Abstract
Objective
Data on pregnancy-associated cancers (PACs) are lacking. The objectives of this study were to determine the incidence of PACs and describe the characteristics and outcomes of pregnancies affected by malignancy at a single tertiary care center in Ottawa, Canada.
Methods
This was a retrospective chart review of individuals with PAC at The Ottawa Hospital (TOH) between 2011-2022. Eligible cases were identified from the TOH Data Warehouse, the TOH instance of the Better Outcomes Registry & Network Ontario, and the TOH Division of Maternal Fetal Medicine’s Perinates database. Chart reviews were conducted to confirm case eligibility and to extract demographic, oncologic, obstetrical, and neonatal measures. The annual incidence of PAC over the 11-year period was reported per 1000 deliveries. Descriptive statistics were used to describe the sample, including frequency (n) and proportions (%) for categorical variables and mean and standard deviation (SD) for continuous variables.
Results
The final cohort included 59 individuals with PAC at TOH between 2011–2022. The annual incidence of PAC ranged from 0.47 to 1.54 per 1000 deliveries. The most common PACs were breast cancer (28.8%), Hodgkin lymphoma (10.2%), and thyroid cancer (8.5%). Common interventions during pregnancy included chemotherapy (33.9%) and surgical intervention (32.2%). A total of 19 individuals (32.2%) did not undergo PAC-related treatment during pregnancy. There were 55 livebirths (91.7%), 2 spontaneous abortions (3.3%), 3 induced abortions (5.0%), and no stillbirths. Among livebirths, the mean gestational age was 37.4 ± 2.8 weeks and the mean birthweight was 2920.3 ± 650.0 g. All neonates had reassuring 5-minute Apgar scores, 18 (32.7%) were admitted to the Neonatal Intensive Care Unit/Special Care Nursery (NICU/SCN), and 8 (14.5%) were noted to have a mild congenital abnormality.
Conclusion
This study shows promising perinatal outcomes for patients with PAC and their neonates. Ongoing surveillance of PAC is needed to better inform care for this patient population.
Introduction
Cancer complicates 0.02% to 0.1% of pregnancies annually [Citation1]. Pregnancy-associated cancers (PACs) are commonly defined as cancer diagnosed during pregnancy and up to 1-year post-partum. Over the past several decades, the incidence of PACs has increased from approximately 1 in 2000 pregnancies [Citation2] to 1 in 1000 pregnancies in Europe and the United States [Citation3–7]. Data from other jurisdictions suggest the same [Citation8–11]. Increases in the incidence of PACs may be attributable to more frequent screening, improved diagnostic methods, increased maternal age in high-income countries, and noted increases in the prevalence of cancers in general [Citation3–5,Citation9,Citation12,Citation13]. Moreover, PACs may be underreported due to miscarriages or induced abortions related to malignancy, for which data are not well-captured [Citation14].
Current treatment guidelines for PAC vary depending on the type and stage of the disease, and the gestational age at diagnosis. Among individuals with non-aggressive cancers diagnosed within the first trimester, guidelines suggest monitoring disease progression regularly and delaying treatment until the second trimester [Citation15]. In contrast, for patients with aggressive or advanced-stage cancer diagnosed during the first trimester, clinical guidelines and other studies advise no delays in treatment[Citation15–19]. Epidemiological analyses have yielded inconsistent findings regarding the safety of antenatal chemotherapy use for fetal and newborn outcomes. In a sample of 157 neonates exposed to chemotherapy in utero, the rates of congenital abnormalities (3.8%), growth restriction (7.7%), preterm delivery (5.8%), and mean gestational age at delivery (36.5 ± 3.3 weeks) were not significantly different compared to those reported for the general population [Citation20]. In contrast, other studies have found adverse outcomes such as lower birthweight [Citation20], small-for-gestational-age [Citation21–23], increased frequency of preterm delivery [Citation9,Citation12,Citation24], increased risk of NICU admission [Citation12,Citation23], stillbirth, intrauterine growth restriction [Citation12,Citation25], and transient myelosuppression [Citation20,Citation22,Citation26] in association with in-utero chemotherapy exposure.
There is also limited evidence regarding the effects of malignancy on perinatal and oncological outcomes. Among individuals with pregnancy-associated breast cancer (PABC), there is an increased risk of induction of labor (adjusted odds ratio [aOR] 2.25, 95%CI 1.9–2.7) but no significant increase in cesarean delivery (aOR 1.16, 95%CI 0.9–1.4)[Citation27]. PAC not limited to breast cancer has also been associated with increased neonatal mortality[Citation24], more frequent antenatal hospitalizations, and an increased risk of labor induction [Citation28], cesarean section, iatrogenic preterm birth, and large-for-gestational-age infants [Citation9]. Moreover, individuals with cancer diagnosed during pregnancy are more likely to experience cancer-related complications such as thromboembolic events, sepsis and severe morbidity [Citation9].
Contemporary Canadian data on PAC are generally lacking and there remains a paucity of data on the incidence of specific PACs. Data from a recent population-based analysis suggest the incidence of PAC to be 156.2 per 100,000 deliveries in Alberta, and 149.4 per 100,000 deliveries in Ontario, comprising the highest reported incidence rates of PAC[29]. Despite scant and inconsistent data on the maternal-fetal impacts of anti-cancer drug therapy in pregnancy, antenatal chemotherapy use has increased over the past 20 years[Citation23]. Evaluation of PAC epidemiology and outcomes in the Canadian healthcare context is needed to better inform clinical decision-making. Moreover, characterization of the breadth of oncological treatment regimens is necessary to guide PAC management options. The objectives of this study were to explore the temporal trends in the incidence of malignancy in pregnancy and describe the oncological, obstetrical, maternal, and neonatal characteristics and outcomes of pregnancies affected by malignancy at a tertiary care facility in Ontario, Canada from 2011 to 2022.
Materials and methods
Study design and setting
This study was a retrospective chart review conducted at The Ottawa Hospital (TOH) in Ottawa, Ontario, Canada. The Ottawa Hospital is a Level 3 maternity care hospital with approximately 6000 deliveries per year (6,319 in 2021–2022). The Ottawa Hospital Cancer Program is a comprehensive cancer center serving a population of 1.5 million individuals across eastern Ontario with over 25,000 visits per year.
Sample
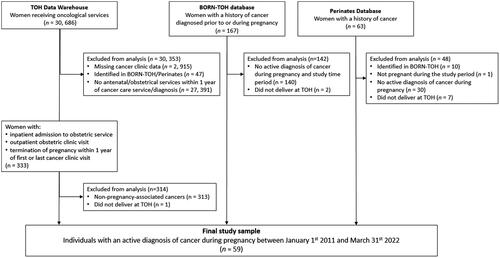
We included all individuals with an active diagnosis of cancer during pregnancy who received care and delivered their live or stillborn infants at TOH between 1 Jan 2011 and 31 March 2022. Eligible cases included pregnant individuals undergoing treatment or active monitoring of any type of malignancy during the index pregnancy and thus included individuals with diagnoses that were made either (1) prior to pregnancy; or (2) between the time of their last menstrual period and delivery. No other exclusion criteria were applied.
Data sources
Identification of cases was completed through TOH Data Warehouse, a large data repository of TOH’s information systems from 2006 onward [Citation30]. Using TOH Data Warehouse, the records of all female individuals receiving oncological services between 1 January 2011 and 31 March 2022 were identified. From this sample, records were selected for further screening if they: (1) received any antenatal/obstetrical or gynaecological services within 12 months of their oncological service or (2) had an estimated date of delivery within 12 months of their oncological service.
Cases were also identified by searching the Better Outcomes Registry & Network (BORN) Ontario and the TOH Perinates Database. BORN Ontario is a registry that collects, interprets, and shares critical data about pregnancy, birth and childhood in the province of Ontario [Citation31]. Institutions submitting data to BORN Ontario have access to institution-specific data within the BORN Information System. Thus, BORN Ontario was used to identify all individuals who delivered at TOH and who were recorded as having any of the following: “cancer diagnosed prior to pregnancy”; “cancer diagnosed during pregnancy”; “chemotherapeutic agents in pregnancy”; or “radiation therapy in pregnancy”. The Perinates database is maintained by the Division of Maternal-Fetal Medicine at TOH and captures information on all patients receiving care through the TOH Maternal Fetal Medicine Clinic.
The charts of potentially eligible cases were then manually screened to confirm case eligibility. Chart reviews were conducted by M.S. using the EPIC system, a proprietary electronic medical record application used at TOH.
Data extraction
Maternal and oncologic characteristics were extracted, as were obstetrical, maternal and neonatal outcomes. Maternal characteristics included age, preexisting comorbid conditions, medications, socioeconomic characteristics, parity, pregnancy complications, number of fetuses, etc. Oncological measures included the type and stage of malignancy and the timing of the diagnosis. Treatment details including the type and timing of administration were captured. Maternal outcome measures included maternal death during pregnancy up until discharge from the delivery hospitalization, antenatal hospitalization, and maternal morbidity. Obstetrical outcome measures included pregnancy outcome, mode of delivery, rates and indications for cesarean section or inductions of labour.
Neonatal outcomes included birth outcome, gestational age at delivery/loss/termination, birth weight, congenital abnormalities, complications at delivery, admissions to the NICU/SCN, length of stay in the hospital, and neonatal death during the birth hospitalization.
Statistical analyses
Descriptive analyses were conducted to report the demographic characteristics of patients, characterize the frequency of specific malignancies, timing of diagnosis, types and timing of treatment regimens, and experienced outcomes. Categorical variables were described as frequencies (n) and percentages (%). Continuous variables were described with means and standard deviations.
Results
Demographic and obstetrical characteristics
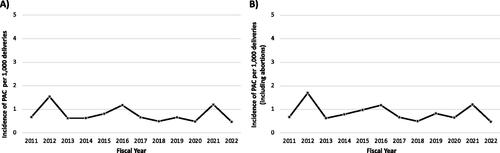
A total of 59 individuals with PAC delivered at TOH between 1 January 2011 and 31 March 2022 (). An additional 10 individuals were diagnosed with PAC and received care at TOH within the study time period, but delivered outside of TOH and were thus excluded from the final sample. The annual incidence rate of PAC ranged from 0.47 to 1.54 per 1000 deliveries between 1 January 2011 and 31 March 2022 ().
Figure 2. Incidence of PAC per 1000 deliveries between 1 January 2011 – 31 March 2022; (A) livebirths and stillbirths only, (B) including abortions.
Sample characteristics are summarized in . The mean maternal age was 34.5 ± 5.9 years at the time of livebirth, stillbirth or spontaneous or induced abortion. The majority of cases were nulliparous (47.5%) and were carrying singletons (98.3%). The most prevalent preexisting maternal comorbidities were depression and/or anxiety (25.4%), asthma (8.5%), and hypothyroidism (6.8%). Medication use in pregnancy (excluding oncological medications) included: prenatal vitamins/folic acid (76.3%), iron supplements (15.2%), salbutamol (8.5%), levothyroxine (8.5%), and opioids (5.1%). 44 individuals (74.6%) were referred to an MFM specialist, with an average gestational age of 21.8 ± 7.9 weeks at the first MFM specialist appointment. There were no instances of maternal death during the perinatal period.
Table 1. Demographic and obstetrical characteristics.
Oncological characteristics and interventions
Oncological characteristics and interventions are described in . A total of 45 individuals (76.3%) were diagnosed with a malignancy during pregnancy, at a mean maternal age of 33.4 ± 5.8 years, and a mean gestational age of 19.7 ± 9.2 weeks. The remaining 14 individuals (23.7%) received diagnoses prior to pregnancy, and the malignancy remained active during the gestational period. For these individuals, the average time from cancer diagnosis to the beginning of their pregnancy was 2.8 ± 3.4 years. A total of 6 individuals (10.2%) had a previous history of cancer, prior to the index PAC. Of those, the PAC represented recurrent disease in 3 individuals. The most common types of PAC were breast cancer (28.8%), Hodgkin lymphoma (10.2%), and thyroid cancer (8.5%).
Table 2. Oncological characteristics and interventions.
Oncological interventions in our sample included chemotherapy, radiotherapy, surgical interventions and radioactive iodine ablation, among others. The most common type of oncological treatment received during pregnancy was chemotherapy (33.9%). The most common chemotherapy regimens during pregnancy were: 1) cyclophosphamide, doxorubicin and paclitaxel (n = 6), and 2) fluorouracil, epirubicin hydrochloride, cyclophosphamide and docetaxel (n = 3) among individuals with breast cancer, and 3) ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) (n = 4) among individuals with Hodgkin lymphoma. Surgical interventions were also common, occurring in 32.2% of the study sample, at a mean gestational age of 21.6 ± 6.8 weeks. Other treatments received during pregnancy included oral targeted therapy (e.g. dasatinib, and gleevec for Philadelphia chromosome-positive leukemia), interferon therapy (e.g. alpha-2B/2A), steroids (e.g. dexamethasone), and naturopathic substances. One individual with breast cancer was treated with tamoxifen and trastuzumab before being aware of their pregnancy. One-third (32.2%) of PAC cases received no treatment during pregnancy.
A total of 27 (45.8%) cases underwent radiotherapy. Among these individuals, one (3.7%) underwent radiotherapy (peptide receptor radionuclide therapy) during pregnancy, and the remaining 26 (96.3%) completed radiotherapy treatment either before or after pregnancy. All radioactive iodine ablation for thyroid cancer was completed post-pregnancy. Other treatments received exclusively outside of pregnancy (with the exception of the individual who received tamoxifen and trastuzumab before knowledge of their pregnancy) included: biological therapy (e.g. pertuzumab, trastuzumab), bisphosphonates (e.g. pamidronate, zoledronic acid), endocrine therapy (e.g. tamoxifen, anastrazole, letrozole, goserelin), oral targeted therapy (e.g. abemaciclib, alectinib, crizotinib, lenalidomide, lorlatinib, palbociclib), and stem cell transplant.
Obstetrical, maternal, and neonatal outcomes
Obstetrical, maternal and neonatal outcomes are summarized in . The study sample included 55 livebirths (91.7%), 2 spontaneous abortions (3.3%), 3 induced abortions (5.0%), and no stillbirths.
Table 3. Obstetrical, maternal, and neonatal outcomes.
Among the induced abortions, 2 were unplanned pregnancies where cancer therapy (teratogenic agents such as trastuzumab and tamoxifen) had already been initiated prior to the patients becoming aware of the pregnancy. The third individual required radiation therapy during pregnancy and the risks to the fetus were unclear. The mean gestational age at delivery was 37.4 ± 2.8 weeks, and the rate of preterm deliveries (<37 weeks gestation) was 29.1%. The majority of individuals (55.9%) delivered vaginally, and without induction of labor (59.3%).
A total of 24 individuals (40.7%) required antenatal hospitalization; of which 69.0% were malignancy-related (e.g. surgical interventions, or complications such as biliary colic, bowel obstruction, pericarditis, etc.). The most common pregnancy complications were abnormal fetal heart rate or fetal surveillance (13.6%), preterm premature rupture of membranes (PPROM) and/or preterm labor (8.5%), and polyhydramnios (5.1%). The most common post-partum maternal complications were post-partum hemorrhage (5.1%), sepsis (3.4%), and endometritis (3.4%). The mean maternal length of stay in the hospital for the delivery admission was 5.0 ± 8.9 days. During the delivery admission, 29 (49.2%) individuals were referred to a lactation consultant.
Neonates had a mean birthweight of 2920.3 ± 650.0 g, and all had reassuring Apgar scores (≥7). Congenital anomalies were documented for 8 neonates (14.5%), the most common of which were musculoskeletal abnormalities (9.1%). The most common neonatal complications were respiratory difficulties (12.7%) and hyperbilirubinemia (7.3%). A total of 18 neonates (32.7%) were admitted to the NICU/SCN. Neonates remained in the hospital for a mean 5.6 ± 12.2 days, and 63.6% received breast milk during the delivery admission. There were no instances of neonatal death during the birth hospitalization.
Discussion
In this single-center retrospective chart review study, we identified 59 cases of PAC over an 11-year time frame. The incidence of PAC at TOH ranged from 0.47 to 1.54 per 1000 deliveries between 1 January 2011 and 31 March 2022. The most common PACs were breast cancer, Hodgkin lymphoma, and thyroid cancer, and the most common interventions during pregnancy were chemotherapy and surgical procedures. The sample included 55 live births, 2 spontaneous abortions and 3 induced abortions. Most livebirths were delivered at term, had appropriate birthweights for gestational age and reassuring Apgar scores.
To date, only one other study has evaluated the incidence of PAC in Canada. A population-based study in two Canadian provinces reported a crude incidence rate of 146.5 PACs per 100,000 deliveries (including livebirths and stillbirths) in Ontario and 153.1 per 100,000 deliveries in Alberta[Citation29]. When abortions were accounted for, the incidence rate increased to 149.4 and 156.2 per 100,000 deliveries in Ontario and Alberta respectively. This is comparable to the PAC incidence rate at our center (0.47 to 1.7 PACs per 1000 deliveries annually, including spontaneous and induced abortions). The frequency of different types of malignancies in this sample is also similar to previous reports. In Canada, breast cancer is the most commonly diagnosed malignancy during pregnancy, followed by thyroid cancer and melanoma [Citation29]. Similar findings are noted in population-based studies in the United States and Australia [Citation9,Citation10].
In this study, the majority of individuals underwent cancer treatment during their pregnancy, including chemotherapy and surgical interventions. The average timing of surgical interventions was during the second trimester. These findings are in line with current guideline recommendations. Although one-third of the sample did not undergo treatment during pregnancy, the reasons underlying the decision to defer treatment are unclear. Our observations are in keeping with those from an international registry of 1170 patients with PAC, in which 67% of patients received treatment during pregnancy; here, 37% of patients received chemotherapy, and 39% underwent surgery during pregnancy. In this same sample, the most common adverse obstetric outcome was PPROM or preterm contractions, which affected 10% of singleton deliveries [Citation23]. Reported rates of preterm deliveries and NICU admission for pregnancies affected by PAC vary widely, ranging from 24.0–61.2% for preterm deliveries [Citation9,Citation32] and 41-51.2% [Citation12,Citation23] for NICU admission. Corresponding rates at our center were comparatively low, but the preterm birth rate was similar to those reported for individuals with PAC in Ontario [Citation29]. Finally, the rate of congenital abnormalities in our sample was higher than reported elsewhere (14.5%) [Citation12,Citation20,Citation23,Citation32], but may be attributable to the inclusion of unconfirmed and non-severe abnormalities in our calculations.
The limitations of this study include its single-center design, small sample size and challenges inherent to the datasets available for analysis. First, the data for this study are derived from a single tertiary care facility in Ottawa, Canada, and accordingly, our findings may not be generalizable to other obstetrical populations in Ontario, Canada or elsewhere. Second, the sample size prevented us from conducting more in-depth analyses to explore the association between malignancy-related factors (e.g. gestational age at diagnoses, type of malignancy, interventions received) with maternal and neonatal outcomes. Finally, as data on spontaneous or induced abortions are not always well captured, our incidence calculations are limited to the incidence of PAC among individuals who had livebirths or stillbirths.
Findings from this retrospective chart review suggest encouraging outcomes for pregnancies affected by malignancy. With limited Canadian data on the topic, our study provides valuable insight into PAC epidemiology and outcomes and highlights the willingness of individuals with PAC to undergo conventional oncological treatment during pregnancy. This has promising implications for the management of malignancy during pregnancy, although further research and longer-term follow-up are required to ascertain sociodemographic, treatment- and malignancy-related factors associated with adverse maternal, obstetrical and neonatal outcomes in order to optimize management. Regular surveillance of the incidence and outcomes of PAC is needed to appropriately manage malignancy in pregnancy and ensure the best outcomes for this unique population.
Ethics statement
This study was reviewed and approved by the Ottawa Health Science Network Research Ethics Board. As per the TCPS 2 Article 3.7 A, a waiver of consent was obtained due to the minimal risk to study participants and the impracticability of obtaining the consent of participants due to the retrospective nature of the study.
Author contributions
DEC and MSQM conceptualized and designed the study. MS collected and analyzed the data. MS drafted and edited the manuscript. DEC, MSQM, and SM critically reviewed and revised the manuscript. DEC has primary responsibility for the final content. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Ruth Rennicks White, RN, BScN, and staff from The Ottawa Hospital Data Warehouse for their support with the identification of eligible records for chart review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Pereg D, Koren G, Lishner M. Cancer in pregnancy: gaps, challenges and solutions. Cancer Treat Rev. 2008;34(4):302–312.
- Williams TJ, Turnbull KE. Carcinoma in situ and pregnancy. Obstet. Gynecol. 1964;24(6):857–864.
- Smith LH, Danielsen B, Allen ME, et al. Cancer associated with obstetric delivery: results of linkage with the California cancer registry. Am J Obstet Gynecol. 2003;189(4):1128–1135.
- Pavlidis NA. Coexistence of pregnancy and malignancy. Oncologist. 2002;7(4):279–287.
- Pentheroudakis G, Pavlidis N. Cancer and pregnancy: poena magna, not anymore. Eur J Cancer. 2006;42(2):126–140.
- Parazzini F, Franchi M, Tavani A, et al. Frequency of pregnancy related cancer: a population based linkage study in lombardy, Italy. Int J Gynecol Cancer. 2017;27(3):613–619.
- Dalmartello M, Negri E, La Vecchia C, et al. Frequency of pregnancy-associated cancer: a systematic review of population-based studies. Cancers. 2020;12(6):1356.
- Eibye S, Kjær SK, Mellemkjær L. Incidence of pregnancy-associated cancer in Denmark, 1977-2006. Obstet Gynecol. 2013;122(3):608–617.
- Lee YY, Roberts CL, Dobbins T, et al. Incidence and outcomes of pregnancy-associated cancer in Australia, 1994–2008: a population-based linkage study. BJOG. 2012;119(13):1572–1582.
- Cottreau CM, Dashevsky I, Andrade SE, et al. Pregnancy-associated cancer: a U.S. Population-based study. J Womens Health. 2019;28(2):250–257.
- Andersson TML, Johansson ALV, Fredriksson I, et al. Cancer during pregnancy and the postpartum period: a population-based study. Cancer. 2015;121(12):2072–2077.
- Van Calsteren K, Heyns L, De Smet F, et al. Cancer during pregnancy: an analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J Clin Oncol. 2010;28(4):683–689.
- Andersson TML, Johansson ALV, Hsieh CC, et al. Increasing incidence of pregnancy-associated breast cancer in Sweden. Obstet Gynecol. 2009;114(3):568–572.
- Maggen C, Wolters VERA, Cardonick E, et al. Pregnancy and cancer: the INCIP project. Curr Oncol Rep. 2020;22(2):17.
- Peccatori FA, Azim JA, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi160–vi170.
- Guidroz JA, Scott-Conner CEH, Weigel RJ. Management of pregnant women with breast cancer. J Surg Oncol. 2011;103(4):337–340.
- PDQ Adult Treatment Editorial Board. Breast Cancer treatment during pregnancy (PDQ®): Health professional version. 2019. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002–2019.
- Wolters V, Heimovaara J, Maggen C, et al. Management of pregnancy in women with cancer. Int J Gynecol Cancer. 2021;31(3):314–322.
- Botha MH, Rajaram S, Karunaratne K. Cancer in pregnancy. Int J Gynecol Obstet. 2018;143:137–142.
- Cardonick E, Usmani A, Ghaffar S. Perinatal outcomes of a pregnancy complicated by cancer, including neonatal follow-up after in utero exposure to chemotherapy: results of an international registry. Am J Clin Oncol. 2010;33(3):221–228.
- Loibl S, Han SN, von Minckwitz G, et al. Treatment of breast cancer during pregnancy: an observational study. Lancet Oncol. 2012;13(9):887–896.
- Zemlickis D, Lishner M, Degendorfer P, et al. Fetal Outcome after in Utero Exposure to Cancer Chemotherapy. Arch Intern Med. 1992;152(3):573–576.
- de Haan J, Verheecke M, Van Calsteren K, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19(3):337–346.
- Lu D, Ludvigsson JF, Smedby KE, et al. Maternal cancer during pregnancy and risks of stillbirth and infant mortality. J Clin Oncol. 2017;35(14):1522–1529.
- Vandenbroucke T, Verheecke M, Van Calsteren K, et al. Fetal outcome after prenatal exposure to chemotherapy and mechanisms of teratogenicity compared to alcohol and smoking. Expert Opin Drug Saf. 2014;13(12):1653–1665.
- Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5(5):283–291.
- Abenhaim HA, Azoulay L, Holcroft CA, et al. Incidence, risk factors, and obstetrical outcomes of women with breast cancer in pregnancy. Breast J. 2012;18(6):564–568.
- Gomez-Hidalgo NR, Mendizabal E, Joigneau L, et al. Breast cancer during pregnancy: results of maternal and perinatal outcomes in a single institution and systematic review of the literature. J Obstet Gynaecol. 2019;39(1):27–35.
- Metcalfe A, Cairncross ZF, Friedenreich CM, et al. Incidence of pregnancy-associated cancer in two Canadian provinces: a population-based study. Int J Environ Res Public Health. 2021;18:1–7.
- Oake N, Taljaard M, Van Walraven C, et al. The effect of hospital-acquired clostridium difficile infection on in-hospital mortality. Arch Intern Med. 2010;170(20):1804–1810.
- Murphy MSQ, Fell DB, Sprague AE, et al. Data resource profile: better outcomes registry & network (BORN) Ontario. Int J Epidemiol. 2021;50(5):1416–1417H.
- Amant F, Vandenbroucke T, Verheecke M, et al. Pediatric outcome after maternal cancer diagnosed during pregnancy. N Engl J Med. 2015;373(19):1824–1834.