Abstract
Objective
To investigate the prenatal diagnostic value of chromosome microarray analysis (CMA) in fetuses with isolated or non-isolated umbilical cord cysts (UCCs) of various locations and numbers.
Methods
Between November 2015 and November 2021, 45 pregnant women carrying fetuses with UCCs underwent amniocentesis and CMA. Fetal prognoses were followed from 6 months to 5 years.
Results
Five cases (11.1%, 5/45) of chromosomal aberrations were detected. No significant difference in total chromosome abnormalities was found between fetuses with isolated and non-isolated UCCs (13.3% [2/15] vs 10% [3/30]; p > .999). No common autosomal aneuploidies were found in fetuses with isolated UCCs. At follow-up, among 45 fetuses, there were 11 (24.4%) pregnancy terminations, 26 (57.8%) live healthy births, 4 (8.9%) postnatal UCC-related surgeries, and 4 (8.9%) live births of fetuses with other diseases. The frequency of postnatal surgeries of the infants with UCCs located adjacent to the anterior abdominal wall was higher than those located adjacent to the fetal surface of the placenta (30.8% [4/13] vs 0% [0/22]; p = .014). All 26 live healthy neonates and 4 neonates that underwent postnatal surgery had an overall good prognosis.
Conclusions
For fetuses with isolated or non-isolated UCC, CMA could be a choice for parents after providing detailed information. Even when surgery was required, pregnancy outcomes and short- and long-term prognoses for fetuses with UCCs were favorable.
Introduction
The widespread use of high-resolution prenatal ultrasonography enables an accurate assessment of a fetus, including the umbilical cord and the placenta. The embryonic vitellointestinal duct (VID), which connects the midgut to the yolk sac, narrows in the 5th gestational week [Citation1]. Mucus accretion in one part of the VID results in umbilical cord cyst (UCC) formation, which can be identified using ultrasonography at various gestational stages. Approximately 0.4%–3.4% of UCCs are identified using ultrasonography during the first trimester [Citation2]. Antenatal ultrasound scans can also identify UCCs in the second or third trimester; however, their prevalence during gestation is unknown [Citation3–6].
UCCs are classified into true or pseudocysts according to whether or not the cyst has an epithelial lining. The true UCCs are covered with an epithelial lining and are derived from either the embryonic allantois or omphalomesenteric duct [Citation7]. The allantois may be associated with omphalocele, persistent urachus, or obstructive uropathy [Citation8–10]. Cysts in the omphalomesenteric duct may be related to an abdominal wall defect or Meckel’s diverticulum. More commonly, the pseudocysts, rather than the true cysts, have no epithelial covering, represent localized edema of the Wharton’s jelly, and are primarily caused by focal degeneration within the Wharton’s jelly [Citation7]. However, it is rarely difficult to accurately differentiate between true cysts and pseudocysts by antenatal ultrasound as their appearance is similar.
Some studies have examined the relationship between fetuses with UCCs and chromosomal abnormalities in terms of prenatal karyotype and have reported the probability of increased chromosomal abnormalities in fetuses with UCCs. However, different prevalence rates for fetal chromosomal abnormalities have been reported to range from 10%–85% in studies with limited sample sizes [Citation3–5]. One study found an increased association between the presence of non-isolated UCCs in the second and third trimesters and chromosomal abnormalities in fetuses examined using ultrasonography compared with fetuses with an isolated UCC [Citation3]. A multicenter cohort study reported an increased risk of fetal growth problems in fetuses identified as having multiple UCCs compared with fetuses with a single UCC, and no association was found between prognosis and gestation week at diagnosis or abnormal karyotype [Citation11]. Though common autosomal aneuploidies like trisomy 18 have been reported in fetuses with UCCs [Citation3], it remains unclear whether there was an association between pathogenic copy number variations (CNVs) and UCCs. Additionally, most studies have not reported pregnancy outcomes or long-term postnatal follow-ups. Given the contrasting conclusions of the existing literature and study limitations in terms of small sample sizes and the development of molecular karyotypes, we performed a retrospective study aiming to evaluate the clinical value of CMA in the prenatal diagnosis of fetuses with isolated or non-isolated UCCs of various locations and numbers and to follow up on pregnancy outcomes.
Methods
Clinical setting
All of the pregnancies involved in this study were registered at the West China Second University Hospital. Our study comprised 45 fetuses with UCCs that had been diagnosed using ultrasonography between November 2015 and November 2021. After professional genetic counseling that included the potential risk of amniocentesis, detection ranges, alternative detection techniques, limitations, and the possible significance of the findings, all pregnant women underwent a detailed ultrasound evaluation at the time of diagnosis. The study was approved by the Ethical Medical Committee of the Second Western China University Hospital, Sichuan University. It was performed in accordance with the principles stated in the Declaration of Helsinki. All of the pregnant women signed informed consent forms for amniocentesis and detection procedures. Parents’ peripheral blood was collected for CMA to identify the source of CNVs if needed.
CMA analysis and prenatal whole exome sequencing (WES)
Fetal DNA obtained from amniotic fluid and peripheral blood DNA obtained from parents were extracted using QIAamp DNA Blood Mini kits (Qiagen, Valencia, CA, USA) and hybridized with Affymetrix® CytoscanTM 750K array (Affymetrix, Santa Clara, CA, USA), which contained single nucleotide polymorphism probes, in accordance with the manufacturer’s instructions. Details concerning this procedure have been presented in a previous study [Citation12]. Data analysis was undertaken according to American College of Medical Genetics and Genomics (ACMG) guidelines [Citation13]. Prenatal fetal WES was conducted using an Illumina NovaSeq6000 platform. Details concerning this procedure have been provided in a previous study [Citation14]. The Burrows-Wheeler Aligner software tool was used to align the sequencing reads with hg38/GRCh38. A GATK Unified Genotyper (broadinstitute.org/) was then used to detect single nucleotide variants and small insertions or deletions. Trio-exome gene sequencing was performed using bioinformatic analysis. Genetic variant assessment was conducted in accordance with ACMG guidelines [Citation15] for fetal phenotypes based on prenatal ultrasonographic findings.
Fluorescence in situ hybridization (FISH) analysis and quantitative fluorescence polymerase chain reaction (QF-PCR)
QF-PCR was used to identify maternal cell contamination and rapidly detect abnormal numbers of chromosomes 13/18/21 and sex chromosomes for double verification with CMA. When the CMA results suggested CNVs ≥5 Mb, karyotype analysis was used for further validation. When mosaicism was detected using CMA, FISH analysis was conducted for further confirmation.
Stratification analysis
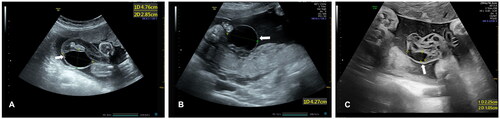
All fetuses with UCCs were categorized into two groups. We first divided all fetuses into isolated and non-isolated groups. The non-isolated groups represented fetuses with UCCs complicated with ultrasonographic abnormalities including soft markers, fetal growth restriction, polyhydramnios/oligohydramnios, or other structural malformations. We then divided all fetuses in terms of single and multiple UCCs. Multiple UCCs were defined as the presence of at least two UCCs simultaneously. Regarding UCC location, all fetuses were divided into those with UCCs on the placental side, those with UCCs on the fetal side, and those with UCCs located in other positions including free-loop, segmental, or whole umbilical cord locations. The UCCs on the placental side were defined as UCCs located adjacent to the fetal surface of the placenta, while those on the fetal side were defined as the UCCs located adjacent to the anterior abdominal wall of the fetus (). Magnetic resonance imaging was performed if necessary.
Clinical follow-up assessments
Clinical follow-up assessments were conducted by telephone, with postnatal medical records used to inquire about pregnancy outcomes. If the fetus was born alive, the parents were asked for information concerning gestational age, fetal weight at birth, type of delivery, childcare after birth for growth and development, and other diseases if appropriate. If a newborn underwent surgery postnatally, the reasons for surgery and the prognosis were investigated.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA) software. Chi-square or Fisher’s exact tests were used to compare differences between the groups. Statistical significance was set at a p-value <.05.
Results
Overall detection conditions of CMA in fetuses with UCC-related abnormalities
In total, 41 singleton pregnancies and two twin pregnancies with fetuses identified as having UCCs underwent amniocentesis and were tested successfully without maternal cell contamination in our center. The average age of the pregnant women was 28.96 (range, 23–40) years, the average gestational age at the time of amniocentesis was 25+3 (range, 19+0–33+3) weeks, and the average gestational age when UCCs were first identified was 22+3 (range, 12+6–33+2) weeks. Five fetuses were diagnosed in the first trimester, and the remaining 40 fetuses were diagnosed in the second or third trimester. The CMA results of fetuses with UCCs are shown in .
Figure 2. Flowchart of CMA in fetuses with umbilical cord cysts. CMA: chromosome microarray, P/LP CNVs: pathogenic or likely pathogenic copy number variants, TOP: termination of pregnancy. a Others: represent diseases not related to UCC, including slight deafness after birth, right auricle deformity, ear canal stenosis, and ventricular septal defect, but no treatment is required currently.
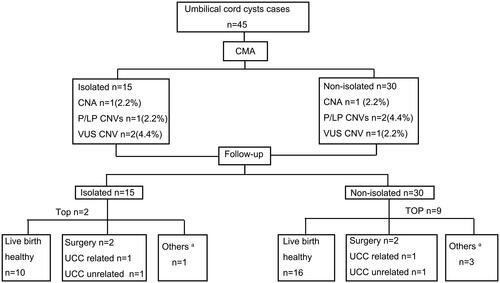
The overall detection rate of chromosome abnormalities was 11.1% (5/45). Two (2/45) fetuses had chromosomal aneuploidies, including one case of trisomy 18 and another case of 47, XXX. Of the two other cases that carried pathogenic CNVs (pCNVs), one fetus had monosomy of the short arm of chromosome 9 and another fetus had a pCNV overlapping with 17p12 recurrent region (including Charcot-Marie-Tooth type 1 A [CMT1A]). Additionally, one case harbored X chromosome short arm terminal duplication (likely pCNV) and chromosome 4 short arm terminal microdeletion (VUS), which could indicate unbalanced translocation. Three (6.7%, 3/45) fetuses had CNVs of unknown significance ().
Table 1. Chromosome abnormalities of fetuses with UCCs.
Two non-isolated cases underwent prenatal trio-WES. Of these, one fetus with a UCC, systemic edema, and abnormal posture of both lower limbs showed negative CMA results but likely had pathogenic parental compound heterozygous variants of the TTN gene (OMIM:188840) (c.32240del, paternal; c.38876-2A > C, maternal), which were detected using WES. Another fetus (case 5, ) with a UCC, mild left lateral ventriculomegaly, and fetal growth restriction was found to have no pathogenic mutation except for CNVs, which was confirmed on CMA.
Detection results of CMA in fetuses with isolated and non-isolated UCCs
The total chromosomal abnormality detection rate in the isolated UCC group was 13.3% (2/15), comprising one case of 47, XXX, and one case of pCNV harboring CMT1A. The total chromosomal abnormality detection rate in the non-isolated UCC group was 10.0% (3/30), comprising one case of trisomy 18, one case of monosomy of the short arm of chromosome 9, and one case of short arm terminal repeat of the X chromosome. No significant difference was observed in terms of total chromosomal aberration frequencies between the isolated and non-isolated groups (13.3% vs 10.0%, respectively; p > .9999) (). Furthermore, the total VUS CNV detection rate did not significantly differ between the isolated and non-isolated UCC groups (13.3% [2/15] vs 3.3% [1/30], respectively; p = .2541) ().
Table 2. The comparison of detection rates of chromosomal anomalies and pregnancy outcomes in fetuses with isolated and nonisolated UCCs.
Detection results of CMA in fetuses with single and multiple UCCs
In the fetuses in the single UCC group, we identified one case of trisomy 18, three cases of P/LP CNVs, and three cases of VUS CNVs. In the fetuses with multiple UCCs, we identified one case of 47, XXX.
Among the fetuses with a single isolated UCC, we identified one case of pCNV (CMT1A) and two cases of VUS CNVs. Among the fetuses with a single non-isolated UCC, we identified one case of trisomy 18, two cases of P/LP CNVs, and one case of VUS (). Among the fetuses with multiple isolated UCCs, we identified one case of 47, XXX. No chromosomal abnormalities were identified in the fetuses with multiple non-isolated UCCs, and no significant difference was found in terms of total chromosomal abnormalities between the single and multiple UCC groups (). Additionally, no significant difference was found in terms of total chromosomal abnormalities between the single isolated and multiple isolated UCC groups, nor between the single non-isolated and multiple non-isolated UCC groups.
Table 3. The comparison of detection rates of chromosomal anomalies and pregnancy outcomes in fetuses with single and multiple UCCs.
Detection results of CMA in fetuses with different UCC locations
None of the fetuses with P/LP CNV had UCCs near the placental side. In the fetuses with UCCs near the fetal side, we found two fetuses with P/LP CNVs (). Among the fetuses with the other positions, three fetuses with chromosomal aberrations (one trisomy 18, one 47, XXX, and one LP CNV) were found (). Total chromosomal aberration in the UCCs in locations other than on the placental or fetal side was found to be higher than that in the UCCs located on the placental side (30%, 3/10 vs 0%, 0/22, p = .024) (). However, no significant difference in terms of total chromosomal aberration was found in the other two groups (placental side vs fetal side; fetal side vs other sides).
Table 4. The comparison of detection rates of chromosomal anomalies and pregnancy outcomes among fetuses with different positions of UCC.
Follow-up results
We followed up on the pregnancy outcomes of all 45 fetuses with UCCs (follow-up time, from 6 months to 5 years; median follow-up time, 2 years 1 month).
shows the pregnancy outcomes after prenatal diagnosis of the fetuses with UCCs. None of the pregnant women were lost to follow-up. Eleven pregnant women opted to terminate their pregnancies partly due to the adverse CMA results or other malformations including omphalocele, fetal heart disease, and multiple deformities. Among the pregnant women who chose to continue their pregnancies, 26 babies were healthy at birth and four underwent surgery immediately after birth due to omphalocele, umbilical cord hemangioma with infection, or a patent urachus presenting as an allantoic cyst. Another four babies lived with other abnormalities including mild deafness, a unilateral auricle deformity, a narrow ear canal, and a ventricular septal defect. The postoperative prognosis for all babies was favorable.
Between the isolated and non-isolated UCC groups, no significant differences were found in relation to terminations of pregnancy, live healthy births, postnatal surgeries, and other diseases (p > .05, ). Data of the 16 fetuses who were healthy after birth in the non-isolated UCCs group are shown in Table S1.
Between the fetuses with single and multiple UCCs, no significant differences were found in terms of the number of pregnancies terminated, live healthy births, postnatal surgeries, and other diseases, which included total single and total multiple UCC groups, single isolated and single non-isolated UCC groups, and multiple isolated and multiple non-isolated UCC groups (p > .05; ).
Four babies with fetal side UCCs underwent surgeries after birth. Two were due to UCC-related disorders (umbilical cord cyst hemangioma with infection and a patent urachus presenting with an allantoic cyst), while the others were for non-UCC-related disorders (omphalocele). The frequency of surgeries after birth in the fetal side group (30.8%, 4/13) was higher than that in the placental side group (0%, 0/22), and a significant difference was found (p = .0137; ). The rate of live healthy births for fetuses identified as having UCCs on the placental side (16/22, 72.7%) was higher than that on the fetal side (5/13, 38.5%), although this difference was not significant (p = .075).
Discussion
In this study, 45 fetuses were diagnosed with UCCs using CMA prenatally. The overall detection rate of chromosomal abnormalities in fetuses with UCCs diagnosed using CMA was 11.1%. The total CMA detection rate for UCCs in locations other than the placental and fetal side was significantly higher than that of UCCs located on the placental side. Pregnancy outcomes and prognoses were better for fetuses with UCCs located close to the placental side than for those with UCCs located elsewhere.
Fetuses with UCCs have been suggested to be associated with chromosomal aneuploidy based on prenatal karyotype in previous studies [Citation11,Citation16,Citation17]. However, no chromosomal anomalies have been reported in fetuses with isolated UCCs [Citation4,Citation11,Citation18]. In our study, with the exception of one case of 47, XXX, common autosomal aneuploidies were not found in fetuses with isolated UCCs. Based on limited data, the risk of autosomal aneuploidy appears not to increase in fetuses with isolated UCCs. For fetuses with non-isolated UCCs, various chromosomal aneuploidies including trisomy 18 were consistently found in both the present and previous studies [Citation4,Citation11,Citation16–18]. As a result, prenatal diagnosis is recommended for fetuses with non-isolated UCCs for common autosomal aneuploidies.
Our finding of no significant difference in total chromosome abnormalities between fetuses with isolated and non-isolated UCCs was inconsistent with the previous study [Citation18] which showed a significantly higher rate of chromosomal anomalies in fetuses with non-isolated UCC than in isolated UCC. After analyzing the specific circumstances of the cases included in the two studies, we found more concomitant soft markers and minor structural deformities were included in the non-isolated group in our study. On the other hand, the limited sample size may also have contributed to the non-differential results. Therefore, larger studies are needed for further confirmation.
In our comparison of the incidence of P/LP CNVs in fetuses with isolated and non-isolated UCCs, no significant difference was found. Similar to what Liu et al. previously reported [Citation18], we found the same P CNV (17p12 duplication) in a fetus with isolated UCC. The 17p12 duplication covers a triplosensitivity (17p12 recurrent hereditary neuropathy with pressure palsies [HNPP)/CMT1A]) region (includes peripheral myelin protein 22), which was associated with CMT1A characterized by chronic progressive neuropathy of the motor and sensory nerves [Citation19]. Unfortunately, the parent in our study chose to terminate the pregnancy due to the P CNV, while the parents in the previous study [Citation18] chose to continue, and the baby had normal survival growth and development in the first 2 years of life by the end of follow-up. Liu et al. also reported one fetus with UCCs, hydramnios, subependymal cysts, a hyperechogenic bowel, and 9p34.3 microdeletions (576 kb) [Citation18], which implied Kleefstra syndrome (intellectual disability and autism) in relation to the EHMT1 gene. In our study, we found a fetus with non-isolated UCCs (case 3, ) that had monosomy of 9p (9p24.3p22.1 deletion) (pCNV), which had a larger deletion size in 9p than that reported in a previous study [Citation18]. Additionally, we first found a de novo terminal repeat of Xp (LP CNV) and VUS in a female fetus with non-isolated UCCs (case 5, ). Fortunately, the baby had no obvious abnormalities during the first 6 months of life. Based on the above findings, CMA should be recommended when fetuses present with non-isolated UCCs. However, as no severe clinical phenotype of CNV was found in fetuses with isolated UCCs, CMA might be considered when pregnant women and their families have been fully informed in consultation with professional medical clinicians.
The presence of multiple UCCs has been reported to be associated with an increased risk of miscarriage and aneuploidy than single UCCs [Citation20]. Sepulveda et al. reported that the presence of multiple UCCs combined with structural abnormalities in late pregnancy appeared to be associated with chromosomal abnormalities such as trisomy 18 and trisomy 13 [Citation5]. However, they did not study the association between the number of UCCs and the long-term prognosis of fetuses [Citation5]. In our study, no significant difference in terms of chromosomal abnormalities was found in fetuses with single and multiple UCCs. There was also no significant difference in prognosis between fetuses identified with single and multiple UCCs in our study. Future prospective studies involving larger sample sizes are needed to validate our findings.
We also considered the incidence of chromosomal abnormalities and pregnancy outcomes in fetuses with different locations of UCCs. One study reported that an increased incidence of fetal abnormalities was associated with UCCs located near the end of the umbilical cord (fetal or placental insertion) in early pregnancy [Citation21]. However, that study did not explore the association between UCC location and fetal chromosomal abnormalities. In our study, we found better prognoses in fetuses with UCCs located on the placental side with no chromosomal abnormalities, none underwent postnatal surgery, and this group had a higher rate of healthy living babies after birth than the group with fetal side UCCs. Additionally, the total rate of chromosome anomalies in fetuses with UCCs located in other positions was higher than that of UCCs located in the placental side (30.0% vs 0%, p = .024). These findings indicate that UCC position may be a factor in terms of pregnancy outcomes.
This study had some limitations. The sample size in our study was limited partly due to the extremely low incidence of UCCs. A relatively small sample size makes it difficult to avoid type I or type II statistic errors and make an assertion that a real difference existed in those compared groups. In addition, follow-ups were mainly conducted by telephone or using medical records, and this may have resulted in bias. Therefore, a larger sample size and more prospective studies are warranted for further validation.
Conclusion
Based on our findings, CMA is recommended when fetuses present with non-isolated UCCs. Given that no severe pathogenic CNVs were detected in the fetuses with isolated UCCs, CMA might be considered after parents have been consulted and well-informed. The overall prognosis for fetuses with UCCs was favorable; however, those with UCCs located close to the placental side might have better pregnancy outcomes than those with UCCs located in other positions.
Supplemental Material
Download MS Word (16.9 KB)Acknowledgments
We thank Min Luo in our center for helping with the rearrangement of data spreadsheets. We also thank the patients and their family members for participating in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data analyzed in this study can be made available upon reasonable request from the authors.
Additional information
Funding
References
- Chiang LS. Vitelline duct remnant appears as a hemorrhagic umbilical mass. JAMA. 1982;247(20):2812–2813.
- Bunch PT, Kline-Fath BM, Imhoff SC, et al. Allantoic cyst: a prenatal clue to patent urachus. Pediatr Radiol. 2006;36(10):1090–1095.
- Zangen R, Boldes R, Yaffe H, et al. Umbilical cord cysts in the second and third trimesters: significance and prenatal approach. Ultrasound Obstet Gynecol. 2010;36(3):296–301.
- Smith GN, Walker M, Johnston S, et al. The sonographic finding of persistent umbilical cord cystic masses is associated with lethal aneuploidy and/or congenital anomalies. Prenat. Diagn. 1996;16(12):1141–1147.
- Sepulveda W, Gutierrez J, Sanchez J, et al. Pseudocyst of the umbilical cord: prenatal sonographic appearance and clinical significance. Obstet Gynecol. 1999;93(3):377–381.
- Stella A, Babbo GL. Omphalocele and umbilical cord cyst. Prenatal diagnosis. Minerva Ginecol. 2000;52(5):213–216.
- Frazier HA, Guerrieri JP, Thomas RL, et al. The detection of a patent urachus and allantoic cyst of the umbilical cord on prenatal ultrasonography. J Ultrasound Med. 1992;11(2):117–120.
- Emura T, Kanamori Y, Ito M, et al. Omphalocele associated with a large multilobular umbilical cord pseudocyst. Pediatr Surg Int. 2004;20(8):636–639.
- Sepulveda W, Bower S, Dhillon HK, et al. Prenatal diagnosis of congenital patent urachus and allantoic cyst: the value of color flow imaging. J Ultrasound Med. 1995;14(1):47–51.
- Weissman A, Jakobi P, Bronshtein M, et al. Sonographic measurements of the umbilical cord and vessels during normal pregnancies. J Ultrasound Med. 1994;13(1):11–14.
- Ruiz Campo L, Cornudella S, Gamez Alderete R, et al. Prenatal diagnosis of umbilical cord cyst: clinical significance and prognosis. Taiwan J Obstet Gynecol. 2017;56(5):622–627.
- Zhang Z, Hu T, Wang J, et al. Pregnancy outcomes of fetuses with congenital heart disease after a prenatal diagnosis with chromosome microarray. Prenat Diagn. 2022;42(1):79–86.
- Riggs ER, Andersen EF, Cherry AM, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American college of medical genetics and genomics (ACMG) and the clinical genome resource (ClinGen). Genet Med. 2020;22(2):245–257.
- Yang M, Xu B, Wang J, et al. Genetic diagnoses in pediatric patients with epilepsy and comorbid intellectual disability. Epilepsy Res. 2021;170:106552.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–424.
- Kuwata T, Matsubara S, Izumi A, et al. Umbilical cord pseudocyst in a fetus with trisomy 18. Fetal Diagn Ther. 2003;18(1):8–11.
- Bahado-Singh RO, Choi SJ, Oz U, et al. Early second-trimester individualized estimation of trisomy 18 risk by ultrasound. Obstet Gynecol. 2003;101(3):463–468.
- Liu Q, Wei R, Lu J, et al. A retrospective cohort analysis of the genetic assay results of foetuses with isolated and nonisolated umbilical cord cyst. IJGM. 2022; 15:5775–5784.
- Salpietro V, Manole A, Efthymiou S, et al. A review of copy number variants in inherited neuropathies. Curr Genomics. 2018;19(6):412–419.
- Ghezzi F, Raio L, Di Naro E, et al. Single and multiple umbilical cord cysts in early gestation: two different entities. Ultrasound Obstet Gynecol. 2003;21(3):215–219.
- Ross JA, Jurkovic D, Zosmer N, et al. Umbilical cord cysts in early pregnancy. Obstet Gynecol. 1997;89(3):442–445.