Abstract
Perinatal tuberculosis is a rare disease with high mortality and a challenging diagnosis. We reported a 56-day-old female infant with cough and wheezing. Her mother had miliary tuberculosis. Gastric aspirate smear, tuberculin skin test, blood and sputum culture of the infant were negative. Thoracic computed tomography demonstrated several consolidated patches with diffuse high-density nodular opacities in bilateral lungs. Fiberoptic bronchoscopy was performed to obtain bronchoalveolar lavage fluid, reduce secretion and restore airway patency on 2 days after admission. Mycobacterium tuberculosis was detected by bronchoalveolar lavage fluid Xpert MTB/RIF and rifampicin resistance was negative on 3 days after admission. Appropriate anti-tuberculosis drug was chosen. The infant made a good recovery. Fiberoptic bronchoscopy plays a vital role in diagnosing rapidly and treating perinatal tuberculosis. And it could be promoted as an important approach to the management of perinatal tuberculosis.
Introduction
Congenital tuberculosis is a rare disease that is considered to infect Mycobacterium tuberculosis (Mtb) from the placenta to the fetus through the umbilical vein or ingestion of the amniotic fluid which is contaminated by placental or genital infection [Citation1]. However, it is difficult to distinguish between intrauterine or postnatal infection. In addition, differentiation is only of epidemiological importance, but the modes of clinical presentation, treatment, and prognosis do not differ [Citation2]. Therefore, the infection with Mtb which occurs in utero, at birth, or during the early newborn period is collectively referred to perinatal tuberculosis [Citation3]. It is difficult to diagnose perinatal tuberculosis early because of the variable and nonspecific clinical findings, which result in a high mortality and poor prognosis. Fiberoptic bronchoscopy (FOB) is widely used in the clinical practice of pediatrics and has demonstrated fundamental value in clinical diagnoses and treatment [Citation4]. However, as an invasive procedure, the use of FOB is limited due to concerns regarding the tolerance of the procedure and the possible complications in neonates [Citation5]. Here, we reported an infant who was infected with perinatal tuberculosis and diagnosed on the third day of admission. After diagnosis, the patient received treatment with a good prognosis.
Case report
A 56-day-old female infant presented with cough and wheezing for 9 days. She was delivered vaginally at 39+4 weeks with a birth weight 2650 g and received bacillus of calmette-guerin (BCG) at birth. Her mother was diagnosed with miliary tuberculosis about 20 days after delivery and was under regular treatment. On admission, her vital signs were as follows: temperature 36.8 °C, respiratory rate 55 breaths/min, heart rate 175 beats/min, blood pressure 68/35 mmHg and transcutaneous oxygen saturation 85%. She was weak and had grunting, shortness of breath with intercostal retractions, cyanosis around the mouth and normal liver. A chest radiograph showed bilaterally patchy high-density shadows (). Hemoglobin was 125 g/L, white cell counts 20.06 × 109/L (neutrophils 49.6%, lymphocytes 39.9%, monocytes 10.7%) and platelets 363 × 109/L. Serum C-reactive protein was 16 mg/L. IgG, IgA, IgM, and IgE levels and CD4/CD8 cell counts were normal. Her human immunodeficiency virus (HIV), tuberculin skin test (TST), blood and sputum culture were negative. In three consecutive days, the acid-fast bacilli stain of gastric lavage aspirates was negative. Abdominal computed tomography (CT) demonstrated liver was normal.
Figure 1. Radiographic findings. A. Thoracic CT at first admission revealed diffuse high-density nodular opacities of various sizes in both lungs. B. Chest radiograph at admission showed bilaterally patchy high-density shadows, which were more severe in the right lung. C. Chest radiograph within 24 h after fiberoptic bronchoscopy displayed improvement over admission. D. Chest radiograph reviewed in the tuberculosis clinic were normal. E. Picture taken by fiberoptic bronchoscopy demonstrated blockage of the airway by a lot of secretions.
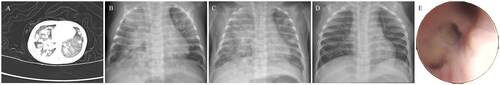
After admission, oxygen inhalation and broad-spectrum antibiotic were performed. Her condition became increasingly serious with heart and respiratory failure. After endotracheal intubation, mechanical ventilation and corresponding symptomatic treatment, her condition improved. Thoracic CT demonstrated scattered consolidated patches with diffuse high-density nodular opacities of various sizes in bilateral lungs, and enlarged nodes were seen in the mediastinum and hilum of the right lung (). Pulmonary tuberculosis couldn’t be excluded. To further clarify the diagnosis, reduce secretion and restore airway patency, FOB was performed on the second day of admission. After the fiberoptic bronchoscope, the infant’s vital signs were stable, which were as follows: temperature 36.6 °C, respiratory rate 50 breaths/min, heart rate 165 beats/min, blood pressure 70/42 mmHg and transcutaneous oxygen saturation 93%. Bronchoalveolar lavage fluid (BALF) Xpert MTB/RIF showed that Mtb nucleic acid detection was positive and rifampicin-sensitive resistance genes were negative on the third day of admission. As a result, anti-tuberculous therapy was commenced with isoniazid, rifampicin, pyrazinamide and ethambutol. Mtb was detected by BALF metagenomic next-generation sequencing (mNGS) on the fourth day of admission. After significant improvement in most indicators, the infant was discharged 23 days after admission and regularly followed up in the tuberculosis clinic of our hospital. Anti-tuberculous drugs were discontinued six months after discharge. At the age of two, she was in good health and had a normal presentation on a chest radiograph ().
Discussion
We presented the case of a child with a positive history of Mtb contact. The presentation of cough and wheezing didn’t appear until the infant was 47 days old. And the liver of the baby was normal which didn’t find a primary hepatic complex or caseating hepatic granulomas. Because some methods for detecting Mtb were not sensitive or required more time, infection with Mtb couldn’t be confirmed rapidly. The infant was proved to have tuberculous lesions by BALF Xpert MTB/RIF on the third day of admission, and the rifampicin resistance was negative. Eventually, perinatal tuberculosis was diagnosed. Meanwhile, irrigation and suctioning via the FOB were used to remove secretion and purulent material. Chest radiograph on the first day after FOB revealed a better recovery of her right lung than that on admission (). Through this case, we believe that Xpert of BALF obtained by FOB is beneficial for early diagnosis and the selection of anti-tuberculosis drugs. Meanwhile, clearing respiratory secretions through FOB is also an effective treatment.
Perinatal tuberculosis is an uncommon infection. To date, there have been approximately over 300 reported cases published in the English language [Citation6]. It is challenging to distinguish congenital from postnatally acquired tuberculosis. Generally, congenital tuberculosis could be diagnosed with one of the following conditions [Citation7]: onset of symptoms in the first week of life, a primary liver lesion or a caseous liver granuloma, tuberculous infection of the placenta or tuberculous infection of the maternal genital tract. But in this case, the infant presented with cough and wheezing on 47 days old after birth, and her liver wasn’t found a primary hepatic complex or caseating hepatic granulomas. In addition, tuberculous infection of the placenta or tuberculous infection of the maternal genital tract weren’t found. Therefore, congenital tuberculosis couldn’t be diagnosed. Her mother was diagnosed with miliary tuberculosis, and they had lived together during the early newborn period. Infection of Mtb by women might result in four possible outcomes: the immediate clearing of the organism, latent infection, onset of an active disease, or onset of an active disease years later. Recently published studies indicate that postpartum women have been found to be twice as likely to contract an active pulmonary disease or become symptomatic as nonpregnant women [Citation8]. Additionally, some mothers whose infants had active tuberculosis remained asymptomatic postnatally. Only a few women have been diagnosed with tuberculosis during the third trimester of pregnancy. The infant and her mother’s specific Mtb infection condition couldn’t be identified. Consequently, perinatal tuberculosis was diagnosed. If the infant’s mother were infected with Mtb, the possibility of perinatal tuberculosis should be highly vigilant. The history of Mtb infection of patient’s mother might play an important part in the diagnosis of tuberculosis.
In the case of suspected perinatal tuberculosis, an accurate examination must be done promptly in order to identify the infection of Mtb. Most patients have abnormal chest imaging, such as pneumonia (76%), multiple pulmonary nodules (43%), and military pattern (38%), which is nonspecific and not a definitive diagnostic method [Citation9]. The sensitivity and specificity of TST are low because the infant’s immune system is immature and BCG is usually given at birth [Citation10]. Interferon-gamma release assay is superior to TST in the detection of Mtb infection. The positive rate of Mtb detected by gastric or sputum smear is higher than that of TST or interferon-gamma release assay in neonates [Citation11], but it is difficult to acquire the specimens. Blood and sputum cultures need a long time and have a low rate of positivity. Moreover, the infants are young and their clinical manifestation changes rapidly. The survival rate can be improved by early diagnosis and treatment. Therefore, rapid and accurate diagnostic methods are required.
In recent years, with the development of FOB, BALF assay has become a new method to diagnose perinatal tuberculosis. BALF is more sensitive to Mtb than sputum due to its proximity to the lesion [Citation12]. A recent study has demonstrated that BALF should be used to obtain specimens for the diagnosis of pulmonary tuberculosis in sputum-scarce or smear-negative cases [Citation13]. Benefiting from the widespread applications of molecular biology, Mtb has been detected more quickly and accurately by Xpert MTB/RIF, Xpert MTB/RIF Ultra and mNGS to reduce misdiagnosis and missed diagnosis. Xpert assay is faster than mycobacterium culture and more sensitive than sputum smears [Citation14]. In addition, it can also detect resistance of rifampicin. With the worldwide increase of multidrug-resistant tuberculosis and extremely resistant tuberculosis, it has become imperative to facilitate drug susceptibility testing to ensure children receive the appropriate tuberculosis antibiotics [Citation15].
FOB is crucial to treat perinatal tuberculosis. The presence of airway compression, airway obstruction, and fistula in the bronchus can be seen more visually by FOB (). At the same time, the lung can be irrigated to reduce sputum plugs and restore airway patency [Citation16]. Lymphobronchial tuberculosis is particularly common in children [Citation17]. Airway compression by lymphadenopathy causes downstream parenchymal pathology, which may ultimately result in irreversible lung destruction if not treated timeously [Citation18]. Bronchoscopic lymph node decompression is operated by bronchoscopes to improve airway compression symptoms and avoid irreversible lung destruction [Citation19]. Antituberculous therapy or combined with surgery treatment should be given if a bronchial fistula is discovered [Citation20].
FOB indications for infants were classified as the following [Citation21]: performance of BALF for detection of pathogens, therapeutic lavage (removal of bronchial mucus plugs causing persistent pneumonia and atelectasis), diagnosis of suspected airway disease (infants with stridor, unexplained tachypnea, retractions, and/or a history of prior intubation), bronchoscope-assisted intubation and extubation. In this case, FOB was used to obtain BALF for the detection of Mtb and therapeutic lavage.
Perinatal tuberculosis progresses rapidly. The prognosis is poor and mortality is high. Infants should receive isoniazid (10–15 mg/kg/d), rifampin (10–20 mg/kg/d), pyrazinamide (15–30 mg/kg/d), and either streptomycin (20–30 mg/kg/d) or ethambutol (15–25 mg/kg/d) for the first 2 months, followed by isoniazid and rifampin for 4–10 months, depending on the severity of the disease [Citation1]. The mortality rate of congenital tuberculosis is only 15.38% after diagnosis and anti-tuberculosis treatment [Citation7]. Therefore, early treatment may improve the prognosis.
Conclusions
In conclusion, the possibility of perinatal tuberculosis should be highly vigilant, if the infant has symptoms of infection and the mother is infected with Mtb. It is also important to use molecular biology techniques to improve the diagnostic sensitivity of perinatal tuberculosis. Moreover, FOB can not only visualize the airway and irrigate airway secretions, but also collect respiratory samples to detect Mtb and the resistance of the drug by molecular biology techniques. As a result, the application of FOB might be a complementary approach to the management of perinatal tuberculosis.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Tianjin Children’s Hospital.
Consent for publication
Written informed consent was obtained from the patient’s parents for the publication of this case report and the accompanying images.
Acknowledgements
The authors are grateful to the patient who took part in the study and all staff members.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cantwell MF, Shehab ZM, Costello AM, et al. Brief report: congenital tuberculosis. N Engl J Med. 1994;330(15):1051–1054.
- Singh M, Kothur K, Dayal D, et al. Perinatal tuberculosis a case series. J Trop Pediatr. 2007;53(2):135–138.
- Lackmann GM. Congenital tuberculosis. N Engl J Med. 1994;331(8):548; author reply 548–9.
- Mackanjee HR, Naidoo L, Ramkaran P, et al. Neonatal bronchoscopy: role in respiratory disease of the newborn-A 7 year experience. Pediatr Pulmonol. 2019;54(4):415–420.
- Yan C, Hu Y, Qiu G, et al. The clinical safety and efficacy of flexible bronchoscopy in a neonatal intensive care unit. Exp Ther Med. 2020;20(5):95.
- Ferreras-Antolin L, Caro-Aguilera P, Perez-Ruiz E, et al. Perinatal tuberculosis: is it a forgotten disease? Pediatr Infect Dis J. 2018;37(3):E81–E83.
- Li CF, Liu LL, Tao YH. Diagnosis and treatment of congenital tuberculosis: a systematic review of 92 cases. Orphanet J Rare Dis. 2019;14(1):131.
- Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis. 2012;55(11):1532–1549.
- Yeh JJ, Lin SC, Lin WC. Congenital tuberculosis in a neonate: a case report and literature review. Front Pediatr. 2019;7:255.
- Jenum S, Selvam S, Mahelai D, et al. Influence of age and nutritional status on the performance of the tuberculin skin test and QuantiFERON-TB gold in-tube in young children evaluated for tuberculosis in Southern India. Pediatr Infect Dis J. 2014;33(10):e260-9–e269.
- Saramba MI, Zhao D. A perspective of the diagnosis and management of congenital tuberculosis. J Pathog. 2016;2016:8623825.
- Liu X, Hou XF, Gao L, et al. Indicators for prediction of Mycobacterium tuberculosis positivity detected with bronchoalveolar lavage fluid. Infect Dis Poverty. 2018;7(1):22.
- Kim YW, Kwon BS, Lim SY, et al. Diagnostic value of bronchoalveolar lavage and bronchial washing in sputum-scarce or smear-negative cases with suspected pulmonary tuberculosis: a randomized study. Clin Microbiol Infect. 2020;26(7):911–916.
- Atherton RR, Cresswell FV, Ellis J, et al. Xpert MTB/RIF ultra for tuberculosis testing in children: a mini-review and commentary. Front Pediatr. 2019;7:34.
- Goussard P, Gie R. The role of bronchoscopy in the diagnosis and management of pediatric pulmonary tuberculosis. Expert Rev Respir Med. 2014; 8(1):101–109.
- Singh V, Singhal KK. The tools of the trade – uses of flexible bronchoscopy. Indian J Pediatr. 2015; Oct82(10):932–937.
- Furin J. Advances in the diagnosis, treatment, and prevention of tuberculosis in children. Expert Rev Respir Med. 2019;13(3):301–311.
- Andronikou S, Lucas S, Zouvani A, et al. A proposed CT classification of progressive lung parenchymal injury complicating pediatric lymphobronchial tuberculosis: from reversible to irreversible lung injury. Pediatr Pulmonol. 2021;56(12):3657–3663.
- Goussard P, Gie RP, Janson JT, et al. Decompression of enlarged mediastinal lymph nodes due to mycobacterium tuberculosis causing severe airway obstruction in children. Ann Thorac Surg. 2015;99(4):1157–1163.
- Tautz E, Wagner D, Wiesemann S, et al. Treatment of a broncho-esophageal fistula complicated by severe ARDS. Infection. 2019;47(3):483–487.
- Atag E, Unal F, Yazan H, et al. Pediatric flexible bronchoscopy in the intensive care unit: a multicenter study. Pediatr Pulmonol. 2021;56(9):2925–2931.
