Abstract
Introduction
The efficacy of myo-inositol supplementation to treat gestational diabetes remains controversial, and this meta-analysis aims to study the efficacy of myo-inositol supplementation on metabolic status for gestational diabetes.
Methods
Several databases including PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases were systemically searched from inception to October 2021, and we included the randomized controlled trials (RCTs) assessing the effect of myo-inositol supplementation on the outcomes of women with gestational diabetes. Gestational diabetes was diagnosed if at least one threshold of glucose concentration was exceeded and the three thresholds included 92, 180, and 153 mg/dl for 0, 1 and 2 h, respectively, after a 75-g, 2-h glucose tolerance test.
Results
Four RCTs and 317 patients were included in this meta-analysis. Compared with routine treatment in pregnant women with gestational diabetes, myo-inositol supplementation could lead to remarkably decreased treatment requirement with insulin (odd ratio [OR] = 0.24; 95% confidence interval [CI] = 0.11–0.52; p = .0003) and homeostasis model assessment of insulin resistance (HOMA-IR, standard mean difference [SMD]= −1.18; 95% CI= −1.50 to −0.87; p < .00001), but demonstrated no obvious impact on birth weight (SMD= −0.11; 95% CI= −0.83 to 0.61 g; p = .76), cesarean section (OR = 0.82; 95% CI = 0.46–1.47; p = .51) or the need of NICU (OR = 0.88; 95% CI = 0.03–26.57; p = .94).
Conclusions
Myo-inositol supplementation is effective to decrease the need of insulin treatment and HOMA-IR for gestational diabetes.
Introduction
Gestational diabetes mellitus is defined as any degree of glucose intolerance with an onset during pregnancy [Citation1–4]. Pregnancy is associated with significant changes in hormonal and metabolic elements in order to ensure adequate fetal nutrition [Citation5,Citation6]. Dysregulation of insulin levels may increase the risk of gestational diabetes [Citation4,Citation7–9]. Gestational diabetes can cause some complications for women and offspring, including hypertensive disorders, fetal macrosomia, shoulder dystocia and neonatal hypoglycemia [Citation10–13].
The treatment of gestational diabetes is very crucial for maternal and neonatal outcomes. Inositol, an insulin sensitizing agent, was reported to have the potential in modulating insulin sensitivity in animals and humans with insulin resistance [Citation14,Citation15]. As one isomer of inositol, myo-inositol can produce insulin sensitizing effects and decrease the occurrence of gestational diabetes in women with the family history of type 2 diabetes mellitus [Citation16,Citation17]. Myo-inositol supplements were documented to substantially improve maternal and fetal outcomes in patients with high risk for gestational diabetes [Citation15,Citation18,Citation19].
Several studies explored the efficacy of myo-inositol supplements for pregnant women with gestational diabetes, but the results remained controversial [Citation20–22]. Therefore, we performed this meta-analysis to study the efficacy of myo-inositol on metabolic status for gestational diabetes.
Materials and methods
This meta-analysis was performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [Citation23]. Since this was a meta-analysis of previously published studies, thus ethical approval and patient consent were not required.
Search strategy and study selection
We have searched the databases including PubMed, EMbase, Web of science, EBSCO and Cochrane library databases from inception to October 2021 by using the keywords: “myo-inositol” AND “gestational diabetes”. The inclusive selection criteria were presented as follows: (i) study design was RCT, (ii) patients were diagnosed with gestational diabetes; (iii) intervention treatments were myo-inositol supplementation versus no myo-inositol supplementation. All women obtained the routine supplementation with folic acid, iron, calcium and vitamin D.
Data extraction and outcome measures
The following information were collected: author, number of women, age, gestational age, body mass index, fasting glucose, detail methods, time for treatment initiation and treatment duration between two groups. Data were extracted independently by two investigators, and discrepancies were resolved by consensus. The primary outcomes were treatment requirement with insulin and homeostasis model assessment of insulin resistance (HOMA-IR). HOMA-IR was evaluated at 8 weeks after the treatment invention. Secondary outcomes included birth weight, cesarean section and the need of Neonatal Intensive Care Unit (NICU) which suggested the serious neonates who required the care of NICU.
Quality assessment in individual studies
We evaluated the methodological quality of the included studies by using the modified Jadad scale [Citation24], which consisted of three items: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points). The score of Jadad Scale varied from 0 to 5 points. Jadad score ≤ 2 suggested low quality, while Jadad score ≥ 3 indicated high quality [Citation25].
Statistical analysis
We calculated standard mean difference (SMD) with 95% confidence interval (CI) for continuous outcomes and odd ratio (OR) with 95% CIs for dichotomous outcomes. I2 statistic was applied to assess heterogeneity, and I2 > 50% indicated significant heterogeneity [Citation26]. Random-effects model was used regardless of heterogeneity. We detected the potential heterogeneity via omitting one study in turn for the meta-analysis or performing subgroup analysis. All statistical analyses were performed using Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
Results
Literature search, study characteristics and quality assessment
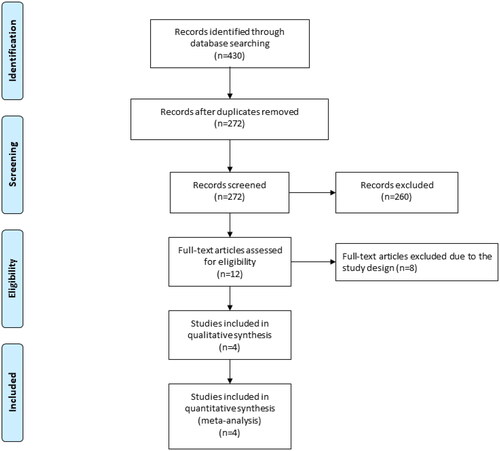
demonstrated the flowchart of the search and selection results. Initially, 430 relevant articles were found, and four eligible RCTs were finally included in the meta-analysis [Citation15,Citation20–22]. showed the baseline characteristics of the eligible RCTs in the meta-analysis. They were published between 2011 and 2021, and total sample size was 327. The doses of myo-inositol ranged from 2000 to 4000 mg daily.
Table 1. Characteristics of included studies.
Among the four studies included here, three studies reported treatment requirement with insulin [Citation20–22], two studies reported HOMA-IR [Citation15,Citation21], three studies reported birth weight and cesarean section [Citation20–22] and two studies reported the need of NICU [Citation20,Citation21]. All included studies were thought to have high quality because their Jadad scores were 3.
Primary outcomes: treatment requirement with insulin and HOMA-IR
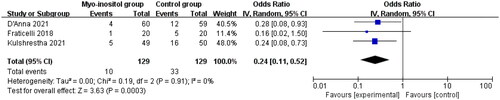
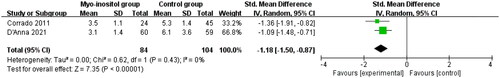
Compared with routine treatment for gestational diabetes, myo-inositol supplementation was associated with significantly reduced treatment requirement with insulin (OR = 0.24; 95% CI = 0.11–0.52; p = .0003) with no heterogeneity remained among the studies (I2=0%, heterogeneity p = .91, ) and HOMA-IR (SMD= −1.18; 95% CI= −1.50 to −0.87; p < .00001) with no heterogeneity remained among the studies (I2=0%, heterogeneity p = .43, ).
Sensitivity analysis and publication bias
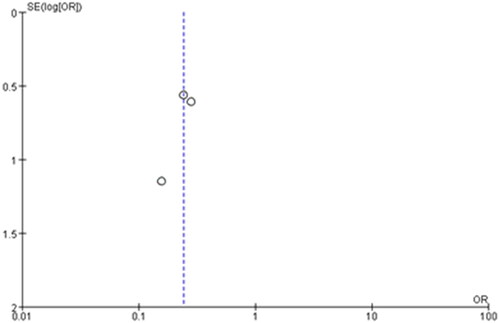
No heterogeneity was seen among the included studies, and thus we did not perform the sensitivity analysis by omitting one study in turn. The funnel plots were relatively symmetrical, and all studies almost fell within the 95% CI axis. There was little evidence of publication bias ().
Secondary outcomes
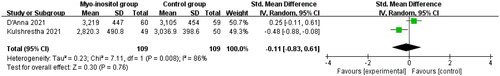
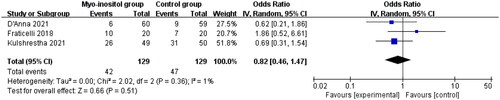
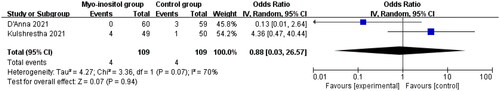
Compared to control group in gestational diabetes, myo-inositol supplementation showed no impact on birth weight (SMD= −0.11; 95% CI= −0.83 to 0.61 g; p =.76; ), cesarean section (OR = 0.82; 95% CI = 0.46–1.47; p = .51; ) or the need of NICU (OR = 0.88; 95% CI = 0.03–26.57; p = .94; ).
Discussion
Our meta-analysis included four RCTs and 327 patients with gestational diabetes. The results found that myo-inositol supplementation can significantly reduce the need of insulin treatment and HOMA-IR, but resulted in no improvement in other outcomes including birth weight, cesarean section or the need of NICU.
Pregnancy commonly leads to physiologically hyperinsulinemia and insulin resistance [Citation27], and insulin sensitivity is reduced to 50–70% lower compared with the pre-pregnancy status during the third trimester [Citation28]. Inositol has nine stereoisomers, and myo-inositol is the most common one [Citation15]. Inositol and inositol derivatives display an important role in both insulin mimics and second messengers. Their complex interactions benefit to both glucose and lipid metabolism across tissue types and in many species [Citation29]. The efficacy of myo-inositol to treat gestational diabetes relies on its function as an insulin sensitizer substance to improve glucose homeostasis [Citation30,Citation31].
Our results confirmed the efficacy of myo-inositol supplementation to reduce treatment requirement of insulin and HOMA-IR in women with gestational diabetes, suggesting that myo-inositol supplementation should be recommended for the translation into clinical practice. However, myo-inositol was administered at the doses ranging from 2000 to 4000 mg daily among the included RCTs, and more studies should be conducted to find the ideal dose of myo-inositol supplementation. In addition, it is necessary to study the best time points to begin myo-inositol supplementation for pregnant women with gestational diabetes in order to improve its efficacy. In addition, the window of opportunity to treat gestational diabetes is narrow after the diagnosis, since typically it is only diagnosed at 24–28 weeks and 8 weeks of treatment is need.
There are several potential limitations. Firstly, our analysis is based on only for RCTs with small sample sizes and more RCTs are needed to confirm our findings. Secondly, although no significant heterogeneity remained in this meta-analysis, different doses and combination of myo-inositol supplementation may produce some bias. Thirdly, a major limitation of all the trials is the lack of blinding, which may cause some heterogeneity for the pooling results. Fourthly, different risk levels in pregnant women with gestational diabetes (e.g. weight, BMI, obesity and lipid metabolism) can affect the efficacy assessment of myo-inositol supplementation to treat gestational diabetes. Fifthly, the included RCTs did not provide the information about the ineffectiveness of lifestyle changes, which may affect the efficacy assessment of myoinositol. Sixthly, only Italian women were included for the primary outcomes, and thus more other populations were needed to confirm our findings.
Conclusions
This meta-analysis confirms that myo-inositol supplementation is effective to reduce the need of insulin treatment and HOMA-IR in women with gestational diabetes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wei J, Heng W, Gao J. Effects of low glycemic index diets on gestational diabetes mellitus: a meta-analysis of randomized controlled clinical trials. Medicine. 2016;95(22):e3792-e3792. doi:10.1097/MD.0000000000003792.
- Chiefari E, Arcidiacono B, Foti D, et al. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. 2017;40(9):899–909. doi:10.1007/s40618-016-0607-5.
- Dias S, Pheiffer C, Abrahams Y, et al. Molecular biomarkers for gestational diabetes mellitus. IJMS. 2018;19(10):2926. doi:10.3390/ijms19102926.
- Xu J, Ye S. Influence of low-glycemic index diet for gestational diabetes: a meta-analysis of randomized controlled trials. J Mater Fetal Neonat Med. 2020;33(4):687–692. doi:10.1080/14767058.2018.1497595.
- Johns EC, Denison FC, Norman JE, et al. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29(11):743–754. doi:10.1016/j.tem.2018.09.004.
- Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207–2225. doi:10.1001/jama.2017.3635.
- McIntyre HD, Catalano P, Zhang C, et al. Gestational diabetes mellitus, nature reviews. Disease Primers. 2019;5(1):47.
- Mack LR, Tomich PG. Gestational diabetes: diagnosis, classification, and clinical care. Obstet Gynecol Clin North Am. 2017;44(2):207–217. doi:10.1016/j.ogc.2017.02.002.
- Moon JH, Kwak SH, Jang HC. Prevention of type 2 diabetes mellitus in women with previous gestational diabetes mellitus. Korean J Intern Med. 2017;32(1):26–41. doi:10.3904/kjim.2016.203.
- Schwartz N, Green MS, Yefet E, et al. Modifiable risk factors for gestational diabetes recurrence. Endocrine. 2016;54(3):714–722. doi:10.1007/s12020-016-1087-2.
- Spaight C, Gross J, Horsch A, et al. Gestational diabetes mellitus. Endocrine Develop. 2016;31:163–178.
- Dominguez-Vigo P, Alvarez-Silvares E, Alves-Perez MT, et al. Incidence and clinical risk factors for the development of diabetes mellitus in women with previous gestational diabetes. Ginecologia y Obstetricia de Mexico. 2016;84(4):228–242.
- Rasmussen L, Poulsen CW, Kampmann U, et al. Diet and healthy lifestyle in the management of gestational diabetes mellitus. Nutrients. 2020;12(10):3050. doi:10.3390/nu12103050.
- Larner J, Brautigan DL, Thorner MO. D-chiro-inositol glycans in insulin signaling and insulin resistance. Mol Med. 2010;16(11–12):543–552. doi:10.2119/molmed.2010.00107.
- Corrado F, D'Anna R, Di Vieste G, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med. 2011;28(8):972–975. doi:10.1111/j.1464-5491.2011.03284.x.
- D'Anna R, Scilipoti A, Giordano D, et al. myo-Inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: a prospective, randomized, placebo-controlled study. Diabetes Care. 2013;36(4):854–857. doi:10.2337/dc12-1371.
- Costabile L, Unfer V. Treatment of gestational diabetes mellitus with myo-inositol: analyzing the cutting edge starting from a peculiar case. Eur Rev Med Pharmacol Sci. 2017;21(2 Suppl):73–76.
- Matarrelli B, Vitacolonna E, D’angelo M, et al. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial. J Mater Fetal Neonat Med. 2013;26(10):967–972. doi:10.3109/14767058.2013.766691.
- Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. doi:10.1056/NEJMoa042973.
- Kulshrestha V, Balani S, Kachhawa G, et al. Efficacy of myoinositol in treatment of gestational diabetes mellitus in Asian Indian women: a pilot randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2021;260:42–47. doi:10.1016/j.ejogrb.2021.02.017.
- D'Anna R, Corrado F, Loddo S, et al. Myoinositol plus α-lactalbumin supplementation, insulin resistance and birth outcomes in women with gestational diabetes mellitus: a randomized, controlled study. Sci Rep. 2021;11(1):8866. doi:10.1038/s41598-021-88329-x.
- Fraticelli F, Celentano C, Zecca IA, et al. Effect of inositol stereoisomers at different dosages in gestational diabetes: an open-label, parallel, randomized controlled trial. Acta Diabetol. 2018;55(8):805–812. doi:10.1007/s00592-018-1157-4.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi:10.1016/j.jclinepi.2009.06.005.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi:10.1016/0197-2456(95)00134-4.
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135(11):982–989. doi:10.7326/0003-4819-135-11-200112040-00010.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi:10.1002/sim.1186.
- Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Semin Fetal Neonatal Med. 2009;14(2):66–71. doi:10.1016/j.siny.2008.09.004.
- Catalano PM, Tyzbir ED, Roman NM, et al. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165(6 Pt 1):1667–1672. doi:10.1016/0002-9378(91)90012-g.
- Watkins OC, Yong HEJ, Sharma N, et al. A review of the role of inositols in conditions of insulin dysregulation and in uncomplicated and pathological pregnancy. Crit Rev Food Sci Nutr. 2022;62(6):1626–1673. doi:10.1080/10408398.2020.1845604.
- Morgante G, Massaro MG, Di Sabatino A, et al. Therapeutic approach for metabolic disorders and infertility in women with PCOS. Gynecol Endocrinol. 2018;34(1):4–9. doi:10.1080/09513590.2017.1370644.
- Giordano D, Corrado F, Santamaria A, et al. Effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome: a perspective, randomized, placebo-controlled study. Menopause. 2011;18(1):102–104. doi:10.1097/gme.0b013e3181e8e1b1.