Abstract
Objective
Isthmin 1 (ISM1) is an adipokine that improves hyperglycemia by increasing glucose uptake in a non-insulin-dependent manner. Studies have shown that ISM is associated with the development of type 2 diabetes mellitus. Based on this, we aimed to investigate serum ISM1 concentrations of pregnant women with gestational diabetes mellitus (GDM).
Methods
This case-control study was conducted with 80 pregnant women who applied to the Gynecology and Obstetrics Clinic of Umraniye Training and Research Hospital between April 2022 and November 2022. While 40 pregnant women diagnosed with GDM according to 75 g OGTT results formed the GDM group, 40 pregnant women with normal OGTT results formed the control group. The two groups were compared in terms of serum ISM1 concentrations.
Results
Both groups were similar in terms of demographic characteristics (p > 0.05). Fasting blood glucose levels, 1st-hour and 2nd-hour blood glucose levels in 75 g OGTT, fasting insulin levels, and HOMA-IR were significantly higher in the GDM group (p > 0.05, for each). Both groups were similar in terms of maternal waist circumference, periumbilical, and epigastric subcutaneous adipose tissue thickness (p > 0.05, for each).
Both groups were similar in terms of the gestational week at blood sampling for ISM1 (p = 0.253). The median maternal serum ISM1 concentration was found to be 3243.94 pg/ml in the GDM group, while it was determined as 2785.29 pg/ml in the non-GDM group (p = 0.026).
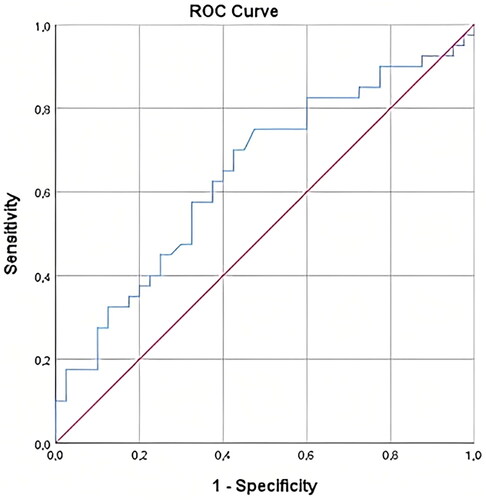
ROC analysis was performed to determine the value of maternal serum ISM1 concentration in predicting GDM. AUC analysis of maternal serum ISM1 for estimation of GDM was 0.645 (p = 0.026, 95% CI = 0.523 − 0.766). The optimal threshold value for maternal serum ISM1 concentration was determined as 3124.41 pg/ml with 62.5% sensitivity and 62.5% specificity.
Conclusions
Serum ISM1 concentrations were found to be higher in pregnant women with GDM than in healthy controls. Whether or how ISM1 participates in the pathophysiology of GDM remains to be investigated.
Introduction
Gestational diabetes mellitus (GDM) traditionally refers to abnormal glucose tolerance that begins during pregnancy or is first diagnosed during pregnancy. GDM is known to be a general public health problem that impairs the health of several million women worldwide [Citation1]. The first criteria of the oral glucose tolerance test (OGTT) used for GDM screening were reported in 1964 by O'Sullivan and Mahan [Citation2]. Later, various organizations defined different OGTT criteria for GDM screening [Citation3]. Depending on the different screening tests used in the countries and the characteristics of the screened population, the prevalence of GDM reported in the literature ranges from 1% to 30% [Citation4,Citation5].
Although it is associated with serious maternal and fetal complications, a considerable number of pregnant women do not want to have OGTT for various reasons. In a very recent study from our country, the acceptance rate of the OGTT test was reported as 79.4%. The most common reasons given by those who reject the OGTT are that the OGTT is unnecessary and unimportant or the belief that it is harmful [Citation6]. This situation has led scientists to investigate the availability of various molecules that are not in daily routine use in GDM screening [Citation7–10].
Isthmin 1 (ISM1) was first identified in 2002 as a protein secreted at the isthmus of Xenopus embryos (clawed frog), with a role during early brain development [Citation11]. Later studies have shown that ISM1 is also expressed in different cells and organs of adult mice, including the brain, lung, vascular system, skin, and immune cells [Citation12]. ISM1 belongs to the isthmin gene family that contains ISM1 and Isthmin 2 (ISM2). ISM1 gene is located on chromosome 20, while the ISM2 gene is located on chromosome 14q24.3 in humans [Citation13]. Phylogenetic analysis showed that ISM1 and ISM2 proteins are highly conserved in different organisms, suggesting that the isthmin protein family is involved in important cellular events [Citation14].
Nineteen years after its discovery, Jiang et al. identified ISM1 as an adipokine expressed in mature adipocytes that plays an important role in glucose and lipid metabolism. In mouse models, they showed that ISM1 suppressed lipid synthesis in hepatocytes and increased glucose uptake in adipocytes, thereby reducing lipid accumulation in the liver while improving hyperglycemia [Citation15].
There are few studies examining the relationship between ISM1 and diabetes mellitus (DM) in the literature, and conflicting results have been reported [Citation16,Citation17]. However, the role of ISM1 in the etiopathogenesis of GDM is currently unknown. Therefore, in this study, we aimed to investigate maternal serum ISM1 concentrations in pregnant women with GDM and to investigate the usability of ISM1 in GDM screening.
Materials and methods
This case-control study was conducted with 80 pregnant women aged between 20 and 40 years who applied to the Umraniye Training and Research Hospital, Department of Obstetrics and Gynecology between April 2022 and November 2022 and had their pregnancy follow-up and delivery in our hospital.
The GDM group consisted of 40 pregnant women who had 75 g OGTT between the 24th and 28th weeks of pregnancy and were diagnosed with GDM. The control group consisted of 40 healthy pregnant women who had a normal 75 g OGTT result. To avoid confounding factors, the control group and GDM group were matched in terms of age, body mass index (BMI), and gestational week at blood sampling.
Multiple pregnancies, smokers, and those who conceived by in vitro fertilization method were not included in the study. Those who had any pregestational disease, or who developed hypertension, preeclampsia, or intrahepatic cholestasis during pregnancy, or who had preterm delivery were not included in the study. Pregnant women whose newborns developed transient metabolic diseases such as hyperbilirubinemia or hypoglycemia were not excluded from the study, as it was thought that there would be no confounding effect on maternal serum ISM1 concentration.
75 g OGTT was applied to all participants between 24 and 28 weeks of gestation. OGTT results were evaluated according to the criteria recommended by the International Association of Diabetes and Pregnancy Study Groups. Accordingly, GDM was diagnosed when a single threshold value was met or exceeded; fasting value, 92 mg/dL; 1-h value, 180 mg/dL; 2-h value, 153 mg/dL [Citation18]. In addition, fasting insulin levels, HOMA-IR, and HbA1c levels were measured in the participants. HOMA-IR was calculated using fasting glucose (mg/dL) x fasting insulin (mU/L)/405 formula.
Jiang et al. stated that ISM1 is an adipokine that plays an important role in lipid metabolism as well as glucose metabolism [Citation15]. Therefore, participants’ waist circumference, periumbilical, and epigastric subcutaneous fat tissue thicknesses were measured on the day of OGTT. Subcutaneous fat tissue thicknesses were measured by the same clinician with an Esaote MylabX6 model ultrasound device. Periumbilical subcutaneous adipose tissue thickness measurement was made from a 2 cm lateral part of the umbilicus using a thin flat-tipped ultrasound probe without including cutaneous tissue. Epigastric subcutaneous fat tissue thickness was measured with a flat-tipped ultrasound probe at the level of the lower end of the xiphoid bone in the midline of the abdomen without including cutaneous tissue.
To investigate the maternal serum ISM1 concentration after 8 h of fasting, approximately 5 ccs of blood samples were drawn from the antecubital vein of the participants in non-anticoagulant biochemistry tubes. Blood samples for ISM1 were obtained at the time the blood sample was taken for fasting blood glucose in OGTT. Blood samples were centrifuged at 1000 rpm for 20 min. After centrifugation, the remaining serum in the upper part of the biochemistry tube was transferred to the Eppendorf tube and stored at −80 degrees. ISM1 concentrations in blood samples were studied with the Human Isthmin-1 (ISM1) ELISA Kit (Wuhan Fine Biotech Co., Ltd. Wuhan, Hubei, China, Lot number: EH4520) using the enzyme-linked immunosorbent assay method. For the Human ISM1 ELISA Kit used in the study, a measurement value of 15.625-1000 pg/ml and a sensitivity of 9.375 pg/ml were determined.
Turkey Umraniye Training and Research Hospital Local Ethics Committee approved this study (Ethics Committee Approval Number: B.10.1.TKH.4.34.H.GP.0.01/68, Date: 16/03/2022). The study protocol was maintained by the Declaration of Helsinki. Written informed consent was obtained from all participants.
Statistical analysis
Power analysis was performed using the G*Power (v3.1.9) program to determine sample sizes. The power of the study is expressed as 1-β (β = Type II error probability) and has 80% power. According to Cohen’s effect size coefficients, assuming that the evaluations to be made between two independent groups would have a large effect size (d = 0.80), it was calculated that at least 34 participants should be in the groups to achieve 90% power at the α = 0.05 level. Considering that there may be dropouts during the study, it was decided to include 40 participants in both groups. However, since there was no dropout, the study was conducted on 80 participants (40 for the GDM group and 40 for the control group).
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) version 25.0. The Kolmogorov-Smirnov test was used to determine whether the data were distributed normally or not. Descriptive statistical methods (mean, standard deviation, median, IQR, frequency, ratio) were used while evaluating the study data. Independent t-test was used for the comparison of two groups showing parametric distribution. Mann-Whitney U test was used for the comparison of two groups showing non-parametric distribution. The direction and level of the relationship between numerical variables were determined by Pearson correlation. The receiver operating curve (ROC) was used to determine the effectiveness of ISM1 in predicting GDM and its significant threshold. Statistical significance was accepted as p < 0.05 for all values.
Results
Both groups were similar in terms of age, BMI, weight gain up to the gestational week at which blood samples were drawn, gravida, and parity (p > 0.05, for each) ().
Table 1. Demographic characteristics of the GDM and non-GDM groups.
Gestational week at blood sampling was similar for both groups (p = 0.253). Fasting blood glucose level, 1st-hour and 2nd-hour blood glucose levels in 75 g OGTT were found to be significantly higher in the GDM group compared to the non-GDM group. (p = 0.000, for each). While fasting insulin level and HOMA-IR were significantly higher in the GDM group, there was no difference between the two groups in terms of HbA1c levels (p = 0.001, p = 0.000, p = 0.673, respectively). The median maternal serum ISM1 concentration was found to be 3243.94 pg/ml in the GDM group, while it was determined as 2785.29 pg/ml in the non-GDM group (p = 0.026). Both groups were similar in terms of maternal waist circumference, periumbilical, and epigastric subcutaneous adipose tissue thickness (p = 0.157, p = 0.760, p = 0.620, respectively) ().
Table 2. Comparison of GDM and non-GDM groups in terms of laboratory and ultrasound findings.
While the gestational week at delivery was significantly lower in the GDM group, the birth weight was significantly higher than in the non-GDM group (p = 0.009, p = 0.028, respectively). While both groups were similar in terms of the 1st-minute Apgar score, the 5th-minute Apgar score was higher in the GDM group than in the non-GDM group (p = 0.890, p = 0.028, respectively). There was no difference between the two groups in terms of neonatal hypoglycemia and hyperbilirubinemia and neonatal intensive care unit admission (p > 0.05, for each) ().
Table 3. Comparison of GDM and non-GDM groups in terms of perinatal outcomes.
ROC analysis was performed to determine the value of serum ISM1 concentration in predicting GDM. AUC analysis of serum ISM1 for estimation of GDM was 0.645 (p = 0.026, 95% CI = 0.523 − 0.766). The optimal threshold value for serum ISM1 concentration was determined as 3124.41 pg/ml with 62.5% sensitivity and 62.5% specificity ().
Figure 1. ROC analysis for sensitivity, specificity, and positive and negative predictive value of isthmin 1 in GDM.
According to Pearson correlation analysis, a positive correlation was detected between serum ISM1 concentration and BMI, maternal waist circumference, periumbilical and epigastric subcutaneous adipose tissue thickness (r = 0.400, p = 0.000; r = 0.410, p = 0.000; r = 0.288, p = 0.010; r = 0.293, p = 0.008, respectively). Also, a positive correlation was detected between serum ISM1 concentration and 75 g OGTT 2nd-hour blood glucose level, HOMA-IR, and HbA1c levels (r = 0.281, p = 0.016; r = 0.358, p = 0.001; r = 0.292, p = 0.008, respectively). No correlation was found between maternal age, fasting blood glucose level, 75 g OGTT 1st hour glucose level, and maternal serum ISM1 concentrations ().
Table 4. Correlation between maternal serum Isthmin-1 concentrations and GDM-related parameters.
Discussion
It is now known that nutritional, hormonal, immunological, genetic, and epigenetic factors are effective in the pathophysiology of GDM [Citation19]. Although the basic pathophysiological mechanisms have been extensively described to date, it has been shown in recent years that some adipokines may be involved in the metabolic changes underlying GDM. Leptin, adiponectin, tumor necrosis factor α, adipocyte fatty acid binding protein, retinol-binding protein 4, resistin, chemerin, progranulin, apelin, and omentin are some of the adipokines that have been investigated in GDM so far [Citation20].
Discovered as a crucial protein in the early brain development of Xenopus embryos, ISM1 was later reported to be an adipokine and participates in glucose metabolism [Citation13]. The first noteworthy study to examine the relationship of ISM1 with glucose metabolism was reported in 2021 by Jiang et al. By RNA sequencing and bioinformatics analysis in mice, they found that ISM1 was highly expressed in mature adipocytes. In this study, they treated mouse adipocytes, human adipocytes, and skeletal muscle cells with recombinant ISM1. They found that ISM1-mediated glucose uptake was via a non-insulin-dependent pathway. It was determined that ISM1-mediated glucose uptake capacity differed between different cell types. It has been suggested that this may be related to different cell type-specific receptors or glucose transporter proteins. In vitro, experiments have shown that ISM1 promotes glucose transporter 4, a glucose transporter protein in adipose and skeletal muscle cells, translocation from the cytoplasm to the plasma membrane. Also, ISM1 was shown to induce pAKTS473 phosphorylation in mature adipocytes and human skeletal muscle cells. Furthermore, treatment with phosphatidylinositol 3-kinase (PI3K) inhibitors revealed a complete blockade of ISM1-induced glucose uptake, suggesting that ISM1 requires PI3K to induce glucose uptake in adipocytes. As a result, it was understood that ISM1 exerts its effect not via the insulin receptor, but instead by binding to an as yet unknown receptor, and shares insulin signaling and downstream phosphorylation targets such as p-AKTSer473 [Citation15].
Just one year later, Wang et al. investigated ISM1 concentrations in the serum of individuals with newly diagnosed type 2 DM and compared it with healthy individuals without DM. They determined that individuals with type 2 DM had a significantly lower serum ISM1 concentration than normoglycemic controls. Additionally, logistic regression analysis showed that serum ISM1 concentration is an independent protective factor for type 2 DM. Therefore, they suggested that serum ISM1 could be used to create models to predict diabetes risk in the population [Citation16].
In another study published in 2022, the relationship between serum ISM1 concentrations and the severity of albuminuria in individuals with type 2 DM was examined. Serum ISM1 concentrations were found to be significantly higher in the group with macroalbuminuria than in the groups with normal albuminuria and microalbuminuria [Citation21]. In the previous study, it was claimed that serum ISM1 was protective against the development of type 2 DM. However, in this study, it was shown that high serum ISMI1 concentrations were associated with macroalbuminuria, which is an indicator of the severity of diabetic nephropathy. Based on these contradictory results, we think that ISM1 may play different roles in the onset and progression of type 2 DM.
In another study published in 2023, Liao et al. investigated serum ISM1 concentration in diabetic patients and its relationship with diabetic peripheral neuropathy. Contrary to the above-mentioned study by Wang et al. in this study, the authors found significantly higher serum ISM1 concentrations in individuals with type 2 DM than in healthy controls. In the correlation analysis between baseline features in type 2 DM and serum ISM1, a positive significant relationship was found with gender and age, while the authors did not find a significant relationship with BMI, HOMA-IR, and HbA1c [Citation17]. Similar to this study, we found that the serum ISM1 concentration in the group with GDM was significantly higher than in the group without GDM. On the other hand, we could not find any significant relationship between serum ISM1 concentration and age in the correlation analysis we performed. However, we found a significant relationship between serum ISM1 and BMI, waist circumference, periumbilical and epigastric subcutaneous adipose tissue thickness, 75 g OGTT 2nd-hour blood glucose level, HOMA-IR, and HbA1c levels.
Although ISM1 was discovered in 2002, there are very few studies investigating the relationship between DM and ISM1 and contradictory results have been reported. There is no study in the literature that investigated serum ISM1 concentrations during pregnancy. In addition, it is not yet known whether ISM1 is expressed in the placenta. To the best of our knowledge, this is the first study in the literature to examine maternal serum ISM1 concentration in pregnant women with GDM.
This single-center preliminary study has some limitations. This study was conducted with a limited number of participants, and the serum ISM1 concentrations of the participants were evaluated only once at the time of initial diagnosis. The change in serum ISM1 concentrations during the pregnancy is unknown. Changes in serum ISM1 concentrations after achieving normoglycemia with anti-diabetic treatment in the GDM group were also not evaluated. The expression pattern of ISM1 in the placenta and its contribution to maternal serum were not evaluated. Another limitation of this study is that the cost-effectiveness was not calculated when serum ISM1 was used instead of OGTT in GDM screening.
Conclusion
In this study, the median serum ISM1 concentration in pregnant women with GDM was found to be significantly higher than in the non-GDM group. However, the sensitivity and specificity of serum ISM1 for GDM screening were found to be 62.5%, and the cost-effectiveness of using serum ISM1 instead of OGTT for GDM screening is not yet clear. While conflicting results have been reported in studies evaluating the relationship between type 2 DM and ISM1, more in-depth studies are needed to understand whether and how ISM1 is involved in the pathogenesis of GDM.
Ethical approval and consent to participate
Turkey Umraniye Training and Research Hospital Local Ethics Committee approved this study (Ethics Committee Approval Number: B.10.1.TKH.4.34.H.GP.0.01/68, Date: 16/03/2022). The study protocol was maintained by the Declaration of Helsinki and informed consent was obtained from all participants.
Acknowledgments
We thank all participants who voluntarily participated in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s). The authors received no funding or grants and reported no conflicts of interest. The authors alone are responsible for the content nd writing of this article.
During the manuscript submission, the ICMJE disclosure form was uploaded to the system.
Data availability statement
Data supporting the findings of this study are available in the OSFHOME data repository with DOI identifier 10.17605/OSF.IO/GFBRJ.
Additional information
Funding
References
- ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49–e64.
- O’sullivan JB, Mahan CM. Crıterıa for the oral glucose tolerance test ın pregnancy. Diabetes. 1964;13:278–285.
- Lende M, Rijhsinghani A. Gestational diabetes: overview with emphasis on medical management. Int J Environ Res Public Health. 2020;17(24):9573. doi: 10.3390/ijerph17249573.
- Hartling L, Dryden DM, Guthrie A, et al. Screening and diagnosing gestational diabetes mellitus. Evid Rep Technol Assess (Full Rep). 2012;(210):1–327.
- Sweeting A, Wong J, Murphy HR, et al. A clinical update on gestational diabetes mellitus. Endocr Rev. 2022;43(5):763–793. doi: 10.1210/endrev/bnac003.
- Sezer H, Yazici D, Canbaz HB, et al. The frequency of acceptance of oral glucose tolerance test in Turkish pregnant women: a single tertiary center results. North Clin Istanb. 2022;9(2):140–148. doi: 10.14744/nci.2021.80588.
- Arslan E, Gorkem U, Togrul C. Is there an association between kisspeptin levels And gestational diabetes mellitus? Gynecol Obstet Reprod Med. 2020;26(3):179–183. doi: 10.21613/GORM.2019.946.
- Qu X, Zhuang J, Xu C, et al. Maternal serum pentraxin 3 level in early pregnancy for prediction of gestational diabetes mellitus. Ann Transl Med. 2019;7(23):722–722. doi: 10.21037/atm.2019.12.25.
- Mierzyński R, Poniedziałek-Czajkowska E, Dłuski D, et al. Nesfatin-1 and vaspin as potential novel biomarkers for the prediction and early diagnosis of gestational diabetes mellitus. Int J Mol Sci. 2019;20(1):159. doi: 10.3390/ijms20010159.
- Kirlangic MM, Eraslan Sahin M, Sahin E, et al. First-trimester maternal serum betatrophin levels are decreased in pregnancies complicated by gestational diabetes mellitus. Placenta. 2022;124:1–4. doi: 10.1016/j.placenta.2022.05.001.
- Pera EM, Kim JI, Martinez SL, et al. Isthmin is a novel secreted protein expressed as part of the fgf-8 synexpression group in the xenopus midbrain–hindbrain organizer. Mech Dev. 2002;116(1–2):169–172. doi: 10.1016/s0925-4773(02)00123-5.
- Heeren J, Scheja L. Isthmin 1—a novel insulin-like adipokine. Nat Rev Endocrinol. 2021;17(12):709–710. doi: 10.1038/s41574-021-00569-z.
- Hu M, Zhang X, Hu C, et al. A brief overview about the adipokine: ısthmin-1. Front Cardiovasc Med. 2022;9:939757. doi: 10.3389/fcvm.2022.939757.
- Shakhawat HM, Hazrat Z, Zhou Z. Isthmin—a multifaceted protein family. Cells. 2022;12(1):17. doi: 10.3390/cells12010017.
- Jiang Z, Zhao M, Voilquin L, et al. Isthmin-1 is an adipokine that promotes glucose uptake and improves glucose tolerance and hepatic steatosis. Cell Metab. 2021;33(9):1836–1852.e11. doi: 10.1016/j.cmet.2021.07.010.
- Wang J, Du J, Ge X, et al. Circulating Ism1 reduces the risk of type 2 diabetes but not diabetes-associated NAFLD. Front Endocrinol. 2022;13:890332. doi: 10.3389/fendo.2022.890332.
- Liao J, Li Y, Gui X, et al. Serum ısthmin-1 was ıncreased in type 2 diabetic patients but not in diabetic sensorimotor peripheral neuropathy. Diabetes Metab Syndr Obes. 2023;volume16:2013–2024. doi: 10.2147/DMSO.S411127.
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682.
- Sharma AK, Singh S, Singh H, et al. Deep ınsight of the pathophysiology of gestational diabetes mellitus. Cells. 2022;11(17):2672. doi: 10.3390/cells11172672.
- Fasshauer M, Blüher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014;2(6):488–499. doi: 10.1016/S2213-8587(13)70176-1.
- Wang C, Xu M, Feng R, et al. Serum isthmin-1 levels are positively and independently correlated with albuminuria in patients with type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2022;10(5):e002972. doi: 10.1136/bmjdrc-2022-002972.
