Abstract
Background
The purpose of this study was to improve diagnostic and therapeutic standards by examining the clinical features, treatment, and prognosis of fetal meconium peritonitis (FMP), as well as the diagnostic efficacy of ultrasound for FMP.
Methods
The clinical data of 41 infants and pregnant women diagnosed with meconium peritonitis (MP) and treated at the Fujian Maternal and Child Health Hospital from January 2013 to January 2020 were analyzed retrospectively. Clinical data, imaging data, complications, treatment strategies, pregnancy outcomes, neonatal prognoses, and follow-up outcomes were all analyzed.
Results
The MP prenatal diagnosis rate was 56.1% (23/41), the neonatal surgery rate was 53.7% (22/41), and the survival rate was 85.4% (35/41). Intraperitoneal calcification (23 pregnant women, 56.1%), intestinal dilatation (13 pregnant women, 31.7%), peritoneal effusion (22 pregnant women, 53.7%), intraperitoneal pseudocyst (7 pregnant women, 17.1%), and polyhydramnios were diagnosed via prenatal ultrasound (18 pregnant women, 43.9%). Twenty-two pregnant women were assigned to the surgical treatment (operation) group, while 18 were assigned to the conservative treatment group. In the operation group, there were 9 cases of ileal atresia (40.9%), 7 cases of jejunal atresia (31.8%), 2 cases of atresia at the jejunum-ileum junction (9.1%), 2 cases of ileal perforation (9.1%), 1 case of ileal necrosis (4.5%), and 1 case of adhesive obstruction (4.5%). There was no statistically significant difference (p > .05) in the occurrence of various prenatal ultrasound findings by etiology.
Conclusion
Multiple prenatal ultrasound markers have been identified for MP. To improve the efficacy of newborn treatment for FMP and reduce neonatal mortality, dynamic monitoring of ultrasound image alterations and strengthened integrated perinatal management are necessary.
Background
Intestinal perforation during pregnancy and subsequent meconium leakage into the abdominal cavity during the fetal period can lead to a condition called fetal meconium peritonitis (FMP), an aseptic chemical peritonitis [Citation1]. FMP is one of the most prevalent acute abdominal conditions in neonates. Symptoms include abdominal distention and vomiting shortly after birth, caused by peritonitis and intestinal blockage. FMP affects about one in every 30,000 live births [Citation2, Citation3]. Prenatal ultrasound scans are typically used to detect FMP because they enable a dynamic assessment in conjunction with Doppler tests. Neonates with a prenatal diagnosis of FMP require continuous monitoring and can benefit from customized treatment right after birth to improve their chances of survival. Hence, extensive multidisciplinary coordination and standardized, integrated perinatal management and treatment are crucial.
Recent reports indicate that the neonatal survival rate has risen to an estimated 80–92.3% [Citation4, Citation5]. As there have been only a few documented cases of FMP, most medical professionals are in the dark about the disease’s prognosis. Neonates frequently require emergency intestinal blockage surgery due to abdominal distention and vomiting. The elevated diaphragm, the compression of the heart and lungs, and the development of pulmonary dysplasia are all major factors in a poor prognosis and are brought on by the extensive ascites caused by persistent leakage of meconium [Citation6]. The objective of this study was to learn more about FMP by retrospectively analyzing the medical records of 41 pregnant women who were diagnosed with FMP and were treated at the Fujian Maternal and Child Health Hospital, which is affiliated with Fujian Medical University. This paper serves as a useful resource for the early detection, supervision, intervention, and treatment of FMP by summarizing the clinical characteristics, imaging features, complications, treatment approaches, and pregnancy outcomes associated with this condition.
Data and methods
This was a retrospective study. In this study, we included 41 fetuses and pregnant women who were diagnosed with FMP between January 1, 2013 and December 31, 2020, either by prenatal ultrasound scan or surgical exploration (i.e. FMP was confirmed and classified via surgical exploration and diagnosis). All pregnant women gave informed consent for prenatal ultrasound examination. A retrospective review was conducted on 41 fetuses with FMP to examine their prenatal ultrasonographic characteristics, neonatal clinical characteristics, postoperative diagnoses, and outcomes. In one case, an amniotic injection of Rivanol was used to induce labor for an abortion; hence, the neonatal outcome in this case was not discussed. Cases where emergency surgery was required based on the imaging findings or symptoms of intestinal obstruction or peritonitis (abdominal distension, vomiting, etc.) were included in the surgical treatment (operation) group. The rest were assigned to the conservative treatment group. Surgical exploration and diagnosis were used to classify fetal intestinal atresia and the perforation site. We compared the results of prenatal ultrasound scans with those of surgical explorations to arrive at our diagnoses.
Diagnostic criteria
Based on the presence of intraperitoneal calcification, prenatal ultrasound scan was used to diagnose FMP. The following ultrasound indications of calcification were observed either alone or in combination with one another: (1) peritoneal effusion, (2) intestinal dilatation, (3) intraperitoneal pseudocysts, and (4) polyhydramnios ( and ) [Citation7]. At least two ultrasound physicians with prenatal diagnosis training performed routine fetal ultrasound examinations, assessed fetal growth indicators and determined the fetus’ gestational age. If aberrant fetal abdominal cavity echoes were identified, an ultrasound scan was performed every 2–4 weeks [Citation6].
Figure 1. Intraperitoneal calcification in a prenatal ultrasound scan. AS: ascites; STO: stomach. The bold arrows indicate calcification on the surface of the liver and intestines.
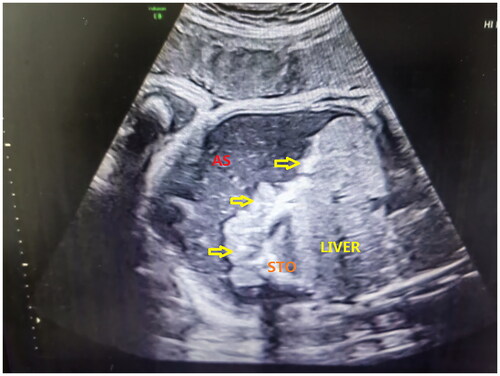
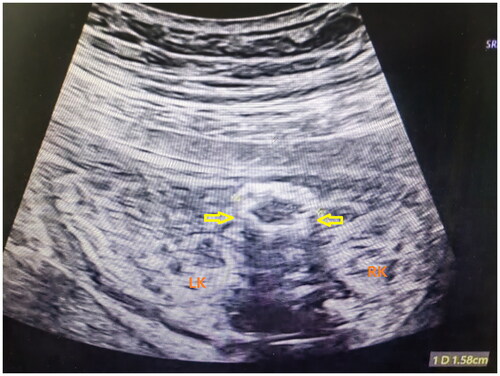
Figure 2. Intestinal dilatation in a prenatal ultrasound scan. RK: right kidney; LK: left kidney; Thick arrows represent calcified, thickened, and dilated intestinal tube walls.
In this paper, we classified cases based on the following ultrasound manifestations: Grade 0 was assigned to patients with a single abnormal ultrasound manifestation, Grade 1 to those with two abnormal ultrasound manifestations, Grade 2 to those with three abnormal ultrasound manifestations, and Grade 3 to those with four or more abnormalities or an abnormal ultrasound appearance. Cases with varying grades in multiple ultrasound examinations were categorized based on the results of the most recent scan.
Clinical intervention
The delivery method of FMP fetuses was determined based on obstetric considerations, and physicians and surgeons were present in the delivery room at the time of birth. The condition was evaluated using plain abdominal standing X-ray films and abdominal ultrasound scans. When it was deemed necessary, abdominal computed tomography was used to make a definitive diagnosis. If diagnostic imaging and clinical evaluation indicated complete intestinal obstruction or intestinal perforation, surgery was performed. The neonates were subject to routine monitoring.
Grouping
Forty-one pregnant women had a prenatal diagnosis of FMP, and all of them were dynamically monitored during pregnancy; however, one case received a Rivanol amniotic injection to induce labor for abortion, and so the neonatal outcome of this case is not discussed and included in the group. An ultrasound was used as a diagnostic tool prior to the birth. Peritonitis, or intestinal blockage, is exhibited postnatally by symptoms such as abdominal distention and vomiting. During the imaging examinations, doctors investigated the possibility of an intestinal obstruction or perforation. Some cases require immediate surgical intervention, and these patients were included in the surgical treatment group. The remaining pregnant women were assigned to the conservative treatment group. The surgical therapy group included 22 pregnant women, while the conservative treatment group included 18 women.
Observation indexes
The medical history, clinical manifestations, laboratory results, imaging data, genetic diagnosis, pregnancy complications, timing and method of pregnancy termination, perinatal delivery outcome, postnatal intervention, and prognosis of the pregnant women were systematically collected and documented. The imaging data were reread and graded based on the diagnostic criteria.
Statistical analysis
The data was analyzed using SPSS 22.0 statistical software. Multiple means were compared using a univariate analysis of variance for normally distributed measurement data expressed as mean ± standard deviation (x ± SD). Count data are expressed as frequency or percentage and evaluated with the chi-squared test or Fisher’s exact probability test. A p value <.05 was considered statistically significant.
Results
Between January 1, 2013 and December 30, 2020, 114,376 pregnant women delivered or underwent an abortion at the Fujian Maternal and Child Health Hospital. Forty-one of these pregnancies, or 0.013% were affected by FMP. These pregnant women ranged in age from 19 to 42 years, with a mean age of 28.5 years. Twenty primipara and twenty-one multipara pregnancies were conceived normally. In one instance involving a female infant delivered vaginally, abdominal obstructive dystocia occurred during labor, and neonatal intestinal obstruction was identified after birth. Despite this, the treatment was terminated due to severe asphyxia. Another case was terminated by Rivanol-induced labor at 33+ weeks of gestation. When comparing the general data of neonates in the surgical treatment group and the conservative treatment group, there were no significant variations between the two groups, such as the age of the mothers ().
Table 1. Comparison of general data of neonates between the operation group and conservative treatment group (X ± SD).
The initial FMP diagnosis was made via ultrasound between 20 and 37 weeks of gestation. Twenty-four women (24/41, 58.6%) were diagnosed between 20 and 25 weeks of gestation; 16 women (16/41, 39.0%) were diagnosed between 25 and 34 weeks of gestation; and 1 woman (1/41, 2.4%) was diagnosed after 34 weeks of gestation. The initial ultrasound imaging results of 41 pregnant women diagnosed with meconium peritonitis (MP) were as follows: 15 pregnant women (15/41, 36.6%) had intra-abdominal calcification, 14 (14/41, 34.1%) had peritoneal effusion, 14 (14/41, 34.1%) had intestinal dilatation, 9 (9/41, 22.0%) had hydramnios, and 1 (1/41, 2.4%) had pseudocysts. When ultrasound abnormalities were initially detected, 17 (17/41, 41.5%) of the women had two abnormal ultrasound manifestations, 16 (16/41, 39.0%) had one abnormal ultrasound manifestation, 6 (6/41, 14.6%) had three abnormal ultrasound manifestations, and 2 (2/21, 4.9%) had four abnormal ultrasound manifestations. Of the 41 pregnant women, 39 were monitored using dynamic ultrasound monitoring up until the time of delivery. Out of these, nineteen showed worsening abnormal ultrasound findings, four showed findings identical to the first ultrasound, and six showed improvement, with indicators such as fetal peritoneal effusion, polyhydramnios, intestinal dilatation, and pseudocysts regressing on their own.
According to the prenatal ultrasound examination, there were statistically significant differences between the surgical treatment and conservative treatment groups in the occurrences of intestinal dilatation (odds ratio [OR] 3.167, 95% confidence interval [CI]: 1.034–9.699), intraperitoneal calcification (OR: 4.894, 95% CI: 1.691–14.166), and peritoneal effusion (OR: 0.531, 95% CI: 0.283–0.999). Local echo enhancement of the intestinal tract, intraperitoneal pseudocysts, and polyhydramnios were not significantly different (p > .05) (). The ultrasound indexes of five neonates in the conservative group increased throughout the dynamic follow-ups.
Table 2. Results of prenatal ultrasonography in the operation group and conservative treatment group.
The nuchal translucency measurements of 15 pregnant women revealed no abnormalities. Fifteen pregnant women underwent interventional amniocentesis, single-nucleotide polymorphism array monitoring, cytomegalovirus (CMV) testing, and human parvovirus B19 testing. Consequently, it was revealed that two pregnant women had chromosomal 9 inversion and one had CMV infection in the amniotic fluid. All six pregnant women subjected to noninvasive DNA testing were found to be at low risk. By examining the prenatal infection index, seven cases of prior CMV infection and six cases of past rubella infection were identified.
There were a total of 41 births, 26 of which were male and 15 were female. One patient had an abortion induced with a Rivanol amniotic injection because of fetal abdominal calcification, significant peritoneal effusion, intestinal dilatation, and hydramnios, all of which occurred in the third trimester. Twenty-two pregnant women had cesarian sections due to medical complications such as scarred uterus, fetal distress, intracranial pressure, or placenta previa. Between 34 and 36 weeks, 17 babies were born prematurely, while the rest of the pregnant women went into labor normally. There were 23 full-term pregnancies, and the average birth weight of these infants was 3239.83 ± 89.98 g. Seven pregnant women had grade III amniotic fluid, while five had grade II amniotic fluid. There were also three cases of neonatal asphyxia. Chorionic inflammation was found in six of the 17 cases when a pathological investigation of the placenta was conducted.
The correlation between ultrasound performance grade and the frequency of operations was as follows: Of the 41 cases of FMP, 11 were classified as grade 0, 16 were grade 1, 7 were grade 2, and 7 were grade 3. Among them, 3, 10, 5, and 4 cases were surgically treated, respectively. The rate of surgery did not vary substantially across groups (p = .247) ().
Due to the severity of their condition, treatment for three neonates was discontinued, while 22 underwent surgical treatment and 15 received conservative treatment. Three infants were diagnosed with bilateral testicular hydroceles, and four were diagnosed with unilateral testicular hydroceles. Surgery on 22 neonates revealed ileal atresia in 9 cases (40.9%), jejunal atresia in 7 (31.8%), atresia at the jejunum-ileum junction in 2 (9.1%), and ileal perforation in 2 (9.1%). There was one case each (4.5%) of intestinal dysplasia, ileal necrosis, adhesive obstruction, short-small intestine, Meckel diverticulum, and intestinal dysplasia. Intestinal atresia was diagnosed in 13 neonates, while 5 had resection and anastomosis and 3 had enterostomy (). One infant’s surgery was terminated due to congenital intestinal atresia type IV, and in another case, treatment was discontinued after surgery. Postoperative pathology confirmed the FMP diagnosis.
Table 3. Surgical method in the operation group.
All neonates were subjected to follow-ups. Ten pregnant women in the surgical treatment group reported symptoms such as diarrhea, vomiting, and intestinal congestion; seven of these pregnant women were hospitalized for reoperation. Four of these seven pregnant women underwent intestinal adhesion relaxation, while three underwent fistula closure. Low-grade incomplete intestinal blockage and amputation syndrome were some of the frequent postoperative complications following the second operation. However, after receiving symptomatic treatment, all of the newborns made a full recovery and were released. In the conservative treatment group, one infant died 13 h and 30 min after birth, one infant underwent right testicular descent fixation 9 months after birth, and the remaining infants developed normally without experiencing any discomfort during subsequent follow-ups ().
Table 4. The maternal complications in different groups.
Discussion
Approximately half of FMP cases have idiopathic causes, which means their causes are unknown [Citation8]. CMV infection is typically linked to congenital intrauterine infection, which causes mesenteric vasculitis, which in turn causes intestinal necrosis and perforation, and hence FMP. Children with congenital toxoplasmosis are typically identified as having the condition more than a month after birth. These include hepatosplenomegaly, ascites, pericarditis, pneumonia, diarrhea, hypothermia, jaundice, and bleeding [Citation9, Citation10]. Meconium intestinal obstruction occurs in the first few weeks of life and is the earliest symptom of cystic fibrosis (10–20% of cases) [Citation11]..
In the present study, the ratio of male to female neonates was 26:15. The increased incidence among males is consistent with prior research findings [Citation6, Citation12]. Familial intrahepatic cholestasis syndrome has been linked to the emergence of MP [Citation13, Citation14]. In this study, 10 neonates with MP were born to mothers with hypercholesterolemia during pregnancy. Cholic acid, which binds taurine, may promote inflammation by stimulating the production of proinflammatory cytokines by hepatocytes, which then leads to the release of inflammatory mediators and cell damage [Citation15, Citation16]. In this study, AHP was present in 6 of the moms of MP newborns, and ICP was present in 4 of them. Pregnant women with AHP had elevated total bile acid (TBA) levels, which were discovered at less than 24 weeks of pregnancy.
An ultrasound scan is the primary prenatal diagnostic test for MP. Furthermore, ultrasound manifestations of MP vary and fluctuate in unpredictable ways. Inflammatory ascites develop when meconium leaks through an intestinal rupture and into the abdominal cavity. The perforation can be sealed by fiber adhesion if calcium salt is applied at the spot. However, a pseudocyst forms if the exudate collects locally and adheres to the greater omentum and intestine. The most prominent symptom of FMP is intraperitoneal calcification, which is a result of the disease. However, the absence of calcification does not rule out the possibility of FMP. There are also limitations associated with ultrasound examinations. For instance, fetal intestinal atresia is notoriously hard to pinpoint using ultrasound technology. However, magnetic resonance imaging (MRI) makes it much simpler to pinpoint the exact location of intestinal atresia and perforation since it reveals the fetal abdomen in high resolution. When MP is detected during prenatal ultrasound, an MRI can help confirm the diagnosis.
Ultrasound clinical manifestations and their relationship with prognosis
Ultrasound diagnosis of FMP is most common after 20 weeks of gestation due to the timing of prenatal malformation screening, which is typically between 20 and 24 weeks [Citation4]. Additionally, meconium cannot enter the abdominal cavity easily by intestinal rupture before 20 weeks because the fetus lacks intestinal peristalsis [Citation17,Citation18]. FMP was diagnosed in 61.0% of cases before 28 weeks of gestation and in 38.9% of instances after 28 weeks. This shows that the likelihood of neonatal surgical treatment for FMP increases with an earlier diagnosis. There was no statistically significant difference between the conservative and operation groups with regard to the average gestational age at first detection of abnormal ultrasound characteristics, which was 26.11 ± 3.41 weeks gestation in the conservative group and 27.72 ± 4.97 weeks gestation in the operation group (t = −1.171, p = .249). Possible explanations for the discrepancies between this study and prior research include the relatively small number of FMP cases, increased uniformity in prenatal testing, and the advent of color Doppler ultrasound technology. To improve the prognosis of neonates with MP, it is clear that prenatal screening, consultation, and diagnosis must adhere to strict standards.
Since intestinal dilatation, peritoneal effusion, intra-abdominal calcification, and intra-abdominal cysts are typically treated surgically, it is necessary to monitor changes in ultrasound features during follow-ups in cases where fetal intestinal dilation and obstruction are initially detected [Citation18–20]. The most prevalent cause of MP is fetal congenital intestinal obstruction, and it has been discovered that the rate of neonatal surgery correlates closely with the rate of ultrasound-observed intestinal dilatation [Citation19]. Fetal peritoneal effusion, abdominal calcification, and polyhydramnios accounted for the bulk of the cases in which abnormal ultrasound signs were identified during dynamic ultrasound monitoring, which occurred in 19 cases in this study. Intraperitoneal calcification is the primary sign of simple MP without additional symptoms. Abnormal ultrasound images showing one or more MP indications and intra-abdominal calcification are both present in patients with complex MP (CMP). Surgery is necessary for 50% of CMP cases. A higher incidence of surgeries after delivery has been linked to more abnormal ultrasound images being captured during pregnancy [Citation7, Citation21]. Therefore, the final ultrasound images taken just prior to birth have a higher direct diagnostic value for evaluating the fetal prognosis [Citation22]..
During the perinatal period, specialists in obstetrics, neonatal pediatrics, and pediatric surgery can work together to care for newborns who have received a definitive prenatal diagnosis [Citation23]. In this study, neonates with FMP were closely monitored, and their condition was communicated to neonatologists and pediatric surgeons in advance so that they could prepare for delivery. Neonates were transferred immediately to the pediatric surgery department right after delivery to undergo fasting, total parenteral nutrition, electrolyte imbalance treatment, and gastrointestinal decompression if necessary.
In this study, there was an evaluation of the diagnostic value of ultrasound in the diagnosis of FMP by comparing neonates with various treatment modalities and assessing preoperative ultrasound examinations. However, there are numerous restrictions. First, this was a retrospective study, and there may have been potential for selection bias. Participants were screened for FMP using ultrasound; if no lesion was found, they were excluded. Therefore, FMP diagnosis relies on the technical competence of the ultrasound specialist and the accuracy of the ultrasound equipment, without which cases may be missed or misdiagnosed. Therefore, it is possible that there could have been a missed prenatal diagnosis. Second, the follow-up procedure for this study was conducted via telephone, which leaves room for error in the professional interpretation of follow-up results. Thirdly, the follow-up period in the study was relatively brief, which creates difficulties for professional assessment of gastrointestinal function. More extensive long-term follow-ups are needed to objectively and accurately assess the complications of FMP.
Prenatal ultrasound can detect a range of FMP presentations. Conservative FMP treatment is indicated for a favorable prognosis in pregnant women without gastrointestinal symptoms, although surgery is the primary treatment for FMP and can enhance the survival rate. In order to improve the efficacy of neonatal treatment for FMP and reduce neonatal mortality, it is crucial to dynamically monitor changes in ultrasound images and increase perinatal management. More focus should be placed on nutrient intake throughout infancy for neonates with FMP to prevent growth and development issues.
Ethical approval
I confirm that I have read the Editorial Policy pages. This study was conducted with approval from the Ethics Committee of Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants’ guardian.
Authors contributions
ZXQ and WDM conceived the idea and conceptualized the study. CX, LJX and WXC collected the data and analyzed the data. RKH, LHQ and YJY drafted the manuscript, then ZXQ and XRL reviewed the manuscript. All authors read and approved the final draft.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Additional information
Funding
References
- Eckoldt F, Heling KS, Woderich R, et al. Meconium peritonitis and pseudo-cyst formation: prenatal diagnosis and post-natal course. Prenat Diagn. 2003;23(11):1–8. doi: 10.1002/pd.720.
- Saleh N, Geipel A, Gembruch U, et al. Prenatal diagnosis and postnatal management of meconium peritonitis. J Perinat Med. 2009;37(5):535–538. doi: 10.1515/JPM.2009.097.
- Wang K, Cai S, He L, et al. Pediatric gastric perforation beyond neonatal period: 8-year experience with 20 patients. Pediatr Neonatol. 2019;60(6):634–640. doi: 10.1016/j.pedneo.2019.03.006.
- Nam SH, Kim SC, Kim DY, et al. Experience with meconium peritonitis. J Pediatr Surg. 2007;42(11):1822–1825. doi: 10.1016/j.jpedsurg.2007.07.006.
- Tsai M-H, Chu S-M, Lien R, et al. Clinical manifestations in infants with symptomatic meconium peritonitis. Pediatr Neonatol. 2009;50(2):59–64. doi: 10.1016/s1875-9572(09)60034-6.
- Shyu M-K, Shih J-C, Lee C-N, et al. Correlation of prenatal ultrasound and postnatal outcome in meconium peritonitis. Fetal Diagn Ther. 2003;18(4):255–261. doi: 10.1159/000070806.
- Zangheri G, Andreani M, Ciriello E, et al. Fetal intra-abdominal calcifications from meconium peritonitis: sonographic predictors of postnatal surgery. Prenat Diagn. 2007;27(10):960–963. doi: 10.1002/pd.1812.
- Tseng J, Chou M, Ho E. Meconium peritonitis in utero: prenatal sonographic findings and clinical implications. J Chin Med Assoc. 2003;66(6):355–359.
- Desmonts GD, Couvreur JC. Congenital toxoplasmosis. A prospective study of 378 pregnancies. N Engl J Med. 1974;290(20):1110–1116. doi: 10.1056/NEJM197405162902003.
- Koppe JG, Rothova A. Congenital toxoplasmosis. A long-term follow-up of 20 years. Int Ophthalmol. 1989;13(6):387–390. doi: 10.1007/BF02306486.
- Casaccia G, Trucchi A, Nahom A, et al. The impact of cystic fibrosis on neonatal intestinal obstruction: the need for prenatal/neonatal screening. Pediatr Surg Int. 2003;19(1-2):75–78. doi: 10.1007/s00383-002-0781-8.
- Uchida K, Koike Y, Matsushita K, et al. Meconium peritonitis: prenatal diagnosis of a rare entity and postnatal management. Intractable Rare Dis Res. 2015;4(2):93–97. doi: 10.5582/irdr.2015.01011.
- Hillemeier AC, Hen J, Riely CA, et al. Meconium peritonitis and increasing sweat chloride determinations in a case of familial progressive intrahepatic cholestasis. Pediatrics. 1982;69(3):325–327. doi: 10.1542/peds.69.3.325.
- Feng D, He W. Asymptomatic elevated total serum bile acids representing an unusual form of intrahepatic cholestasis of pregnancy. Int J Gynaecol Obstet. 2016;134(3):343–344. doi: 10.1016/j.ijgo.2016.04.004.
- Keitel V, Donner M, Winandy S, et al. Expression and function of the bile acid receptor TGR5 in kupffer cells. Biochem Biophys Res Commun. 2008;372(1):78–84. doi: 10.1016/j.bbrc.2008.04.171.
- Gorelik J, Shevchuk A, de Swiet M, et al. Comparison of the arrhythmogenic effects of tauro- and glycoconjugates of cholic acid in an in vitro study of rat cardiomyocytes. BJOG. 2004;111(8):867–870. doi: 10.1111/j.1471-0528.2004.00166.x.
- Wax JR, Hamilton T, Cartin A, et al. Congenital jejunal and ileal atresia: natural prenatal sonographic history and association with neonatal outcome. J Ultrasound Med. 2006;25(3):337–342. doi: 10.7863/jum.2006.25.3.337.
- Zhu J, Yang ZJ, Wang L, et al. Prenatal diagnosis and prognosis of meconium peritonitis. Chin J Perinat Med. 2016;19(06):432–435.
- Zerhouni S, Mayer C, Skarsgard ED. Can we select fetuses with intra-abdominal calcification for delivery in neonatal surgical centres? J Pediatr Surg. 2013;48(5):946–950. doi: 10.1016/j.jpedsurg.2013.02.006.
- Karimi A, Gorter RR, Sleeboom C, et al. Issues in the management of simple and complex meconium ileus. Pediatr Surg Int. 2011;27(9):963–968. doi: 10.1007/s00383-011-2906-4.
- Staebler M, Donner C, Van Regemorter N, et al. Should determination of the karyotype be systematic for all malformations detected by obstetrical ultrasound? Prenat Diagn. 2010;25(7):567–573. doi: 10.1002/pd.1187.
- Chen C-W, Peng C-C, Hsu C-H, et al. Value of prenatal diagnosis of meconium peritonitis: comparison of outcomes of prenatal and postnatal diagnosis. Medicine. 2019;98(39):e17079. doi: 10.1097/MD.0000000000017079.
- Ping LM, Rajadurai VS, Saffari SE, et al. Meconium peritonitis: correlation of antenatal diagnosis and postnatal outcome – An institutional experience over 10 years. Fetal Diagn Ther. 2017;42(1):57–62. doi: 10.1159/000449380.

