?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Presently, the efficacy of neonatal resuscitation techniques via interventions such as oral, nasal, and endotracheal suction for preventing meconium aspiration syndrome (MAS) after delivery has not been satisfactory.
Objective
This study aimed to investigate the role of intratracheal instillation of budesonide on oxidative stress in MAS.
Methods
Sixty-two neonates with MAS admitted to Huai’an Maternity and Child Healthcare Hospital from January 2018 to June 2020 were divided into a study group (intratracheal instillation of 2 ml budesonide suspension; n = 31) and a control group (intratracheal instillation of 2 ml normal saline; n = 31). Collect data from two groups of patients and evaluate clinical outcomes, including oxygenation index (OI), as well as serum total oxidant status (TOS), total antioxidant capacity (TAC), oxidative stress index (OSI) and 8-Isoprostane before treatment and 72h after admission.
Results
We found no statistical differences in mortality, complication rate, total oxygen inhalation time, OI before treatment and 72h after admission between the two groups of neonates with MAS, while the duration of invasive respiratory support in the study group was significantly shorter than in the control group. Also, serum TAC, TOS, OSI and 8-isoprostane levels were not statistically different before treatment between the two groups. After 72h of admission, OSI and 8-Isoprostane in neonates with MAS in the study group were much lower than those in the control group. TOS, OSI, 8-Isoprostane in the control group and 8-Isoprostane in the study group were significantly higher than those before treatment. As for TAC and TOS, no significant differences were observed between the two groups.
Conclusion
Intratracheal instillation of budesonide was shown to alleviate oxidative stress and shorten invasive ventilation time in neonates with MAS.
Introduction
Meconium aspiration syndrome (MAS) is neonatal respiratory distress caused by aspiration of meconium-contaminated amniotic fluid at birth, and approximately 11.2% of newborns with meconium-contaminated amniotic fluid suffer MAS [Citation1]. There are also large differences in mortality of MAS neonates among different grades of neonatal intensive care unit (NICU), with the highest mortality reaching up to 39% [Citation2]. While as for survivors, about 21% of MAS infants suffer neurological complications [Citation3].
Pulmonary inflammation caused by meconium plays an important role in the pathogenesis of MAS. Specifically, inhaled meconium triggers inflammation via the TLR4/MD-2 CD14 signaling complex, resulting in increased nuclear factor kappa B (NF-kB) [Citation4,Citation5]. Increased NF-κB induces pro-oxidative and inflammatory cascades, excessive activation of inflammatory cells such as neutrophils release, cytotoxic factors, immune activators such as reactive oxygen species, and cationic peptides through the oxidative system. The combined effects of excessive oxidative stress and inflammation induce alveolar collapse by increasing alveolar-capillary leakage and inactivating alveolar surfactant [Citation6]. All in all, inhibition of excessive oxidative stress and inflammation contributes to the improvement of MAS [Citation7].
Glucocorticoids can inhibit NF-kB, NF-kB-dependent pro-inflammatory gene expression, neutrophil activation, respiratory burst, reduce oxidative stress, and maintain endothelial integrity and vascular permeability [Citation8]. Budesonide, developed in 1970, is a superactive non-halogenated corticosteroid widely applied in airway inflammation and obstructive diseases of the respiratory system [Citation9]. The application of budesonide in the airway of MAS animal models was shown to improve respiratory function and reduce inflammation and oxidative stress [Citation10]. Moreover, significant adverse reactions have not been observed in neonates after intratracheal instillation of budesonide [Citation11,Citation12].
In this study, we hypothesized that intratracheal instillation of budesonide could reduce excessive oxidative stress, improve clinical outcomes, and avoid the adverse effects of intravenous glucocorticoids in newborns with MAS.
Material and methods
General information
In this study, the case data of neonates with MAS admitted to Huai’an Maternity and Child Healthcare Hospital from January 2018 to June 2020 were collected. Those who were given intratracheal instillation of 2 ml budesonide were classified into a study group, while those who were given intratracheal instillation of 2 ml normal saline were grouped as the control group. Inclusion criteria: (1) full-term infants with meconium in the amniotic fluid; weight ≥ 2.5 kg; (2) meconium could be detected or aspirated below vocal cords following endotracheal intubation; (3) respiratory distress started within 6 h of birth; (4) signs of infiltration, hyperinflation or atelectasis by chest x-ray examination; (5) other possible causes of neonatal respiratory distress were excluded; (6) parents/guardians provided informed consent for the anonymous use of their data for research purposes. Exclusion criteria: (1) premature or small for gestational age; (2) severe congenital malformation; (3) parents were unwilling to participate in the study; (4) neonates that died within 24 h or abandoned treatment. Guardians of all included neonates were informed and provided signed informed consent forms, and this study was approved by the Ethics Committee of Huai’an Maternity and Child Health Care Hospital (2017065).
Interventions
Ventilation schemes: neonates in both groups were given mechanical ventilation, and ventilator parameters (pH: 7.35-7.45, partial pressure of oxygen (PO2): 60-80 mmHg, and partial pressure of carbon dioxide (PCO2): 35-55 mmHg) were adjusted according to the results of blood gas analysis. If necessary, nitric oxide (NO) inhalation could be given as a treatment method. The noninvasive ventilation (nasal continuous positive airway pressure or high-flow nasal oxygen inhalation) was transited according to the normal withdrawal procedure. The inspired oxygen concentration was gradually decreased until oxygen inhalation was discontinued.
Conventional therapy: Patients in both groups were given the same anti-infection, microcirculation improvement, sedation, and fluid therapy strategies. The neonates’s vital signs were closely monitored, and the normal range of electrolytes and blood glucose was maintained.
After admission and based on the conventional treatment, 2 ml normal saline was instilled intratracheally into the neonates in the control group, and 2 ml of budesonide suspension was instilled intratracheally into neonates in the study group. Additionally, 3ml of venous blood was drawn from the two groups of neonates before treatment and 72 h after admission, respectively. Specifically, the blood was collected into ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes, centrifuged at 2000 rpm, collected respectively, and then stored in the refrigerators at -80 °C for the following examination.
Observational indicators
Indicators of clinical efficacy included pneumothorax, pulmonary hemorrhage, the incidence of persistent pulmonary hypertension of the newborn (PPHN), and mortality.
The oxygenation index (OI) was estimated using the following equation: OI = FiO2×MAP × 100/PaO2. Here, OI refers to before treatment and 72h after admission. In addition, the duration of invasive respiratory support (h) and total oxygen inhalation time were also recorded.
Serum-related indicators included total oxidant status (TOS), total antioxidant capacity (TAC), 8-Isoprostane, and oxidative stress index (OSI) before treatment and 72h after admission. The ratio of TOS to TAC was defined as the OSI, and the OSI indicated the degree of oxidative stress [Citation13]. TOS reflects the overall level of oxidants present in the serum, providing an aggregate measure of oxidative stress, which is a crucial factor in the pathophysiology of MAS. It includes various oxidized molecules such as hydrogen peroxide, lipid peroxides, and free radicals. TAC represents the serum’s overall capacity to counteract these oxidants, encompassing various antioxidants that neutralize harmful oxidative species. Evaluating TAC provides insights into the body’s defense mechanism against oxidative stress, particularly in the context of neonatal conditions like MAS. Enzyme-linked immunosorbent assay (ELISA) kits were purchased from Shanghai Kanglang Biotechnology Co., Ltd, and the test was performed according to the instructions.
Statistical analysis
Data processing and statistical analysis were performed using the SPSS v17.0 software; normally distributed measurement data are described as mean ± standard deviation (SD), and the t-test was used to compare the two groups. In addition, enumeration data were expressed as n or (%), and the comparison was checked by the χ2 test. For data not following a normal distribution, the Mann-Whitney U test was used. p < 0.05 was considered statistically significant.
Results
General information
A total of 62 neonates with MAS were enrolled in this study, including 33 males and 29 females. Based on the treatments given, 31 neonates were allocated to the study group and 31 to the control group. As shown in , there were no statistical differences in general clinical characteristics such as gender ratio, delivery mode, Apgar score, and presence or absence of delivery room intubation between the two groups. The outcomes of indicated that neonates in the study group and control group were comparable. Flow chart of patient screening ().
Table 1. Comparison of general information between the two groups (±S).
Comparison of clinical efficacy between the two groups of neonates with meconium aspiration syndrome (MAS)
The clinical efficacy and OI of the two groups of neonates with MAS after treatment were compared and the results are shown in . There were 3 cases of deaths in the study group (mortality rate: 9.68%) and 6 in the control group (mortality rate: 19.35%), and the mortality of neonates with MAS between the two groups was not statistically different (p = 0.28). Furthermore, the study group had 3 cases of pneumothorax, 1 case of pulmonary hemorrhage, and 6 cases of PPHN, while the control group had 4 cases of pneumothorax, 2 cases of pulmonary hemorrhage, and 9 cases of PPHN. No significant differences were observed in complication rates between the study and control groups (p = 0.37).
Table 2. Comparison of the clinical efficacy and oxygenation index (OI) of the two groups of neonates.
The comparison outcomes of the OI between the MAS neonates in the two groups revealed that invasive respiratory support time in the study group was lower than in the control group, and the difference had statistical significance (p = 0.03). After 72 h of treatment, the OI of the two groups was lower than before treatment, but there was no significant difference in the OI of the two groups of neonates with MAS before (p = 0.916) and after 72h of treatment (p = 0.637) (). Moreover, there was no significant difference in total oxygen inhalation time between the two groups of neonates with MAS (p = 0.66).
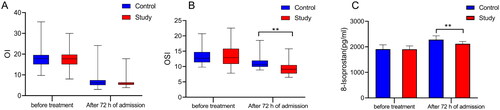
Figure 2. Comparison of serum OI, OSI, and 8-Isoprostane levels between control and study groups before treatment and After 72 h of admission. (A) Serum OI levels in the two groups. (B) Serum OSI levels in both groups. (C) Serum 8-Isoprostane levels in both groups.
OI, oxygenation index; OSI, oxidative stress index.
Comparison of serum indicators between the two groups before treatment and after 72h after admission
The level of serum-related indicators before treatment and 72 h after admission was compared between the two groups of neonates with MAS (). Before treatment, there were no significant differences in the level of serum TAC (p = 0.508), TOS (p = 0.464), OSI (p = 0.588), and 8-Isoprostane (p = 0.911). After 72 h of admission, the level of OSI (p < 0.001) and 8-Isoprostane (p < 0.001) of neonates with MAS in the study group was much lower than in the control group, and the difference was statistically significant. As for the level of TAC (p = 0.118) and TOS (p = 0.078), there were no statistically significant differences between the two groups.
Table 3. Comparison of serum-related indicator levels in the two groups before treatment.
Serum-related indicators were further compared between the two groups of neonates with MAS before treatment and 72 h after admission, and the results are shown in . In the control group, TOS (p = 0.04) and OSI (p < 0.001) () were decreased 72 h after admission compared with those before treatment. In the study group, the level of TAC (p = 0.001) and serum 8-Isoprostane (p < 0.001) () was increased 72 h after admission compared with that before treatmen. Additionally, there was no statistical difference in other serum-related indicators before treatment and 72 h after admission between the study and control groups.
Table 4. Serum-related indicators before treatment and 72 h after admission in both groups.
Discussion
With the popularization of neonatal resuscitation techniques, interventions (oral, nasal, and endotracheal suction) for preventing MAS after delivery have not been satisfactory [Citation14]. MAS remains one of the leading causes of respiratory distress in full-term neonates and has a high mortality rate. Zhu et al. reported that the mortality rate of neonates with severe MAS was nearly 40% in the 1990s [Citation15], and a study from Chongqing Medical University reported that the mortality of MAS within 21 years was 29.25% [Citation16]. With the widespread application of alveolar surfactants, high-frequency oscillatory ventilation, and NO inhalation during PPHN, the mortality rate of MAS has gradually decreased. In this study, the mortality of neonates with MAS reached 14.52%, higher than the incidence of PPHN in neonates with MAS in the United States (5.67%) reported by Thornton et al. [Citation17]. The above difference may be related to the failure of extracorporeal membrane oxygenation (ECMO) in the medical institutions of this study. Besides, the mortality in the study group was lower than in the control group. Although the difference was not statistically significant, it suggested that early intratracheal instillation of budesonide may reduce mortality in neonates with MAS. There was no significant difference in the incidence rate of pneumothorax, pulmonary hemorrhage and PPHN between the two groups, but the indicators in the study group were lower than those in the control group. Compared with the control group, intratracheal instillation of budesonide in the study group was mainly associated with reduced invasive respiratory support time (p = 0.03) but not the total oxygen inhalation time (p = 0.66) ().
Meconium aspiration first obstructs airways, resulting in ventilation-perfusion mismatch and hypoxemia, and in the following hours, severe pulmonary inflammatory reactions emerge and gradually worsen [Citation18]. Specifically, after meconium aspiration, many neutrophils flood into the airways and alveoli, these neutrophils activate alveolar macrophages to produce oxygen-free radicals, induce alveolar epithelial cell death and apoptosis, and then cause intense inflammation and lung injury [Citation19]. Moreover, excessive oxidative stress and inflammatory responses can accelerate alveolar surfactant consumption and further aggravate lung injury. Notably, intratracheal application of budesonide reduces the migration of neutrophils to the lung, relieves inflammatory responses and oxidation, and improves lung function in MAS animals [Citation20]. Budesonide can inhibit neutrophil chemotaxis and pro-inflammatory protein production by inhibiting vascular cell adhesion molecule-1 (VCAM-1) expression [Citation21]. Further, the addition of budesonide to the surfactants can reduce meconium-induced neutrophil migration to the lung, inhibit pro-inflammatory cytokine production and oxidative modification of proteins and lipids, relieve oxidative damage to the lung, and alleviate pulmonary edema [Citation20]. In this study, we found no significant difference in the level of TOS, TAC and OSI before treatment between the two groups. In the study group, TOS, TAC, and OSI were increased after 72h of hospitalization compared with those before treatment, but the differences were not statistically significant.
As a product formed by free radical-mediated peroxidation of arachidonic acid, 8-Isoprostane is stably present in various body fluids such as plasma. It was reported that 8-Isoprostane, a noninvasive biomarker of oxidative stress in vivo, is a reliable tool for assessing oxidative stress [Citation22]. Further, 8-Isoprostane is a sensitive indicator of oxidative stress and lipid peroxidation in airway inflammation, such as ARDS, asthma, and pneumonia [Citation23,Citation24]. In this study, the two groups did not exhibit a significant difference in the level of serum 8-Isoprostane before treatment, while after 72h of hospitalization, the level of 8-Isoprostane was lower in the study group than in the control group. Besides, serum 8-Isoprostane notably increased 72h after treatment compared to that in both groups, and the increase in the control group was more significant. The above outcomes of this study indicated that budesonide reduced the oxidation level in the respiratory system.
Based on the pathophysiology of MAS, treatments to reduce inflammation and cytokine production should benefit patients with MAS. The instillation of budesonide with surfactant has been shown to improve the respiratory status in animal studies [Citation25]. Management of MAS in infants mainly involves supportive respiratory and cardiovascular care, with other modalities such as surfactants [Citation26]. Considering the viscous and sticky physical properties of meconium, to effectively design new treatments for MAS drugs, researchers could focus on drugs that could liquefy meconium to make it less viscous and sticky, thus, easier to be removed from the tracheobronchial tree. As such, even if the meconium stays in the lungs, the liquefied meconium has a lesser risk of causing mechanical obstruction and a ball-valve effect in the airways.
In a previous study by Garg et al. who conducted a randomized control study to investigate the efficacy of early nebulized Budesonide in the clinical course and outcome (morbidity and mortality) of neonates with meconium aspiration, they similarly found that budesonide nebulization in meconium aspiration results in significant early improvement in general condition (early improvement in respiratory distress and early normalization of Downe’s score) of the newborn with lesser oxygen requirement, thus early discharge from NICU [Citation27]. In addition, inhaled budesonide has also been shown to effectively decrease the risk of chronic lung disease in newborns with a gestational age of <28 weeks with respiratory distress syndrome [Citation28]. However, similar research has been limited. Thus, our study confirms and sheds light on the potential clinical significance of this treatment in such neonates.
Although some interesting findings were observed in this study, there were still some limitations worth mentioning. First, this was a retrospective study with a relatively limited number of cases. Second, we only investigated the effects of intratracheal instillation of budesonide while other routes, such as intravenous or the use of other drugs such as methylprednisolone, dexamethasone, etc., were not investigated. Lastly, although the baseline characteristics between intergroup comparisons were not statistically significant, some intragroup heterogeneity existed in terms of gender, delivery room intubation, and mode of delivery. Thus, a larger cohort of patients using randomized and prospective settings might provide a higher evidence level of data.
This study showed that intratracheal instillation of budesonide could significantly decrease OSI and oxidative stress and shorten invasive ventilation time in neonates with MAS. Thus, intratracheal instillation of budesonide might be a promising alternative to commonly used existing treatments. In addition, although there are currently some theoretical investigations on drugs that could potentially alter meconium’s properties, studies on the underlying mechanisms of meconium pathogenicity in the MAS are urgently needed to improve the treatments of these neonates.
Authors’ contributions
Aijuan Qiu and Jing Wang conceptualized and designed the study, Lili Yang and Xiuli Lu, carried out the initial analyses, Wenjie Zhang and Zhaojun Pan drafted the initial manuscript and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
Ethics approval and consent to participate
Guardians of all included neonates were informed and signed informed consent forms. Besides, this study was approved by the Ethics Committee of Huai’an Maternity and Child Healthcare Hospital (2017065).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Lee J, Romero R, Lee KA, et al. Meconium aspiration syndrome: a role for fetal systemic inflammation. Am J Obstet Gynecol. 2016;214(3):1 e361–8. doi: 10.1016/j.ajog.2015.10.009.
- Natarajan CK, Sankar MJ, Jain K, et al. Surfactant therapy and antibiotics in neonates with meconium aspiration syndrome: a systematic review and meta-analysis. J Perinatol. 2016;36 Suppl 1(Suppl 1):S49–S54. (doi: 10.1038/jp.2016.32.
- Beligere N, Rao R. Neurodevelopmental outcome of infants with meconium aspiration syndrome: report of a study and literature review. J Perinatol. 2008;28 Suppl 3:S93–S101. doi: 10.1038/jp.2008.154.
- Salvesen B, Stenvik J, Rossetti C, et al. Meconium-induced release of cytokines is mediated by the TRL4/MD-2 complex in a CD14-dependent manner. Mol Immunol. 2010;47(6):1226–1234. doi: 10.1016/j.molimm.2009.12.015.
- Anand V, Basu S, Yadav SS, et al. Activation of toll-like receptors in meconium aspiration syndrome. J Perinatol. 2018;38(2):137–141. doi: 10.1038/jp.2017.169.
- Schuliga M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules. 2015;5(3):1266–1283. doi: 10.3390/biom5031266.
- Kopincova J, Mikolka P, Kolomaznik M, et al. Selective inhibition of NF-kappaB and surfactant therapy in experimental meconium-induced lung injury. Physiol Res. 2017;66(Suppl 2):S227–S236. doi: 10.33549/physiolres.933678.
- Adcock IM, Mumby S. Glucocorticoids. Handb Exp Pharmacol. 2017;237:171–196. doi: 10.1007/164_2016_98.
- Tashkin DP, Lipworth B, Brattsand R. Benefit: risk profile of budesonide in obstructive airways disease. Drugs. 2019;79(16):1757–1775. doi: 10.1007/s40265-019-01198-7.
- Mokra D, Mokry J, Drgova A, et al. Intratracheally administered corticosteroids improve lung function in meconium-instilled rabbits. J Physiol Pharmacol. 2007;58 Suppl 5(Pt 1):389–398.
- Kothe TB, Sadiq FH, Burleyson N, et al. Surfactant and budesonide for respiratory distress syndrome: an observational study. Pediatr Res. 2020;87(5):940–945. doi: 10.1038/s41390-019-0663-6.
- Yeh TF, Chen CM, Wu SY, et al. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2016;193(1):86–95. doi: 10.1164/rccm.201505-0861OC.
- Yuksel M, Ustabas Kahraman F, Selek S, et al. Oxidant and antioxidant levels and DNA damage in tuberous sclerosis. Brain Dev. 2019;41(3):245–249. doi: 10.1016/j.braindev.2018.10.014.
- Gandhi CK. Management of meconium-stained newborns in the delivery room. Neonatal Netw. 2018;37(3):141–148. doi: 10.1891/0730-0832.37.3.141.
- Zhu JX, Zhou XL, Zhang XD, et al. Analysis of clinical characteristics and death related factors of severe meconium aspiration syndrome-A multi-center retrospective survey. Chinese J Pract Pediatr. 2001;16(5):277–279.
- Huang Y. Clinical analysis of 612 meconium aspiration syndrome and their risk factors of complications [master’s thesis]. China: ChongQing Medical University; 2015. p. 1–39.
- Thornton PD, Campbell RT, Mogos MF, et al. Meconium aspiration syndrome: incidence and outcomes using discharge data. Early Hum Dev. 2019;136:21–26. (doi: 10.1016/j.earlhumdev.2019.06.011.
- Haakonsen Lindenskov PH, Castellheim A, Saugstad OD, et al. Meconium aspiration syndrome: possible pathophysiological mechanisms and future potential therapies. Neonatology. 2015;107(3):225–230. doi: 10.1159/000369373.
- Vidyasagar D, Zagariya A. Studies of meconium-induced lung injury: inflammatory cytokine expression and apoptosis. J Perinatol. 2008;28 Suppl 3:S102–S107. doi: 10.1038/jp.2008.153.
- Mikolka P, Kopincová J, Košútová P, et al. Lung inflammatory and oxidative alterations after exogenous surfactant therapy fortified with budesonide in rabbit model of meconium aspiration syndrome. Physiol Res. 2016;65(Suppl 5):S653–S662. doi: 10.33549/physiolres.933529.
- Strandberg K, Blidberg K, Sahlander K, et al. Effect of formoterol and budesonide on chemokine release, chemokine receptor expression and chemotaxis in human neutrophils. Pulm Pharmacol Ther. 2010;23(4):316–323. doi: 10.1016/j.pupt.2010.03.004.
- Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10 Suppl 1:S10–S23. doi: 10.1080/13547500500216546.
- Zhang WB, Zhou SJ, Wang G, et al. Exhaled breath condensate 8-isoprostane of patients with or at risk for ARDS in ventilation. Chinese J Respiratory and Crit Care Med. 2018;17(1):66–70.
- Wu YX, Li XY. Research progress of 8-isoprostane in oxidative stress injury mechanism of respiratory disorders. Int J Respiration. 2019;39(18):1427–1430.
- Mikolka P, Kopincova J, Tomcikova Mikusiakova L, et al. Effects of surfactant/budesonide therapy on oxidative modifications in the lung in experimental meconium-induced lung injury. J Physiol Pharmacol. 2016;67(1):57–65.
- Monfredini C, Cavallin F, Villani PE, et al. Meconium aspiration syndrome: a narrative review. Children (Basel). 2021;8(3):230. doi: 10.3390/children8030230.
- Garg N, Choudhary M, Sharma D, et al. The role of early inhaled budesonide therapy in meconium aspiration in term newborns: a randomized control study. J Matern Fetal Neonatal Med. 2016;29(1):36–40. doi: 10.3109/14767058.2014.985202.
- Sadeghnia A, Beheshti BK, Mohammadizadeh M. The effect of inhaled budesonide on the prevention of chronic lung disease in premature neonates with respiratory distress syndrome. Int J Prev Med. 2018;9(1):15. doi: 10.4103/ijpvm.IJPVM_336_16.