Abstract
Background
Fluid management in newborns undergoing surgery can be challenging due to difficulties in accurately assessing volume status in context of high fluid needs perioperatively and postoperative third-space fluid loss. Fluid overload can be associated with an increase in neonatal morbidity and mortality.
Objective
Our objective was to determine the burden of fluid overload and to evaluate their associations with adverse effects among infants undergoing abdominal surgery at a tertiary perinatal center.
Methods
Patients from our Neonatal Intensive Care Unit who underwent abdominal surgery from January 2017 to June 2019 were included in this retrospective cohort study. Fluid balance was assessed based on the maximum percentage change in body weight at 3- and 7-postoperative days.
Results
Sixty infants were included, with a median [interquartile range] gestational age (GA) of 29 [25–36] weeks and birth weight of 1240 [721–2871] grams. The median daily actual fluid intake was significantly higher than the prescribed fluid intake in the first 7 postoperative days (163 vs. 145 mL/kg, p < .01). The median maximum change of body weight by postoperative days 3 and 7 were 6% [3–13] and 11% [5–17], respectively. A 1% increase in weight within the first 3 postoperative days was associated with a 0.6-day increase for invasive ventilatory support (p = .012). The correlation was still significant after adjusting for GA (p = .033).
Conclusion
Fluid overload within the first 3 postoperative days was associated with an increase in ventilator support among infants. Careful attention to fluid management may affect the optimization of outcomes for newborns undergoing abdominal surgery.
Keywords:
Introduction
Fluid management in newborns undergoing surgery can be challenging due to difficulties in accurately assessing volume status in context of high fluid needs perioperatively and postoperative third-space fluid loss. Preterm infants in particular are more susceptible to fluid overload due to their underdeveloped cardiovascular and renal systems, making them less capable of handling large fluid volumes [Citation1]. On the other hand, available tools for evaluation of fluid balance such as weight, urine output, and renal function tests might not be able to accurately measure effective intravascular and interstitial volume. Perioperative fluid management for newborns requiring abdominal surgery is especially difficult.
In pediatric surgery patients, fluid overload (FO) can be associated with subsequent acute kidney injury (AKI) and increased morbidity [Citation2]. Abdominal surgery is associated with blood and extravascular fluid loss, including evaporative insensible loss from skin and exposed peritoneal surfaces during surgery, third spacing and fluid sequestration in the gastrointestinal tract from postoperative ileus [Citation3]. On the other hand, secretion of antidiuretic hormone may occur postoperatively and promote free water retention [Citation1,Citation3]. In addition, FO can be associated with AKI following surgery or abdominal compartment syndrome, which may interfere with bowel edema resolution, thus leading to mesenteric ischemia [Citation4,Citation5].
There is a paucity of literature evaluating fluid balance in newborns undergoing abdominal surgery. The objective of this study was to assess the burden, risk factors, and short-term outcomes in newborns after abdominal surgery.
Methods
Infants admitted to a tertiary-level neonatal intensive care unit (NICU) at BC Women’s and Children’s Hospital (Vancouver BC, Canada) between January 2017 and June 2019 were included in this retrospective cohort study. Prospectively maintained neonatal surgical databases at the hospital were used to screen eligible patients. We included infants who underwent abdominal surgeries and were admitted to the NICU for postoperative care. Those who had multiple congenital anomalies, including congenital heart or kidney disease, and those who died <24 h after surgery were excluded. Data were extracted from paper and electronic medical charts. Fluid management was assessed from 3 days pre-operation to 7 days post-operation.
The study was reviewed and approved by the Research Ethics Board of the Children’s & Women’s Health Center of British Columbia and the University of British Columbia (Ethics approval number: H19-01951).
Demographic and outcome variables were defined according to the Canadian Neonatal Network Abstractor’s Manual [Citation6]. Gestational age (GA) was defined as the best obstetric estimate based on early prenatal ultrasound, obstetric examination, and obstetric history unless the postnatal pediatric estimate of gestation differed from the obstetric estimate by >2 weeks.
Primary outcomes were fluid balance at post-operative day 3 and day 7. Percent daily weight change was calculated as following: (daily postoperative weight – preoperative weight)/preoperative weight × 100%) [Citation6]. We used a weight-based definition for fluid balance, which is especially useful in infants compared to fluid balance-based definition due to role of insensible fluid losses and difficulties with quantifying urine output accurately [Citation4]. Other outcomes of interest included: mortality, duration of mechanical ventilation, AKI, days to start enteral feeds, post-operative sepsis, length of hospital stay, bronchopulmonary dysplasia (BPD), and high-grade intraventricular hemorrhage (IVH).
Actual fluid intake (mL/kg/day) referred to the total volume of fluid the patient received in the previous 24 h, including parenteral nutrition, fluid boluses, transfusions, flushes through intravenous (IV) lines, medications, and fluid to keep the IV line open. Prescribed fluid intake referred to the amount of fluid ordered on the physicians’ orders on the patient records. Taking physiological shift of extrcellular fluid volume in the neonates into account, we also analyzed the impact of fluid balance among infants with surgery performed <7 days of life (DoL) and ≥7 DoL separately. AKI was defined as urine output less than 1 mL/kg/hour (averaged in a 24-h window) within the first 7 postoperative days or any increase in serum creatinine (Cr) by 1.5-fold or greater from a baseline value within 7 postoperative days or an absolute Cr increase of 26.5 μmol/L [Citation7]. BPD was defined as the receipt of oxygen at 36 weeks’ postmenstrual age or at discharge, whichever came first [Citation8]. IVH was classified according to the Papile’s classification [Citation9]. Periventricular leukomalacia referred to the persistent periventricular echogenicity or echolucency documented on neuroimaging before discharge [Citation10]. Sepsis was indicated by positive bacterial, viral, or fungal culture in blood or cerebrospinal fluid from birth.
Patient demographics and clinical characteristics were reported as median (interquartile range [IQR]) and proportion (%) for continuous and categorical variables, respectively. Differences between the prescribed and actual total fluid intake were compared using the Wilcoxon signed-rank test. Odds ratio and 95% confidence interval were calculated using univariate logistic regression analyses to quantify the association between adverse outcomes and FO. Multivariable logistic regression was performed after adjusting for potential confounders and other covariates based on the findings of the univariate analysis. R (v.4.0.3) using R-studio (v.1.3.1073) was used for statistical analysis, with p < .05 to be considered statistically significant.
Results
Between January 2017 and June 2019, 83 newborns underwent abdominal surgery. Sixty patients were included in this study, with median [IQR] gestational age of 29 weeks [25–36] and median [IQR] birth weight of 1240 g [721–2871]. Surgery was conducted at a median [IQR] of 6 DoL [Citation3–12], with 32 patients having surgery before 7 DoL and 28 patients having surgery on or after 7 DoL. Baseline patient characteristics are presented in .
Table 1. Baseline patient characteristics and surgical data.
The actual median daily fluid intake was 178 mL/kg on post-operative days 0–2 and 163 mL/kg from postoperative days 3 to 7, which were significantly higher than the median prescribed fluid volume of 145 mL/kg (p < .01).
Surgery data and postoperative outcomes are presented in . The maximum median [IQR] percentage of weight change was 6% [Citation3–13] and 11% [Citation5–17] in postoperative days 3 and 7, respectively. Analysis of the cohort when divided into surgery before 7 or after 7 DoL, we found that there were no differences in fluid administration or max weight change between the two groups (Supplementary Table 1).
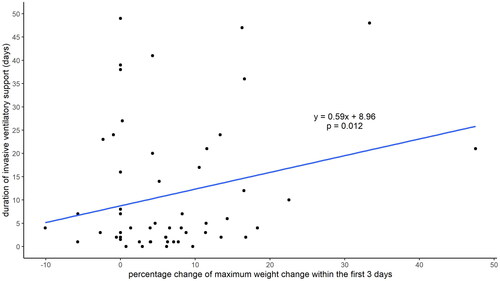
Every 1% increase in weight gain within the first 3 postoperative days was associated with the requirement of additional 0.6 days of invasive ventilator support (p = .012) (). This correlation remained significant after adjusting for gestational age (p = .033). There were 32 (53%) infants requiring respiratory support at 28 DoL, and 20 (33%) developed BPD. We did not identify any correlations between max weight gain within the first 3- or 7-days post-operation and the development of BPD and mortality.
Figure 1. Correlation between 3-day postoperative weight change and duration of invasive ventilator support.
Only 30 (50%) of patients had serum creatinine measured pre- and post-operatively for comparison. Overall, we identified 4 (6.7%) infants who developed AKI, according to the aforementioned criteria.
Discussion
In infants who had undergone abdominal surgeries in our NICU, we found that FO, defined by the maximum weight change 3 days after abdominal surgery, correlated with the duration of invasive ventilatory support even after adjusting for gestational age.
In a study on 362 infants with gastroschisis from the Canadian Pediatric Surgery Network, a direct correlation exists between pre-closure resuscitative fluid volume and days of post-closure ventilation, total parenteral nutrition, length of hospital stay, and episodes of bacteremia [Citation11]. The relationship between FO and adverse outcomes, including longer respiratory support, has been demonstrated in several studies in children and adult critical care units [Citation12], but has been understudied in hospitalized neonatal populations. Based on two separate sub-analyses of the AWAKEN study, the largest multicenter neonatal AKI study to date, Selewski et al. found that both in infants with GA of >36 weeks and <36 weeks, higher peak fluid balance during the first postnatal week was independently associated with the need for mechanical ventilation on postnatal day 7, irrespective of the presence of AKI [Citation7]. The findings were similar in their subgroup analysis in <36 weeks infants where positive fluid balance during the first postnatal week was independently associated with mechanical ventilation at postnatal day 7 and negative fluid balance at postnatal day 7 was associated with lower likelihood of mechanical ventilation [Citation13]. A study on pediatric intensive care unit patients with respiratory failure found that the percentage peak FO was associated with a longer duration of ventilation, and FO could be an independent risk factor for multi-organ dysfunction rather than just a marker of disease severity [Citation14].
The underlying mechanism of association between FO and increase in invasive ventilation durations could be fluid accumulation in the lungs, endothelial dysfunction, increased risk of patent ductus arteriosus (PDA), or pulmonary edema in the context of prematurity [Citation14]. It is also possible that an increase in circulating inflammatory cytokines due to stress of surgery and underlying disease could cause more injury to the lungs in these circumstances. The longer duration of ventilator support could potentially lead to further increase in lung injury and consequently, increase the risk of BPD. However, considering the small sample size, we were unable to assess predictive factors for the development of BPD here.
The increased weight change in our patients is likely due to generous amounts of administered fluid, which was consistently higher than the ordered fluid intake. This finding is consistent with the finding in a retrospective study where Bonasso et al. found that patients received excessive IV fluid volume after undergoing gastroschisis repair [Citation3]. With a range of positive fluid balances from 80 to 120 mL/kg/day, babies received 180 to 270 mL/day in excess fluids [Citation3]. Based on the Cochrane review, it is established that restricted water intake will assist with postnatal weight loss and lower the risk of PDA and necrotizing enterocolitis, with a trend toward decreasing BPD and mortality [Citation15].
In our cohort, only 6.7% of these patients developed AKI. However, only 30 patients had their serum Cr measured during their hospital stay, so the incidence of AKI was likely to be significantly under-reported. Bonasso et al. demonstrated that urine output could be high after surgery and not all AKI cases were oliguric [Citation3]. Additionally, urine output could still be in the normal range despite low intravascular volume in preterm infants due to the inability of premature kidneys to concentrate urine [Citation16,Citation17]. Other potential explanations for a low observed AKI rate are hemodilution of serum creatinine in the face of fluid overload, inability to identify a significant rise in serum Cr when glomerular filtration rate is already lower in the first few days of life, and lack of standardized AKI definitions for premature infants [Citation16]. Other more novel AKI biomarkers may need to be studied further in this population to determine the true burden of AKI in FO.
Our study is one of the very few in the literature to review the post-operative fluid balance in a cohort of preterm infants, highlighting the importance of judicious fluid management peri-operatively. Having said that, our study has a couple of limitations. First, due to the relatively small number of patients, the results of the study may not have adequate power to address all clinical outcomes, such as BPD and death. Second, the urine output calculations may be overestimated as it could be mixed with stool as the majority of infants could not have Foley catheter monitoring peri-operatively. Third, lack of standard serum Cr measurement assessment prevent us from the understanding of how AKI may contribute the fluid overload.
Studies with larger sample size and adequate power are needed to understand the impact of FO on neonatal mortality and morbidities. Unlike in pediatric and adult populations, accurately assessing the intravascular volume status with measurement of central venous pressure in preterm infants is difficult, if not impossible. Targeted neonatal echocardiography may also be utilized to provide an additional tool to assess the intravascular volume status, with careful use of diuretics in this vulnerable populations. Lastly, regular serum Cr and urine output should be monitored peri-operatively to identify early AKI and help guide fluid therapy in this vulnerable population.
Conclusion
In newborns undergoing abdominal surgery, fluid overload in the first 3 postoperative days was positively associated with increased duration of invasive ventilator support. Judicious post-operative fluid management may be beneficial to improve respiratory outcomes.
Supplemental Material
Download MS Word (17.6 KB)Acknowledgments
We are grateful to Ms. Jessica Tang for her editorial support regarding the completion and submission of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s). The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The database for this research article is not available because of identifiable patient particulars in a relatively small number of patients.
Additional information
Funding
References
- Arumainathan R, Stendall C, Visram A. Management of fluids in neonatal surgery. BJA Educ. 2018;18(7):199–203.
- Hassinger AB, Wald EL, Goodman DM. Early postoperative fluid overload precedes acute kidney injury and is associated with higher morbidity in pediatric cardiac surgery patients. Pediatr Crit Care Med. 2014; 15(2):131–138.
- Bonasso PC, Lucke-Wold B, Hobbs GR, et al. Excessive postoperative fluid administration in infants with gastroschisis. Am Surg. 2016;82(8):704–706.
- Selewski DT, Goldstein SL. The role of fluid overload in the prediction of outcome in acute kidney injury. Pediatr Nephrol. 2018;33(1):13–24.
- Oh W, Poindexter BB, Perritt R, et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr. 2005;147(6):786–790.
- Network TCN. The Canadian Neonatal Network abstractor’s manual, CNN v.3.6; 2023. Available from: https://www.canadianneonatalnetwork.org/portal/Portals/0/CNN%20Manuals/CNN%20Manual_20230308.pdf
- Selewski DT, Akcan-Arikan A, Bonachea EM, et al. The impact of fluid balance on outcomes in critically ill near-term/term neonates: a report from the AWAKEN study group. Pediatr Res. 2019;85(1):79–85.
- Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–1955.
- Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534.
- Kwon SH, Vasung L, Ment LR, et al. The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clin Perinatol. 2014;41(1):257–283.
- Jansen LA, Safavi A, Lin Y, et al. Preclosure fluid resuscitation influences outcome in gastroschisis. Am J Perinatol. 2012;29(4):307–312.
- Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–2495.
- Selewski DT, Gist KM, Nathan AT, et al. The impact of fluid balance on outcomes in premature neonates: a report from the AWAKEN study group. Pediatr Res. 2020;87(3):550–557.
- Arikan AA, Zappitelli M, Goldstein SL, et al. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012 May;13(3):253–258.
- Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2008;23(1):Cd000503.
- Askenazi D, Patil NR, Ambalavanan N, et al. Acute kidney injury is associated with bronchopulmonary dysplasia/mortality in premature infants. Pediatr Nephrol. 2015;30(9):1511–1518.
- Visram AR. Intraoperative fluid therapy in neonates. Southern Afr J Anaest Analg. 2016;22(2):46–51.
