA). Continuous variables with normal distribution were presented as mean ± SD, using median plus 25–75 interquartile range (IQR) to show non-normally distributed variables, and categorical variables were presented as percentages and counts. Chi-square or Fisher’s exact test was used for categorical variables such as the incidence of GDM. Under the premise that the continuous variables obey a normal distribution, the one-way ANOVA was used if the inter-group variance is homogeneity. If the variance was inhomogeneity, the rank sum test was used. Post hoc analysis involving pairwise comparisons was performed if there were ≥3 independent groups, using L-S-D correction results, and results with significant differences between the two groups have been shown in tables.
Multiple logistic regression was used to compare the outcomes of pregnant women and fetuses and obstetric complications, such as placental factors, umbilical factors, and macrosomia of different groups. Potential covariates that may be associated with the outcomes of pregnant woman and fetus, abnormal placentation, macrosomia, and other obstetric complications were adjusted in the model, including age and gestational age. In addition, multiple logistic regression was used in one-minute Apgar scores, delivery patterns, OGTT biochemical criteria, and obstetric complications. Thus, other variables are considered to be continuous variables, such as neonatal weight, body length, placental weight, and length. Results are presented as OR or Adjusted OR with 95% CI. No more than 1% of values were missing in the variables used in the statistical analyses. Therefore, none of the imputations were done for the missing data.
Results
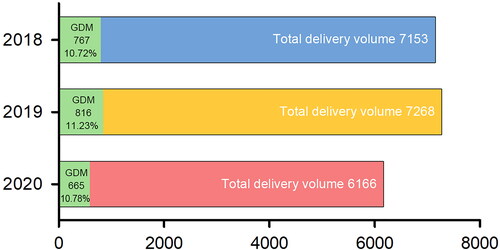
A total of 19,229 pregnant women were excluded from 20,587 pregnant women who gave birth in The First Affiliated Hospital of Chongqing Medical University from 24 February to 24 November of 2018, 2019, and 2020. In total, 1358 patients were included in the final cohort, and all were diagnosed with GDM through the OGTT. Of these, 466 patients in 2018, 468 patients in 2019, and 484 patients in 2020 were included in our study, respectively (). The analysis of GDM incidence in three years showed that the GDM incidence rate in 2019 (11.23%) was the highest, and 2020 (10.78%) was similar to 2018 (10.72%) ().
Clinical characteristics of patients with GDM in 2018, 2019, and 2020
The data showed no significant difference in the incidence of GDM over the three years (). The further study of the clinical characteristic of 1358 patients with GDM showed that patients with GDM in 2020 (home quarantine group) had the maximal average age, 31.69 years (p = .029). However, patients with GDM in 2020 had the minimum gestational weeks, which means their fetuses delivered at earlier gestational ages, 38.91 (p = .025; ).
Table 1. Basic characteristics of patients with GDM.
Maternal outcomes of patients with GDM in 2018, 2019, and 2020
Logistic regression showed that patients with GDM in 2020 have fewer gestational weeks than 2019 (AOR, 1.20; 95% CI, 1.05–1.31; p = .007), and patients with GDM in 2020 had a growing risk of cesarean section rate than 2018 (AOR, 1.62; 95% CI, 1.18–2.22; p = .003) and 2019 (AOR, 1.63; 95% CI, 1.18–2.24; p = .003; ). patients with GDM in 2020 had higher blood glycemic levels than in 2018 (AOR, 0.32; 95% CI, 0.23–0.43; p < .001) and in 2019 on fasting glycemia (AOR, 0.66; 95% CI 0.49–0.90; p = .009; ). Additionally, patients with GDM in 2020 also had higher blood glycemic levels of 60 min glycemia (AOR, 0.71; 95% CI, 0.53–0.96; p = .026) and 120 min glycemia than in 2018 (AOR, 0.58; 95% CI, 0.43–0.79; p = .001; ). Further analysis showed that the incidence of polyhydramnios increased in 2020 than in 2018 (AOR, 0.89, 95%CI, 0.62–0.98; p = .047) and 2019 (AOR, 0.69, 95%CI, 0.59–0.85; p = .041), but there was no significant difference in the incidence of oligohydramnios.
Table 2. Pregnant outcomes of patients with GDM.
Fetal outcomes of patients with GDM in 2018, 2019, and 2020
Logistic regression showed that fetuses of patients with GDM in 2020 had longer abdominal circumferences (AOR, 0.89; 95% CI, 0.88–0.95; p = .044), head circumference (AOR, 0.98; 95% CI, 0.96–0.99; p = .005), and body length (AOR, 0.89; 95% CI, 0.81–0.98; p = .019) than 2018, but there was no difference in fetal outcomes between 2019 and 2020 (). Furthermore, fetuses of patients with GDM in 2020 had thicker (AOR, 0.95; 95% CI, 0.91–0.97; p = .007) and larger size placenta than 201in 8 (width: AOR, 0.89; 95% CI, 0.82–0.98; p = .015; length: AOR, 0.90; 95% CI, 0.83–0.97; p = .008; ). In addition, fetuses of patients with GDM in 2020 had lower one-minute Apgar scores than in 2018 (AOR, 0.49; 95% CI, 0.35–0.69; p < .001) and 2019 (AOR, 0.47; 95% CI, 0.33–0.63; p < .001; ).
Table 3. Fetal outcomes of patients with GDM.
The labor complications were further analyzed to evaluate the effects of home quarantine on fetal outcomes. Multivariate logistic regression was used to control for the following covariates, age, parity, and gestational age. Although there is no significant difference in the incidence of pre-term delivery, which is defined as delivery before 37 weeks of gestation, the data showed that the incidence of fetal macrosomia in 2020 was higher than in 2018 (AOR, 0.82; 95% CI, 0.71–0.98; p = .046; ). Besides, the incidence of the umbilical cord around the neck has also increased in 2020 (AOR, 0.63; 95% CI, 0.44–0.90; p = .003; ).
Table 4. Obstetric complications of patients with GDM.
In addition, only 20 infants with fetal growth restriction (FGR) were found, and there was no significant difference. No other abnormal outcomes were observed among the infants, such as death, stillbirth, dystocia, bone fracture, nerve palsy, admission to the neonatal intensive care unit, or respiratory complications.
Effects of home quarantine during different gestational periods on pregnancy outcomes in patients with GDM
These 1358 patients with GDM were further divided into three groups according to different periods of pregnancy during home quarantine, including first (group 1), second (group 2), and third trimester (group 3). The data showed a significant difference in pre-pregnancy BMI (p = .002) in group 2 between 2018 and 2020, 2019 and 2020 (Supplemental Table 1). Patients with GDM in group 1 of 2020 had a higher glycemic level in fasting glycemia than in 2019 (first, AOR, 0.46; 95% CI, 0.26–0.82; p = .008), while the glycemic in group 2 of 2020 also showed a higher level than 2018 (AOR, 0.40; 95% CI, 0.22–0.71; p = .002) and 2019 (AOR, 0.47; 95% CI, 0.26–0.84; p = .011; Supplemental Table 2).
The cesarean section rate was significantly increased in both group 1 (AOR, 2.51; 95% CI, 1.37–4.58; p = .003) and group 3 of 2020 than in 2018 (AOR, 1.69; 95% CI, 1.04–2.74; p = .036). A significant increase of cesarean section rate was also observed in second (AOR, 1.79; 95% CI, 1.01–3.16; p = .046) and group 3 of patients with GDM in 2020 than 2019 (AOR, 1.85; 95% CI, 1.10–3.10; p = .021; Supplemental Table 2). Patients with GDM in group 1 (AOR, 0.85; 95% CI, 0.74–0.99; p = .033) or group 3 of 2020 have a heavier (AOR: 0.89; 95% CI, 0.79–0.99; p = .049) and thicker placenta than 2018 (AOR, 0.94; 95% CI, 0.89–0.99; p = .025); however, the placenta of patients in group 2 of 2020 was lighter (AOR,1.011; 95% CI, 1.007–1.016; p < .001) and thinner than 2019 (AOR, 1.39; 95% CI, 1.14 = 1.69; p = .001; AOR, 1.16; 95% CI, 1.00–1.35; p = .047; Supplemental Table 2). Besides, patients with GDM in group 2 of 2020 had higher placenta accreta spectrum rate than both 2018 (AOR, 0.17; 95% CI, 0.05–0.60; p = .006) and 2019 (AOR, 0.19; 95% CI, 0.05–0.71; p = .013).
We further analyzed the effects of different pregnant periods during home quarantine on fetal outcomes. The fetus in group 2 of 2020 presented a heavier weight (AOR, 1.01; 95% CI, 1.00–1.02; p = .001) and longer body than 2019 (AOR, 1.32; 95% CI, 1.10–1.58; p = .003). Meanwhile, fetuses of patients with GDM in 2020 had lower one-minute Apgar scores than all trimesters of 2018 (AOR, 0.41; 95% CI, 0.21–0.82; p = .001; AOR, 0.52; 95% CI, 0.29–0.96; p = .007; AOR, 0.39; 95% CI, 0.23–0.64; p < .001) and group 1, 2 of 2019 (AOR, 0.32; 95% CI 0.16–0.63, p = .012; AOR, 0.43; 95% CI, 0.23–0.79; p = .036; Supplemental Table 2). More importantly, although there is no significant difference in the incidence of pre-term delivery among all trimesters, data showed fetuses in group 1 (AOR, 0.05; 95% CI, 0.01–0.43; p = .005) and group 2 of 2020 had a higher incidence of umbilical cord around neck increased than 2018 (AOR, 0.29; 95% CI, 0.14–0.64; p = .002; Supplemental Table 2). In summary, the home quarantine of all three pregnancy periods would bring different degrees of harm to pregnant women and fetuses, especially group 2 had brought a wider impact on pregnancy outcomes.
Discussion
COVID-19 is still raging worldwide, and numerous countries are forced to adopt varying degrees and forms of lockdown to prevent the broader spread of the virus. These lockdowns have brought many challenges to people’s economic development and lives, which have dramatically changed the diet and exercise of people, and even aggravated the symptoms of many diseases, especially metabolic disorders [Citation28–30]. Therefore, this retrospective study of patients with GDM was used to investigate the impacts of home quarantine on patients with GDM and their pregnancy outcomes during the COVID-19 outbreak. The data showed that the incidence of GDM was observed in no significant difference over three years. However, other studies have reported a significant increase in the number of patients with GDM during the lockdown [Citation18,Citation31]. Possible reasons for this phenomenon could be the following: (1) The detection rate of GDM decreased since a significant number of pregnant women reduced their antenatal checkups even though some highly recommended antenatal checkups had been canceled or postponed beyond their opportune gestational age due to the epidemic management policy and fear of infection [Citation32]; (2) the mild GDM may be dismissed/undiagnosed in the 2020 group, and it could further cause the detection rate of GDM decreased in 2020 and the increased prevalence of adverse perinatal outcomes in the GDM group in 2020. Therefore, multicenter data is needed to determine whether home quarantine would change GDM incidence. Although an increased incidence of GDM was not observed, fasting glycemia in 2020 was significantly higher than in 2018 and 2019. Further clinical data from different trimesters showed that home quarantine increased the fasting glycemia of patients with GDM, especially in the second trimester. In addition, the home quarantine group had thicker and heavier placenta than patients with GDM in 2018 and 2019, leading to macrosomia in most patients with GDM [Citation33]. These findings suggested that home quarantine may aggravate the impact of GDM on patients.
Pregnant women had a shorter pregnancy gestational age in 2020 than in 2019; meanwhile, the cesarean section rate of patients with GDM in 2020 was significantly higher than that in 2018 and 2019. This suggests that the increase in cesarean sections in 2020 may lead to a decrease in pregnancy gestational age. Studies have found that pregnant women with GDM are more likely to have placental accreta spectrum but the specific reason is still unknown [Citation34].
Significant increases were also observed in fetal head circumference, body length, and incidence of macrosomia in patients with GDM in 2020. The high-fat and high-calorie diet will not only increase the occurrence of GDM but also increase the probability of macrosomia, while the increase in depression, anxiety, and stress during the lockdown will make pregnant women more inclined to a high-calorie or high-fat diet [Citation35–37]. However, quantitative dietary data still needs to confirm whether the high incidence of macrosomia is caused by dietary changes during home quarantine. A previous study showed that the incidence of the umbilical cord around the neck among patients with GDM was higher than in normal pregnancies [Citation38]. It has been reported that approximately 6%–25% of women with GDM would experience polyhydramnios, and this phenomenon increases the space for the fetus to move in the uterus, thus increasing the risk of the umbilical cord around the neck [Citation39]. Our data also showed that patients with GDM in 2020 had a higher incidence of polyhydramnios, which may be the reason for the higher incidence of the umbilical cord around neck. Studies have found that moderate exercise helps improve GDM condition, especially benefiting from walking [Citation40–42]. The light regulation of circadian rhythm has also been found to be essential for fetal development. On the one hand, home quarantine greatly limited the exercise of patients with GDM; on the other hand, the residential structure and climate in Chongqing greatly limited the light exposure of pregnant women. These might also be the reasons for the decline of various clinical indicators in GDM pregnant women and the increased incidence of macrosomia. Macrosomia will cause postpartum bleeding and vaginal lacerations and lead to an increased risk of fetal dystonia, collarbone fracture, and brachial plexus injury, which further increases the rate of admission to the neonatal intensive care unit [Citation43]. Thus, pregnant women with macrosomia generally adopted the cesarean section in clinical treatment, which gives a possible explanation for the increase in the cesarean section rate in 2020. Moreover, the data confirmed that one-minute Apgar scores in 2020 were significantly lower than in 2018 and 2019, and the incidence of the umbilical cord around the neck increased significantly. Clinically Apgar score is widely used to evaluate various neonatal indexes, such as hypotonia, pulse, skin color, respiration, breath, response to stimulation, and so on [Citation44]. Previous studies have shown that the umbilical cord around the neck could cause breathing difficulties and distress in newborns [Citation45], which may contribute to the decrease in Apgar scores in 2020.
Limitations
Although the research clarified the impact of home quarantine on patients with GDM, it has some limitations. Firstly, the patients with a history of at least one month to three months of home quarantine were included in the analysis. There may not be enough time for significant psycho-environmental changes to occur that impact glycemic control in pregnancies complicated by diabetes. Second, because this study is a retrospective study using routinely collected data, it is hard to obtain quantitative data on the diet, exercise, and mental state of patients with GDM. Lastly, the reasons for c-section were not clearly recorded in electronic medical records, and it is determined by the doctor according to the clinical guidelines, as well as the actual situation of pregnant women.
Conclusion
Our findings suggested that home quarantine will aggravate the condition of GDM pregnant women and further bring more adverse pregnancy outcomes, such as macrosomia and umbilical cord around the neck. More importantly, the analysis of the different trimesters showed that home quarantine in the second trimester would bring more extensive adverse effects on patients with GDM compared to the first and third trimesters, including neonatal weight, body length, placental weight, placental size, amniotic fluid volume, and the risk of placental accreta spectrum. We recommend that patients with GDM should have a proper diet and exercise moderately during home quarantine, especially those in the second trimester. All hospital levels should intensify exercise guidance and dietary management for pregnant women through online consultations during the pandemic lockdown. Besides, accelerating the development of wearable and portable home-care devices and using artificial intelligence to develop disease prediction models can further strengthen disease prevention and nursing to avoid adverse pregnancy outcomes.
Ethics approval and consent to participate
This study was approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University (ID: 20200501). All participants provided informed consent.
Consent for publication
Verbal informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no conflict of interest.
Author contributions
Q.C., X.L., and T.L. designed the study. Q.C., L.R., D.W., H.C., S.Y., and W.L. contributed to data collection and data cleaning. Q.C., Y.Y., and T.L. were involved in the data analysis, interpreted the findings, and wrote the manuscript. Y.Y., L.R., X.L., and T.L. reviewed and edited the manuscript. All authors gave final approval for publication. T.L. is the guarantor of this work and, as such, had full access to all of the data in the study and all authors take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplemental Material
Download MS Word (43.2 KB)Acknowledgments
The authors also thank all involved laboratory technicians for the help with data collection and analysis.
Availability of data and materials
All data are available upon request from the authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ′cytokine storm’ in COVID-19. J Infect. 2020;80(6):607–613.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
- Pietrobelli A, Pecoraro L, Ferruzzi A, et al. Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: a longitudinal study. Obesity. 2020;28(8):1382–1385.
- Di Renzo L, Gualtieri P, Pivari F, et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18(1):229–243.
- Mattioli AV, Sciomer S, Cocchi C, et al. Quarantine during COVID-19 outbreak: changes in diet and physical activity increase the risk of cardiovascular disease. Nutr Metab Cardiovasc Dis. 2020;30(9):1409–1417.
- Cai QY, Yang Y, Wang YH, et al. Home quarantine: a double-edged sword during COVID-19 pandemic for hypertensive disorders of pregnancy and the related complications. Diabetes Metab Syndr Obes. 2022;15:2405–2415.
- Guessoum SB, Lachal J, Radjack R, et al. Adolescent psychiatric disorders during the COVID-19 pandemic and lockdown. Psychiatry Res. 2020;291:113264.
- Blume C, Schmidt MH, Cajochen C. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol. 2020;30(14):R795–R797.
- Bakaloudi DR, Barazzoni R, Bischoff SC, et al. Impact of the first COVID-19 lockdown on body weight: a combined systematic review and a meta-analysis. Clin Nutr. 2021;41:3046–3054.
- Simmons D, Devlieger R, van Assche A, et al. Effect of physical activity and/or healthy eating on GDM risk: the DALI lifestyle study. J Clin Endocrinol Metab. 2017;102(3):903–913.
- Mijatovic-Vukas J, Capling L, Cheng S, et al. Associations of diet and physical activity with risk for gestational diabetes mellitus: a systematic review and meta-analysis. Nutrients. 2018;10(6):698–716.
- Kintiraki E, Goulis DG. Gestational diabetes mellitus: multi-disciplinary treatment approaches. Metabolism. 2018;86:91–101.
- Ehrlich SF, Ferrara A, Hedderson MM, et al. Exercise during the first trimester of pregnancy and the risks of abnormal screening and gestational diabetes mellitus. Diabetes Care. 2021;44(2):425–432.
- Martis R, Crowther CA, Shepherd E, et al. Treatments for women with gestational diabetes mellitus: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2018;8:CD012327.
- Bain E, Crane M, Tieu J, et al. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2015;4(4):CD010443.
- Ponzo V, Fedele D, Goitre I, et al. Diet-Gut microbiota interactions and gestational diabetes mellitus (GDM). Nutrients. 2019;11(2):330–342.
- Chiefari E, Arcidiacono B, Foti D, et al. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. 2017;40(9):899–909.
- Ghesquiere L, Garabedian C, Drumez E, et al. Effects of COVID-19 pandemic lockdown on gestational diabetes mellitus: a retrospective study. Diabetes Metab. 2021;47(2):101201.
- Sidor A, Rzymski P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients. 2020;12(6):1657–1669.
- Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682.
- Feig DS, Corcoy R, Jensen DM, et al. Diabetes in pregnancy outcomes: a systematic review and proposed codification of definitions. Diabetes Metab Res Rev. 2015;31(7):680–690.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
- Zaretsky MV, McIntire DD, Reichel TF, et al. Correlation of measured amnionic fluid volume to sonographic and magnetic resonance predictions. Am J Obstet Gynecol. 2004;191(6):2148–2153.
- Krispin E, Berezowsky A, Chen R, et al. Updating the amniotic fluid index nomograms according to perinatal outcome. J Matern Fetal Neonatal Med. 2020;33(1):113–119.
- Magann EF, Morton ML, Nolan TE, et al. Comparative efficacy of two sonographic measurements for the detection of aberrations in the amniotic fluid volume and the effect of amniotic fluid volume on pregnancy outcome. Obstet Gynecol. 1994;83(6):959–962.
- Horsager R, Nathan L, Leveno KJ. Correlation of measured amniotic fluid volume and sonographic predictions of oligohydramnios. Obstet Gynecol. 1994;83(6):955–958.
- Dubil EA, Magann EF. Amniotic fluid as a vital sign for fetal wellbeing. Australas J Ultrasound Med. 2013;16(2):62–70.
- Conceicao LSR, Gomes CVC, Carvalho VO. Comment on: “the challenge of maintaining metabolic health during a global pandemic.” Sports Med. 2020;50(11):2063–2064.
- King AJ, Burke LM, Halson SL, et al. The challenge of maintaining metabolic health during a global pandemic. Sports Med. 2020;50(7):1233–1241.
- Martinez-Ferran M, de la Guia-Galipienso F, Sanchis-Gomar F, et al. Metabolic impacts of confinement during the COVID-19 pandemic due to modified diet and physical activity habits. Nutrients. 2020;12(6):1549–1566.
- Radan AP, Fluri MM, Nirgianakis K, et al. Gestational diabetes is associated with SARS-CoV-2 infection during pregnancy: a case-control study. Diabetes Metab. 2022;48(4):101351.
- Chen M, Liu X, Zhang J, et al. Characteristics of online medical care consultation for pregnant women during the COVID-19 outbreak: cross-sectional study. BMJ Open. 2020;10(11):e043461.
- Whittington JR, Cummings KF, Ounpraseuth ST, et al. Placental changes in diabetic pregnancies and the contribution of hypertension. J Matern Fetal Neonatal Med. 2020;35:486–494.
- Matsuzaki S, Mandelbaum RS, Sangara RN, et al. Trends, characteristics, and outcomes of placenta accreta spectrum: a national study in the United States. Am J Obstet Gynecol. 2021;225(5):534 e1–534 e38.
- Vambergue A, Fajardy I. Consequences of gestational and pregestational diabetes on placental function and birth weight. World J Diabetes. 2011;2(11):196–203.
- Zhang J, Zhang Y, Huo S, et al. Emotional eating in pregnant women during the COVID-19 pandemic and its association with dietary intake and gestational weight gain. Nutrients. 2020;12(8):2250–2261.
- de la Torre NG, Assaf-Balut C, Jimenez Varas I, et al. Effectiveness of following Mediterranean diet recommendations in the real world in the incidence of gestational diabetes mellitus (GDM) and adverse maternal-foetal outcomes: a prospective, universal, interventional study with a single group. The St Carlos Study. Nutrients. 2019;11(6):1210–1224.
- Ezimokhai M, Rizk DE, Thomas L. Maternal risk factors for abnormal vascular coiling of the umbilical cord. Am J Perinatol. 2000;17(8):441–445.
- Chiou YL, Hung CH, Liao HY. The impact of prepregnancy body mass index and gestational weight gain on perinatal outcomes for women with gestational diabetes mellitus. Worldviews Evid Based Nurs. 2018;15(4):313–322.
- Peters TM, Brazeau AS. Exercise in pregnant women with diabetes. Curr Diab Rep. 2019;19(9):80.
- Bagci S, Sabir H, Muller A, et al. Effects of altered photoperiod due to COVID-19 lockdown on pregnant women and their fetuses. Chronobiol Int. 2020;37(7):961–973.
- Hayashi A, Oguchi H, Kozawa Y, et al. Daily walking is effective for the management of pregnant women with gestational diabetes mellitus. J Obstet Gynaecol Res. 2018;44(9):1731–1738.
- Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metabol. 2015;66(Suppl 2):14–20.
- American Academy of Pediatrics Committee on Fetus and Newborn, American College of Obstetricians and Gynecologists Committee on Obstetric Practice. The Apgar score. Pediatrics. 2015;136(4):819–822.
- Walla T, Rothschild MA, Schmolling JC, et al. Umbilical cord entanglement’s frequency and its impact on the newborn. Int J Legal Med. 2018;132(3):747–752.