Abstract
We report the case of a massive fetal cardiac rhabdomyoma recently occurred at our clinic. A woman at 23 weeks of gestational age was referred to our center for a fetal cardiac echogenic mass of 26 mm detected at the second-trimester screening ultrasound. During pregnancy, though, the mass progressively increased its dimensions until reaching 48 mm in diameter at 37 weeks of gestation. Fetal echoencephalography and brain magnetic resonance did not show any further fetal anomalies, but molecular genetic testing at amniocentesis revealed a heterozygotic missense variant of gene TSC2 associated with Tuberous Sclerosis. The mass was therefore most likely preferable to a single large rhabdomyoma of gradually increasing dimensions. The baby was delivered at term with a cesarean section. Because of the rhabdomyoma remarkable size and newborn ECG electrical alterations, postnatal therapies with Flecainide and Everolimus were started. Everolimus treatment led to a significant and progressive reduction in the cardiac mass volume. This case, therefore, shows the efficacy of what seems to be a promising treatment in pediatric patients with large rhabdomyomas.
Rhabdomyomas may present with different features: most often they appear as multiple masses along the interventricular sept, but they may also appear as a single large thoracic mass.
When a rhabdomyoma is suspected, genetic counseling is recommended.
Both before and after birth, a multidisciplinary approach is useful to choose the appropriate therapy for the newborn.
mTOR inhibitors therapies look like promising therapeutic approaches to stimulate the involution of rhabdomyomas.
Learning points:
Case report
We report the case of a massive fetal cardiac rhabdomyoma recently occurred at our clinic. The mother signed an informed consent and care has been taken in preserving anonymity.
A 31-year-old woman at her first pregnancy was referred to our clinic at 23 weeks of gestational age because of the finding of a large fetal cardiac mass at second-trimester screening ultrasound, suspicious for cardiac fibroma. Noninvasive prenatal testing and nuchal translucency thickness assessed during the first-trimester aneuploidies screening ultrasound were within normal limits.
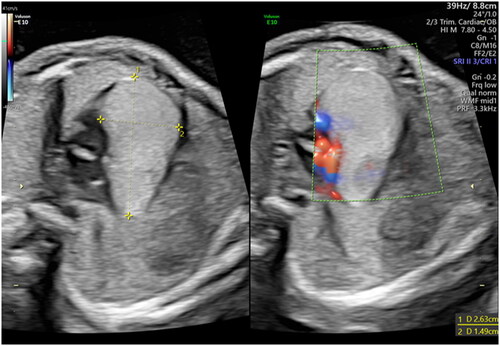
Echocardiographic examination showed a hyperechogenic homogeneous area of 26 × 15 mm located at the atrioventricular junction (). It was extending throughout the whole cardiac diameter and moved synchronically with cardiac chambers (Supplementary material, SCitation1). Pulsed wave and Color Doppler assessment of atrioventricular and semilunar valves showed normal waves form. A modest pericardial effusion was detected but the cardiac frequency was normal. A fetal neuro sonography was performed, showing no visible pathological lesions. Fetal magnetic resonance confirmed the presence of an expansive cardiac neoformation of 29 x 13 x 12 mm.
The woman underwent an amniocentesis, resulting in a normal female karyotype (46 XX) and normal array CGH. However, molecular analysis of genes TSC1 and TSC2 revealed a heterozygotic missense variant of gene TSC2 associated with Tuberous Sclerosis (TS). The same mutation was later found in the asymptomatic mother as well. Considering the results of genetic testing, the mass was most likely referrable to a single large rhabdomyoma of progressively increasing dimensions (supplementary material, S2). At 37 weeks of gestational age, the mass had reached the remarkable size of 48 × 42 mm (), raising the fear of fetal heart failure and malignant arrhythmia.
Figure 2. Echocardiographic appearance of the rhabdomyoma at 25 (a) and 37 (b) weeks of gestational age respectively, demonstrating its progressive enlargement.
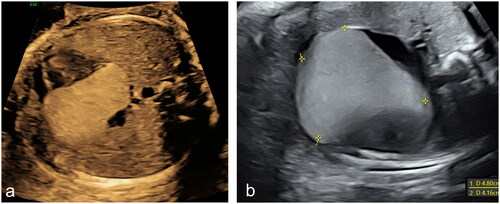
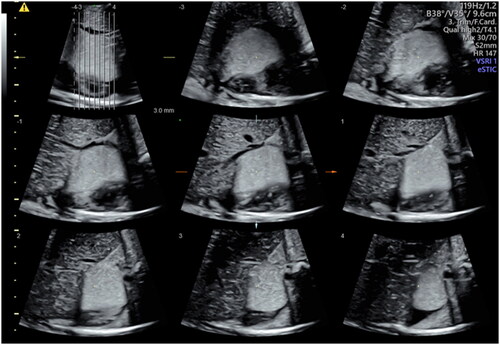
Figure 3. Three-dimensional reconstruction of the rhabdomyoma at 37 weeks by Tomographic Ultrasound Imaging (TUI).
A vaginal labor induction was planned at 39 weeks and 5 days of gestational age, however eventually the patient underwent a caesarean section for failed induction and delivered a female newborn of 2980 grams, with normal umbilical cord gas analysis and an Apgar score of 10 at 5 min. The baby was promptly transferred to Neonatal Intensive Care Unit for cardiac monitoring.
Postnatal electrocardiographic (ECG) evaluation showed a cardiac frequency of 135 bpm, ventricular pre-excitation, short PR interval, wide QRS complexes, and widespread downsloping ST-segment depression. Cardiac ultrasound confirmed the presence of an intrapericardial wide mass of 42 × 28 mm strictly connected to the right ventricle, the right atrium, and the posterior wall of the left ventricle. No obstruction to the effluxes was detected. Both at echoencephalography and at magnetic resonance the brain appeared normal and no major anomalies could be found. Electroencephalography did not demonstrate any atypical neural activity and fundus oculi examination was regular. Dermatological examination and abdominal ultrasound did not show any abnormality either.
Because of the significant dimensions of the mass, prophylactic anti-arrhythmic therapy with Flecainide was started to prevent the occurrence of potentially lethal arrhythmia. The newborn was then discharged, with outpatient follow-up arranged.
At the two-month cardiologic follow-up examination, the rhabdomyoma size was unchanged. An off-label experimental therapy with Everolimus was therefore started to stimulate mass involution. The starting dose was 0.1 mg per day. Three weeks after the beginning of Everolimus administration, the tolerance to treatment was good and the baby’s growth was regular. Cell count, and renal and liver function were monitored regularly and appeared normal. Moreover, a cardiac ultrasound revealed a considerable reduction in the mass dimensions, which at the time measured 37 × 15 mm. At ten weeks follow-up examination, the mass volume decline continued, with normalization of ECG evaluation. Twenty-five weeks after the beginning of the therapy at the unmodified dose of 0.1 mg per day, the mass had further decreased in size, with a maximum dimension of 29 mm. The baby’s tolerance to treatment was adequate and her cardiac function was still optimal. The only laboratory alteration that emerged during treatment was hypertriglyceridemia, while cell count and renal and liver function had always been normal. Everolimus serum levels never exceeded the therapeutic range (3.0–8.0 ng/ml) throughout the treatment. The baby is currently on monthly follow-up and is now asymptomatic.
Discussion
Primary heart tumors are an extremely rare finding during prenatal examination [Citation1]. Fetal cardiac tumors are usually benign, with variable clinical manifestations [Citation2]. Among cardiac tumors, rhabdomyoma are the most common, accounting for approximately 60–70% of cases [Citation1,Citation3]. Other cardiac neoplasms include fibromas, myxomas, teratomas, and hemangiomas [Citation1,Citation4]. At ultrasound examination, they appear as single or multiple round, hyperechogenic homogeneous masses, mostly located in the ventricles [Citation1,Citation5,Citation6]. Even though symptoms and prognosis are determined by their size, location, and rhythm or flow impairment [Citation1,Citation3,Citation7,Citation8], they are mostly asymptomatic and are often diagnosed as incidental findings during the second or third trimester of pregnancy [Citation1]. Rhabdomyomas might increase in size in utero [Citation5,Citation6,Citation9], with possible arrythmias or hemodynamic impairment in case of large tumors [Citation5,Citation7]. However, as rhabdomyomas can regress during postnatal life, surgical intervention is reserved for cases of severe hemodynamic compromise or persistent arrythmias refractory to medical treatment [Citation1–3,Citation6,Citation8,Citation9]. One of the major concerns when a fetal rhabdomyoma is detected is its possible association with TS, which varies from 50% to 80% of cases [Citation1,Citation5,Citation6] and of which rhabdomyomas are often the first manifestation in utero [Citation1,Citation2]. TS is an autosomal dominant trait with a variable expression, with sporadic mutations explaining two-thirds of cases. Mutations leading to TS are detectable by chorionic villus sampling, amniocentesis, or cordocentesis and occur in tumor suppressor genes TSC1 (encoding for protein hamartin) or TSC2 (encoding for protein tuberin); these two proteins are widely expressed in neural, epithelial and cardiac tissues, explaining the neural, cardiac, retinal and renal lesions typically found in affected patients [Citation4,Citation6].
The Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations which were published in 2021 [Citation10] enumerate a list of genetic and clinical criteria to establish tuberous sclerosis complex (TSC) diagnosis. Consistently with the case we reported, it is stated that a pathogenic variant in TSC1 or TSC2 genes is diagnostic for TSC, while cardiac rhabdomyoma are mentioned among the major clinical diagnostic criteria [Citation10].
As a rhabdomyoma may be the first antenatal sign of TS and precede the diagnosis of cerebral or renal lesions, its detection should be considered a warning sign for TS and guide the genetic and obstetric counseling of at-risk families [Citation1,Citation5,Citation8].
Because surgical management may determine significant morbidity and mortality, new treatments with mammalian target of rapamycin (mTOR) inhibitors such as Sirolimus or Everolimus have been proposed to stimulate the involution of rhabdomyoma and to reduce the necessity of intervention in symptomatic neonates [Citation11]. Currently, data on dose, duration, and safety profile in newborns are limited and only a few case reports are reported in literature [Citation12,Citation13]. Nevertheless, their use in the management of TSC manifestations and rhabdomyoma seems promising, as these therapies are often reported to induce a significant reduction in rhabdomyoma size [Citation13].
As for our case, side effects monitoring was performed through seriate cell count, renal, and hepatic function evaluations. Everolimus serum concentration was tested regularly and never occurred to exceed the therapeutic range. As the duration of the therapy was not predetermined, follow-up visits took place every three to four weeks to verify the baby’s tolerance to treatment.
When compared to current literature, our Pediatric Cardiologists opted for a low dose of 0.1 mg/day on daily administration to prevent drug serum level fluctuations. Sagiv et al. [Citation14] described a starting dose of 0.125 mg daily, which is in line with our therapeutic approach. Conversely, Öztunç et al. [Citation15] described a 4 weeks treatment with the dosage of 0.25 mg twice per day two days a week, while Wagner et al. [Citation16] opted for a dosage of 1.5–2 mg/m2 daily. Other examples of therapeutic approaches are 0.4 mg daily [Citation17] or 0.25 mg every 6 h, 2 days per week [Citation18]. However, most of these therapeutic schedules had to be modified or discontinued because of laboratory test alterations or serum drug concentrations exceeding the therapeutic range. As a matter of fact, further studies are needed to better understand and establish the correct therapeutic approach when Everolimus is administrated.
Moreover, in the case of TS, rhabdomyoma are quite often multiple and located along the intraventricular sept. However, the mass described in our article presented peculiar features: it was a single large tumor developing externally along the ventricular wall, making both the differential diagnosis and the management more challenging.
Even though larger studies are needed to finally establish the dosage, safety, and recommended durations of mTOR inhibitor therapies, our case report highlights the efficacy of what seems to be a promising treatment in pediatric patients with large rhabdomyoma.
Supplemental Material
Download MP4 Video (28.6 MB)Supplemental Material
Download MP4 Video (31.5 MB)Acknowledgements
The authors report no acknowledgements.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Isaacs H. Fetal and neonatal cardiac tumors. Pediatr Cardiol. 2004;25(3):252–273.
- Yuan SM. Fetal cardiac tumors: clinical features, management and prognosis. J Perinat Med. 2018;46(2):115–121.
- Morka A, Kohut J, Radzymińska-Chruściel B, et al. Echocardiography and newer imaging techniques in diagnosis and long-term follow-up of primary heart tumors in children. Int J Environ Res Public Health. 2020;17(15):5471.
- Isaacs H. Perinatal (fetal and neonatal) tuberous sclerosis: a review. Am J Perinatol. 2009;26(10):755–760.
- Chao AS, Chao A, Wang TH, et al. Outcome of antenatally diagnosed cardiac rhabdomyoma: case series and a meta-analysis. Ultrasound Obstet Gynecol. 2008;31(3):289–295.
- Shaddy RE, Penny DJ, Feltes TF, et al. Moss and adams’ heart disease in infant, children, and adolescent: including the fetus and young adult. 10th ed. Lippincott Williams & Wilkins, a Wolters Kluwer business; 2021.
- Ide T, Miyoshi T, Katsuragi S, et al. Prediction of postnatal arrhythmia in fetuses with cardiac rhabdomyoma. J Matern Fetal Neonatal Med. 2019;32(15):2463–2468.
- Abuhamad A, Chaoui R. A practical guide to fetal echocardiography: normal and abnormal hearts. 3rd ed. Lippincott Williams, Wolters Kluwer; 2016.
- Fesslova V, Villa L, Rizzuti T, et al. Natural history and long-term outcome of cardiac rhabdomyomas detected prenatally. Prenat Diagn. 2004;24(4):241–248.
- Northrup H, Aronow ME, Bebin EM, et al. Updated international tuberous sclerosis complex diagnostic criteria and surveillance and management recommendations. Pediatr Neurol. 2021;123:50–66.
- Garg A, Gorla SR, Kardon RE, et al. Involution of large cardiac rhabdomyomas with everolimus therapy. World J Pediatr Congenit Heart Surg. 2021;12(3):426–429.
- Lawley C, Popat H, Wong M, et al. A dramatic response to sirolimus therapy in a premature infant with massive cardiac rhabdomyoma. JACC Case Rep. 2019;1(3):327–331.
- Sugalska M, Tomik A, Jóźwiak S, et al. Treatment of cardiac rhabdomyomas with mTOR inhibitors in children with tuberous sclerosis complex – a systematic review. Int J Environ Res Public Health. 2021;18(9):4907.
- Sagiv E, Chikkabyrappa SM, Conwell J, et al. Use of everolimus to treat cardiac rhabdomyomas and incessant arrhythmias in a newborn: benefits and complications. Ann Pediatr Cardiol. 2022;15(1):58–60.
- Öztunç F, Atik SU, Güneş AO. Everolimus treatment of a newborn with rhabdomyoma causing severe arrhythmia. Cardiol Young. 2015;25(7):1411–1414.
- Wagner R, Riede FT, Seki H, et al. Oral everolimus for treatment of a giant left ventricular rhabdomyoma in a neonate-rapid tumor regression documented by real time 3D echocardiography. Echocardiography. 2015;32(12):1876–1879.
- Shibata Y, Maruyama H, Hayashi T, et al. Effect and complications of everolimus use for giant cardiac rhabdomyomas with neonatal tuberous sclerosis. AJP Rep. 2019;9(3):e213–7–e217.
- Demir HA, Ekici F, Yazal Erdem A, et al. Everolimus: a challenging drug in the treatment of multifocal inoperable cardiac rhabdomyoma. Pediatrics. 2012;130(1):e243-247–e247.