Abstract
Background and Aim: Unexplained recurrent pregnancy loss has been a a challenging research task to experts since there is no explicit pathophysiological mechanism and therefore, the treatment remains elusive. Immunological imbalance and morphological abnormalities are under investigation. This study aims to evaluate the implication of MMP-2, MMP-9, EGFR, and IL-8 in recurrent pregnancy loss cases. Materials & Methods: The study was carried out through comparison among two groups; the unexplained miscarriage group which consisted of 22 women, and the control group consisted of 18 women, who had electively terminated their pregnancies. Both groups were in the first trimester of gestation. The specimens included the trophoblast, decidua basalis, and decidua parietalis. The study was conducted via immunohistochemical methods. Antibodies were used against MMP-2, MMP-9, EGFR, and IL-8. The results were presented at a contingency table and were statistically analyzed with the Chi-Square Test (X2). Results: There were remarkable disparities in some cases in the comparison of the two groups. MMP-9 was detected significantly high in recurrent pregnancy loss (RPL) cases, both on trophoblastic and decidual specimens (p-value < .00001), MMP-2 displayed no difference among the two groups (mild to moderate detection on trophoblast and almost negative on decidual tissues). EGFR was highly detected in trophoblastic tissue (p-value = .014). IL-8 detection was particularly different in both trophoblast and decidua parietalis of the two groups (p-value < .01). Conclusion: The study revealed both morphological and immunological dysregulations that might participate in the RPL pathogenesis.
Introduction
Recurrent pregnancy loss (RPL) is the occurrence of two or three repeated abortions, before the 20th week of pregnancy [Citation1,Citation2]. There are several possible culprits responsible for the pathogenetic mechanisms that could lead to RPL [Citation1]. Apart from the known reasons and abnormalities (Parental karyotypes, Intrauterine structural abnormalities, Luteal Phase Deficiency, Infections, Systemic Lupus Erythematosus, Non-APS thrombophilia), there are several cases of RPL which are characterized as “unexplained” [Citation1]. During the last decade, the maternal immunological imbalance has been under extensive investigation, along with possible trophoblastic and decidual molecular and developmental abnormalities [Citation1,Citation2]. However, studies have not managed to elucidate the pathogenesis of these unexplained RPL phenomena.
Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that play a central role in the breakdown and reorganization of extracellular matrix [Citation3]. Remodeling of the extracellular matrix (ECM), which is essential for endometrial decidualization, as well as trophoblast implantation and placentation, is primarily enabled by the enzymes MMP [Citation4,Citation5]. MMP-2 and MMP-9 belong to gelatinases, a subgroup of MMPs, which are involved in various physiological and pathological progress. Important functions for MMP-2 and MMP-9 in placental development are suggested by their well-known spatiotemporal expression patterns [Citation6]. Several studies showed that MMP-2 and MMP-9 were expressed at the early stages of placentation. Specifically, MMP-2 is produced and secreted predominantly by extravillous trophoblasts during the early stages of the first trimester with a peak between the 6th and 8th weeks. Similarly, the expression of MMP-9 was shown in villous cytotrophoblasts and, to a lesser extent, in extravillous trophoblasts at both the mRNA and protein levels [Citation7]. MMP2 and MMP9 have crucial roles in the terminal differentiation of human endometrial stromal cells into decidual cells and as they are detected in decidual tissues throughout pregnancy, they have been assigned key roles in the regulation of trophoblast invasion and angiogenesis [Citation8]. The dysregulation of MMP-2 and MMP-9 could lead to excessive endometrial matrix degradation, thus contributing to RPL [Citation9].
The epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein with endogenous protein phosphotransferase activity, which is located on chromosome 7p and consists of a polypeptide chain of 1186 amino acids [Citation10,Citation11]. The EGFR pathway is related to placental development via the proliferation and differentiation of placental trophoblasts. In addition, EGFR promotes pregnancy maintenance, the implantation of fertilized oocytes, and fetal growth[Citation11]. At the 4th-5th week of placenta development, EGFR is expressed in cytotrophoblast, where proliferation is induced without affecting the ability to secrete human placental lactogen (hPL) and chorionic gonadotropin (hCG). On the contrary, when the development progresses between the 6th and 12th week, EGFR is expressed in the syncytiotrophoblast and as a result, the secretion of hPL and hCG is increased. However, the proliferation of the cytotrophoblast is not affected [Citation12].
Interleukin-8 (IL-8) is a pro-inflammatory cytokine, which recruits and activates immune cells and is involved in the implantation process [Citation13]. More precisely, it is an α-chemokine, functioning as a potent chemoattractant and as an activator of T-lymphocytes and neutrophils [Citation14,Citation15]. Amongst other tissues, it may be produced and received in the endometrium, which is an important element concerning molecular interactions between the embryo and the mother [Citation13,Citation14]. Other areas of the human reproductive tract that IL-8 may be detected in are the placenta, the amnion, the cervix, and the chorio-decidua [Citation14,Citation16]. During the implantation period, the endometrium undergoes a slew of procedures that remodel its structure. To explain, stromal fibroblasts differentiate into decidual cells and an accumulation of innate immune cells in the endometrium is observed [Citation17,Citation18]. It is known that decidual cells secrete high amounts of factors that are pro-invasive during the implantation period, including IL-8 [Citation17]. Moreover, cytokines like IL-8 participate in the recognition of placenta-fetus alloantigen by immune cells and they can act as growth factors for the fetus [Citation13]. Some studies advocate that normally IL-8 is detected in higher amounts in the first and third trimesters [Citation15]. During the first trimester, the permeability of the endometrium must be increased to achieve the trophoblast invasion and higher secretion levels of IL-8 that contribute to this procedure [Citation13–15,Citation17]. During the third trimester, IL-8 is affiliated with on-term delivery and increased levels of the cytokine are detected in the cervix and lower uterus [Citation13].
Based on the suggested evidence from the literature, this study aims to evaluate the implication of MMP-2, MMP-9, EGFR, and IL-8 molecules in the pathogenesis of unexplained RPL.
Materials and methods
The RPL group included 22 women that miscarried during the 1st trimester of gestation, while the control group consisted of 18 women who had proceeded with elective termination of their pregnancies. Demographic characteristics were the same in the two groups (Healthy, Caucasian, Greek women with normal BMI). The age range was higher in the RPL cases. All women from the RPL experimental group had a history of at least three prior miscarriages during the 1st trimester. These RPL cases were of unexplained etiology (all laboratory examinations were normal, including parental karyotype, intrauterine structural study, luteal phase endometrial biopsy, hormone levels, cervical culture, lupus anticoagulant testing, and antibodies to cardiolipin and phosphatidylserine). Women with fewer than three miscarriages (even without an etiology), were excluded from the study. Women with health conditions or with anatomical or systematic abnormalities (explorative pathological examination), were excluded from the study as well. All experiments were performed in accordance with all safety considerations, ethical guidelines and applicable regulations. Informed consent was obtained from all women participating in the experiment. The approval of the Bioethics Committee of the School of Medicine of the Aristotle University of Thessaloniki was granted at an official No.4-198/17-07-2019.
Pathology-examination
Tissues
The tissues were obtained immediately after the elective abortion or the miscarriage and they were washed with distilled water to remove any blood or mucus. After this procedure, the tissues were inspected under a stereoscope to examine whether there are abnormalities or placental lesions on the decidua, villus chorion, or other parts of the embryo. Specimens with the latter conditions were excluded from this study. The samples that were collected from the decidua and the villus chorion underwent microscopic preparation processing, as in similar studies [Citation1,Citation2]. The stained sections of each sample were examined using a microscope to select the most suitable specimens for the immunohistochemical study.
Immunohistochemistry
The antibody cytokeratin (CK7) was used to detect the trophoblastic cells. Moreover, decidual and trophoblastic cells at the fetomaternal interface were discriminated by staining the duplicate sections, with a monoclonal antibody against prolactin, which leads to the visualization of decidual cells. The immunohistochemical protocol as to similar studies was performed [Citation1,Citation2,Citation19]. The antibodies were used were MMP-2 (monoclonal 8B4 SANTA CRUZ), MMP-9 (monoclonal 4A3 SANTA CRUZ), EGFR (Epidermal Growth Factor Receptor monoclonal A10 SANTA CRUZ) and IL-8 (rabbit polyclonal NOVUS BIO).
Observation and statistics
The evaluation was performed microscopically on the cells of the intermediate trophoblast on decidua basalis and decidua parietalis of RPL and elective abortion material. All of the specimens were examined with an optical Zeiss™ microscope. The photographs attached to this study were taken using an Axion 208 color (ZEISS) camera attached to the microscope. The intensity of the staining was evaluated as negative (−), mild (+), moderate (++), and strong (+++), and was determined by two independent researchers. A distinct granular brown stain is scored as positive [Citation20–22]. Positive specimens should present at least 5% of granular stains (mild, up to 20%), followed by moderate (20 to 60%) and strong (>60%) [Citation20–22]. The statistical analysis of the results was performed with the program SPSS, version 24.0 (IBM, SPSS Inc., Chicago, IL, USA). The differences between both groups were presented at a contingency table and statistically evaluated with the Chi-Square Test (X2, p-value at .05).
Results
The comparison among tissue specimens of the two groups (RPL-elective abortion) revealed significant disparities in some cases. More specifically, MMP-9 antibody was significantly different among our two groups, both in trophoblast and the decidua parietalis (; ).
Figure 1. MMP-2 and MMP-9 expression on trophoblastic and decidual tissue specimens among the RPL and elective abortion (control) group. (A) Moderate expression of MMP-2 on RPL trophoblastic tissue (↑) (×10 magnification). (B) Moderate expression of MMP-2 on control trophoblastic tissue (↑) (×10 magnification). (C) Mild expression of MMP-2 on RPL decidual tissue (*) (×10 magnification). (D) Mild expression of MMP-2 on control decidual tissue (*) (×10 magnification). (E) Moderate expression of MMP-9 on RPL trophoblastic tissue (↑) (×10 magnification). (F) Negative expression of MMP-9 on control trophoblastic tissue (↑) (×10 magnification). (G) Moderate expression of MMP-9 on RPL decidual tissue (*) (×10 magnification). (H) Negative expression of MMP-9 on control decidual tissue (*) (×10 magnification). The graphs are an approximate percentage of staining for the specimens presented in this figure.
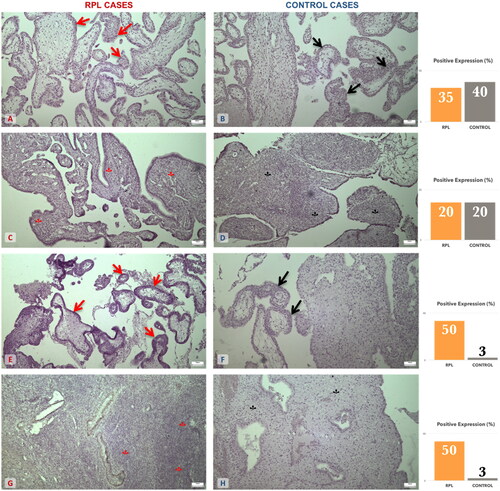
Table 1. MMP-2, MMP-9, EGFR and IL-8 intensities of staining on trophoblastic and decidual tissues. Comparisons among experimental (RPL) and control (electively abortion) groups.
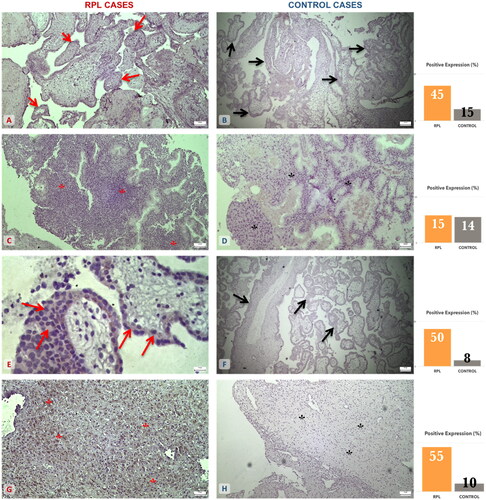
EGFR antibody revealed significant disparities in trophoblastic tissue and not in the decidua parietalis. IL-8 detection was marked statistically different both in the trophoblast and the decidua parietalis of the two groups (; ).
Figure 2. EGFR and IL-8 expression on trophoblastic and decidual tissue specimens among the RPL and elective abortion (control) group. (A) Moderate expression of EGFR on RPL trophoblastic tissue (↑) (×10 magnification). (B) Mild expression of EGFR on control trophoblastic tissue (↑) (×4 magnification). (C) Mild expression of EGFR on RPL decidual tissue (*) (×4 magnification). (D) Mild expression of EGFR on control decidual tissue (*) (×10 magnification). (E) Moderate expression of IL-8 on RPL trophoblastic tissue (↑) (×40 magnification). (F) Mild expression of IL-8 on control trophoblastic tissue (↑) (×4 magnification). (G) Moderate to strong expression of IL-8 on RPL decidual tissue (*) (×10 magnification). (H) Mild expression of IL-8 on control decidual tissue (*) (×4 magnification). The graphs are an approximate percentage of staining for the specimens presented in this figure.
Discussion
In the present study, an attempt was made to evaluate the detection of MMP-2, MMP-9, EGFR, and IL-8 in decidual and trophoblastic specimens of RPL cases. The present study managed to define the possible connection between early unexplained pregnancy loss and the detection of the proteolytic enzymes, matrix metalloproteinases, and the glycoprotein EGFR. Based on their role in the breakdown and reorganization of extracellular matrix and the placental development, this experimental protocol managed to examine both morphological and immunological dysregulation (IL-8), during the RPL phenomena. The present results were extracted through a single laboratory method, the immunohistochemistry of tissue specimens.
Evidence regarding MMP-2 and MMP-9 presents discrepancies about their implication with RPL. While some studies determined that the MMPs and their tissue inhibitors were increased in RPL, others reported decreased levels of these factors [Citation6]. In our study, the detection of MMP-2 was mild to moderate in both the control and abortion groups, while there was no detection of MMP-9 in the control group and moderate detection in the abortion group. Therefore, there are indications of MMP-9 involvement in RPL.
In an in vivo model of induced abortion, it was determined that MMP-2 and MMP-9 detection in induced abortion specimens was lower than those in spontaneous abortion cases, with the lowest detection in the control group [Citation23].
Similarly, an immunohistochemical study of MMP-9 and TIMP-3 observed that MMP-9 was higher in the decidua from spontaneous abortions than that in normal pregnancies [Citation24]. Nissi et al. assessed serum levels of MMP-2, MMP-9, and their tissue inhibitors. They found that serum MMP-9 and MMP-2/TIMP-2 ratios were elevated in spontaneous abortions, whereas tissue inhibitors were at lower levels in normal pregnancy cases [Citation24]. Sundrani et al. [Citation25] reported higher mRNA levels of MMP-1, −2, and −9 in the placentae of those delivering preterm as compared to term labor. The data obtained, showed that MMP-9 played a more important role than other MMPs and that invasion could not occur without MMP-9 in vitro. The findings in our study suggested that increased MMP-9 detection in spontaneous abortion and tubal pregnancy might result from an exaggerated, uncontrolled trophoblastic invasion as part of the RPL pathogenesis.
EGFR is examined in many research projects including those that study tumor angiogenesis, polycystic ovarian syndrome (PCOS), and preeclampsia. More precisely, findings presented that under hypoxic conditions, EGFR is significantly increased in tissue that is derived from preeclamptic placenta [Citation26,Citation27]. In addition, EGFR is involved in the proliferation and apoptosis of human ovarian granulosa cells (KGN) and as a result, it is related to the pathogenesis of polycystic ovary syndrome (PCOS) [Citation28]. Moreover, EGFR is overexpressed in about 50% of tumors in the endometrium and it is significantly related to the decreased survival rates of type II endometrial cancer [Citation29]. However, there is not enough information about a direct correlation between EGFR and RPL. In the present study, EGFR was detected in tissues from all clinical groups. Nevertheless, EGFR detection was increased in RPL cases in comparison with the induced abortion cases. In particular, regarding the RPL cases, the detection of EGFR was higher in decidual cells than in trophoblasts. The elevated level of EGFR in decidual cells is interpreted as exaggerated decidualization and as a reduced maternal immune tolerance. Some of the EGFRs ligands can be produced by several inflammatory cells, such as macrophages, eosinophils, neutrophils, and mast cells. Consequently, the activation of EGFR brings about cell migration, proliferation, and upregulation of cytokines that modulate the innate immune system [Citation30]. In contrast to our findings, Liu and Gong [Citation11], observed that EGFR was significantly decreased in placenta tissues derived from patients with spontaneous abortion compared to those of induced abortion cases. The low expression of EGFR is attributed to the presence of Long non-coding RNA (lncRNA) T-cell leukemia/lymphoma 6 (TCL6), which decreases the viability of trophoblast cells through the suspension of their proliferation ability [Citation11].
Regarding the abovementioned immune response, IL-8 and cytokines in general are the main factors involved in the normal functions of the placenta and play a crucial role in the implantation process. In this experiment, the levels of IL-8 appear to be elevated in the abortion group, in contrast to the control group. IL-8 may be produced by uNK during the implantation period. The presence of IL-8 leads to the release of MMP-2, MMP-9, PAI-1, PAI-2, and uPA, which indicates the activation of multiple protease mechanisms in the trophoblast [Citation17,Citation31]. Caballero-Campo et al. [Citation14] found that the expression of IL-8 was significantly up-regulated in Endometrial Epithelium Cells (EEC) in the presence of blastocysts, in contrast to the control EEC, according to which an embryo does not exist. It appears that IL-8 is mainly expressed in luminal and glandular epithelium and endothelial cells. It is normally elevated when fertilization occurs to achieve the implantation process. Even though IL-8 is not secreted by the embryo, the blastocyst contributes to the maternal mRNA expression and peptide production of IL-8 which leads to the hypothesis that there are two separate “functions”: the first is maternally controlled and the second is embryo related [Citation14]. Regarding the embryonic expression function, IL-8 is exerted through a direct mechanism that involves embryonic factors whose receptors are located in the maternal EEC. In another study, the examination field was a group of women who had not experienced a miscarriage but had a prolonged duration of pregnancy [Citation13]. This study showed that up-regulation of IL-8 in the third trimester leads to term delivery, in opposition to the poor expression which results in prolonged pregnancy. This finding suggests that IL-8 is the unique cytokine that may be associated with the induction of human delivery and its increase in the third term is highly correlated with labor [Citation13,Citation16].
Conclusively, there are indications of MMP-9 involvement in RPL. The findings suggested that increased MMP-9 detection in spontaneous abortion and tubal pregnancy might result from an exaggerated, uncontrolled trophoblastic invasion as part of the RPL pathogenesis. EGFR was detected in tissues from both clinical groups and it was moderately increased in RPL cases in comparison with the control cases. The elevated level of EGFR detection in decidual cells could be interpreted as exaggerated decidualization and as a case of reduced maternal immune tolerance. It is expected that the activation of EGFR is followed by cell migration, proliferation, and upregulation of cytokines that modulate the innate immune system. Indeed, the levels of IL-8 appear to be elevated in the RPL group, in contrast to the control group. Further studies should be conducted to draw safer conclusions regarding the implication of these molecules on the RPL phenomena.
Limitations of the study
This study has some limitations. The lack of other laboratory methods is the main limitation, in terms of raising results’ reliability. Also, the immunohistochemical evaluation of staining may be subjected to a small margin of error that authors try to erase, based on widely known and approved protocols. Finally, the number of participants is limited.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kavvadas D, Karachrysafi S, Anastasiadou P, et al. Immunohistochemical evaluation of CD3, CD4, CD8, and CD20 in decidual and trophoblastic tissue specimens of patients with recurrent pregnancy loss. Clin Pract. 2022;12(2):177–193.
- Papamitsou T, Fotiadou S, Papadopoulou K, et al. Evaluation of VEGF, BCL-2, BCL-6 by immunohistochemistry in the endometrial tissue of patients with recurrent pregnancy loss. Arch Hell Med. 2021;38(2):224–230.
- Anumba DO, El Gelany S, Elliott SL, et al. Circulating levels of matrix proteases and their inhibitors in pregnant women with and without a history of recurrent pregnancy loss. Reprod Biol Endocrinol. 2010;8(1):62.
- Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod. 1997;3(1):27–45.
- Goldman S, Shalev E. The role of the matrix metalloproteinases in human endometrial and ovarian cycles. Eur J Obstet Gynecol Reprod Biol. 2003;111(2):109–121.
- Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. IJMS. 2020;21(24):9739.
- Zhu J-Y, Pang Z-J, Yu Y-H. Regulation of trophoblast invasion: the role of matrix metalloproteinases. Rev Obstet Gynecol. 2012;5(3–4):e137-43.
- Niu R, Okamoto T, Iwase K, et al. Quantitative analysis of matrix metalloproteinases-2 and -9, and their tissue inhibitors-1 and -2 in human placenta throughout gestation. Life Sci. 2000;66(12):1127–1137.
- Shiomi T, Lemaître V, D'Armiento J, et al. Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol Int. 2010;60(7):477–496.
- Voldborg BR, Damstrup L, Spang-Thomsen M, et al. Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol. 1997;8(12):1197–1206.
- Liu L, Gong Y. LncRNA-TCL6 promotes early abortion and inhibits placenta implantation via the EGFR pathway. Eur Rev Med Pharmacol Sci. 2018;22(21):7105–7112.
- Maruo T, Matsuo H, Otani T, et al. Role of epidermal growth factor (EGF) and its receptor in the development of the human placenta. Reprod Fertil Dev. 1995;7(6):1465–1470.
- Ehsani V, Mortazavi M, Ghorban K, et al. Role of maternal interleukin-8 (IL-8) in normal-term birth in the human. Reprod Fertil Dev. 2019;31(6):1049–1056.
- Caballero-Campo P, Domínguez F, Coloma J, et al. Hormonal and embryonic regulation of chemokines IL-8, MCP-1 and RANTES in the human endometrium during the window of implantation. Mol Hum Reprod. 2002;8(4):375–384.
- Pitman H, Innes BA, Robson SC, et al. Altered expression of interleukin-6, interleukin-8 and their receptors in decidua of women with sporadic miscarriage. Hum Reprod. 2013;28(8):2075–2086.
- García-Velasco JA, Arici A. Chemokines and human reproduction. Fertil Steril. 1999;71(6):983–993.
- Sharma S, Godbole G, Modi D. Decidual control of trophoblast invasion. Am J Reprod Immunol. 2016;75(3):341–350.
- Dimitriadis E, Menkhorst E, Saito S. Recurrent pregnancy loss. Nat Rev Dis Prim. 2020;6(1):98.
- Kyratzoulis E, Kaletzi P, Chatzimichail F, et al. Immunohistochemical study of IL-10 and CD46 in placental tissues in recurrent pregnancy loss. Aristotle Biomed J. 2022;4(1):1–6.
- Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue – a review. Diagn Pathol. 2014;9:221.
- Yavasoglu I, Sargin G, Kadikoylu G, et al. Immunohistochemical evaluation of CD20 expression in patients with multiple myeloma. Rev Bras Hematol Hemoter. 2015;37(1):34–37.
- Papadopoulos E, Nikolaidou C, Kotini A, et al. A comparative immunohistochemical investigation of the consequences of chorioamnionitis on the developing human fetal spleen. Clin Exp Obstet Gynecol. 2017;44(1):30–38.
- Liu C, Gao Y, Guo Y. Relationship between the matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-3 in decidua tissue and spontaneous abortion. J Clin Exp Pathol. 2004;20:551–553.
- Nissi R, Talvensaari-Mattila A, Kotila V, et al. Circulating matrix metalloproteinase MMP-9 and MMP-2/TIMP-2 complex are associated with spontaneous early pregnancy failure. Reprod Biol Endocrinol. 2013;11(1):2.
- Sundrani DP, Chavan-Gautam PM, Pisal HR, et al. Matrix metalloproteinase-1 and -9 in human placenta during spontaneous vaginal delivery and caesarean sectioning in preterm pregnancy. Schunck W-H, editor. PLoS One. 2012;7(1):e29855.
- Hastie R, Brownfoot FC, Pritchard N, et al. EGFR (epidermal growth factor receptor) signaling and the mitochondria regulate sFlt-1 (soluble FMS-Like tyrosine kinase-1) secretion. Hypertension. 2019;73(3):659–670.
- Su MT, Lin SH, Chen YC, et al. Gene-gene interactions and gene polymorphisms of VEGFA and EG-VEGF gene systems in recurrent pregnancy loss. J Assist Reprod Genet. 2014;31(6):699–705.
- Zheng Q, Li Y, Zhang D, et al. ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex and improves ovary functions of PCOS rats. Cell Death Dis. 2017;8(10):e3145–e3145.
- Reyes HD, Thiel KW, Carlson MJ, et al. Comprehensive profiling of EGFR/HER receptors for personalized treatment of gynecologic cancers. Mol Diagn Ther. 2014;18(2):137–151.
- Balci M, Ozdemir G. Differential expression of egfr-1, mmp-3, and mmp-9 in spontaneous abortions, induced abortions, and tubal pregnancies. Turkish J Pathol. 2018;35(1):1–8.
- De Oliveira LG, Lash GE, Murray-Dunning C, et al. Role of interleukin 8 in uterine natural killer cell regulation of extravillous trophoblast cell invasion. Placenta. 2010;31(7):595–601.
