Abstract
Objective
Although early evidence shows that epilepsy can increase the risks of adverse pregnancy, some outcomes are still debatable. We performed a systematic review and meta-analysis to explore the effects of maternal and fetal adverse outcomes in pregnant women with epilepsy.
Methods
PubMed, Embase, Cochrane, and Web of Science were employed to collect studies that investigated the potential risk of obstetric complications during the antenatal, intrapartum, or postnatal period, as well as any neonatal complications. The search was conducted from inception to November 16, 2022. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the included original studies. The odds ratio (OR) values were extracted after adjusting for confounders to measure the relationship between pregnant women with epilepsy and adverse maternal or fetal outcomes. The protocol for this systematic review is registered with PROSPERO ID CRD42023391539.
Results
Of 35 articles identified, there were 142,577 mothers with epilepsy and 34,381,373 mothers without epilepsy. Our study revealed a significant association between pregnant women with epilepsy (PWWE) and the incidence of cesarean section, preeclampsia/eclampsia, gestational hypertension, induction of labor, gestational diabetes and postpartum hemorrhage compared with those without epilepsy. Regarding newborns outcomes, PWWE versus those without epilepsy had increased odds of preterm birth, small for gestational age, low birth weight (<2500 g), and congenital malformations, fetal distress. The odds of operative vaginal delivery, newborn mortality, and Apgar (≤ 7) were similar between PWWE and healthy women.
Conclusion
Pregnant women affected by epilepsy encounter a higher risk of adverse obstetric outcomes and fetal complications. Therefore, it is crucial to develop appropriate prevention and intervention strategies prior to or during pregnancy to minimize the negative impacts of epilepsy on maternal and fetal health.
1. Introduction
Epilepsy is a prevalent neurological disorder worldwide indicated by spontaneous recurrent seizures. It affects approximately 70 million people across all age groups globally [Citation1]. Obstetricians and gynecologists frequently encounter epilepsy as a common neurological issue [Citation2]. Advancements in diagnosis and treatment of epilepsy, coupled with improved social adaptation, have enabled the majority of women with epilepsy to marry and conceive. It has been reported that pregnancies in women with epilepsy (PWWE) are considered at high risk, with maternal mortality ten times higher than in women without seizure disorder [Citation3], although more than 90% of PWWE proceed without any apparent problems [Citation3,Citation4]. A number of factors associated with obstetric complications such as increased clearance of several anti-seizure medications during pregnancy [Citation5] contribute to the vulnerable condition of PWWE that requires special consideration in their treatment. Although the majority of women with epilepsy experience uncomplicated pregnancies and normal deliveries, several cohort studies indicate a higher risk of complications and potential alterations in seizure frequency among PWWE [Citation3,Citation6,Citation7].
Previous study [Citation8] had demonstrated that a small yet significant association between epilepsy and adverse pregnancy outcomes such as antepartum and postpartum hemorrhage, spontaneous abortion, cesarean section, any preterm birth, and fetal growth restriction. However, in recent years, with the development and application of anti-epileptic drugs, more people have paid attention to this special group of PWWE. Some recent studies with large sample sizes have provided additional insights, which seemingly contradict certain conclusions drawn by previous studies. This discrepancy presents a significant challenge to the prevention and management of PWWE in clinical practice. Therefore, this study aims to synthesize the available evidence to investigate the link between epilepsy exposure and obstetric complications as well as fetal outcomes, thus providing a reference for the development of specific strategies in medical practice for PWWE.
2. Materials and methods
2.1. Study registration
This study strictly adhered to the preferred reporting items for systematic reviews and meta-analyses guidelines 2020 (PRISMA 2020) and was prospectively registered in the international prospective register of systematic reviews (PROSPERO) with ID: CRD42023391539.
2.2. Eligibility criteria
2.2.1. Inclusion criteria
P (Population): women with detailed records of epilepsy exposure and fetal outcomes.
E (Expose): The exposure factor was epilepsy.
C (Comparison): The control group was women without epilepsy.
O (Outcome): The primary outcome measure in our review was the odds ratio (OR) which reflected the relationship between epilepsy and adverse pregnancy or delivery outcomes. The OR was obtained by adjusting multivariable logistic regression.
S (Study design): a cohort study, case-cohort, randomized clinical trial, cross-sectional study, and nested case-control study.
2.2.2. Exclusion criteria
P (Population): women who had been diagnosed with any type of chronic disease.
E (Expose): Studies that have serious questions about the diagnosis of epilepsy and only provided outcome data for PWWE receiving anti-seizure medications (ASMs) and those not receiving ASMs.
C (Comparison): None.
O (Outcome): (1) The reported studies focused solely on documenting the incidence of adverse maternal and fetal outcomes among patients with epilepsy, without providing information on the relationship between epilepsy exposure and the occurrence of adverse pregnancy outcomes; (2) Research on the controversial definition of adverse pregnancy or delivery outcome; (3) Studies with limited sample sizes may raise concerns about the stability of the regression coefficient in determining the association between epilepsy and adverse maternal and fetal outcomes. Therefore, we eliminated studies with insufficient sample sizes (e.g. studies with fewer than 30 cases); (4) The analysis carried out in the included studies was limited to univariate analysis and multivariate analysis was not carried out to account for potential confounding factors.
S (Study design): Congress abstracts published without peer review.
2.3. Data sources and search strategy
Related studies were searched via PubMed, Embase, Cochrane, and Web of Science with terms and free text terms. The search deadline was November 16, 2022. Detailed search strategies are shown in Table S1. In order to minimize the risk of missing new literature, a supplementary manual search of the above database was conducted in April 2023.
2.4. Study selection and data extraction
Records from PubMed, Embase, Cochrane, and Web of Science including titles and abstracts of the reviews were saved and added to the EndNote database (Version X9). Duplicates were detected and discarded from the literature records. We performed preliminary studies by screening the title and abstract, downloading the full texts, and then examining the full text to select the original studies that fit our systematic review. Prior to data extraction, we developed a standard data extraction spreadsheet, which included information on the title, first author, publication year, design type, author country, patient origin, sampling time, epilepsy diagnostic criteria, antiepileptic treatment, age of pregnant women, name of adverse pregnancy outcome events, number of epilepsy cases, total population, and control confounding factors.
Then, two reviewers screened eligible papers independently and extracted data using predesigned data extraction forms. If case of any discrepancies, disagreements are resolved by one-on-one discussion, and if necessary, a third researcher provides further assessment.
2.5. Assessment of study quality
The Newcastle-Ottawa Scale (NOS) was employed to evaluate the quality of each study [Citation9] and cross-checked their results after completion of the study assessment. Any disagreements are resolved through discussion, and if a disagreement persists, a third reviewer is involved to resolve the conflict. The NOS scale comprises three aspects with eight elements: four items for object selection, one item for intergroup comparability, and three items for outcome measurement. With the exception of the comparability item, each item can receive a maximum score of two points, while the range for other items is 0 to 1 point. Thus, the overall score can range from 0 to 9 points. A score of 7 to 9 suggests high-quality research, a score of 4 to 6 indicates moderate-quality research, and a score of 0 to 3 demonstrates low-quality research. The risk of bias of each included study was assessed independently by two reviewers.
2.6. Outcomes
Maternal outcomes included cesarean section (planned, elective, or emergency), and preeclampsia/eclampsia (mild preeclampsia is characterized by persisting blood pressure ≥ 140/90 mmHg combined with proteinuria ≥ 0.3 g per 24 h. Severe preeclampsia is characterized by high blood pressure ≥ 160/110 mmHg, proteinuria ≥ 3 g per 24 h, oliguria or clinical symptoms of preeclampsia), hypertensive disorders of pregnancy, induction of labor, placental complications, postpartum hemorrhage, diabetes mellitus, vaginal bleeding, operative vaginal delivery (including forceps and vacuum delivery), spontaneous abortion, anemia, breech presentation, and premature rupture of membranes.
Fetal and neonatal outcomes included preterm birth (delivery before 37 complete weeks of gestation), congenital malformations, newborn mortality, Apgar score (an evaluation tool to determine neonatal condition shortly after birth, typically at one and five minutes; a low Apgar score may suggest acidemia and may be indicative of hypoxia) of less than seven at one or five minutes, fetal distress, small for gestational age (SGA: birth weight less than the 10th percentile based on a standard fetal growth curve), low birth weight (< 2500 g), fetal growth restriction (IUGR < 10th percentile), and neonatal intensive care unit admission.
The study primarily focused on presenting OR values of adverse outcomes among pregnant women with epilepsy relative to healthy women. Detailed outcome indicators can be found in the additional materials.
2.7. Synthesis methods
Data were analyzed with Stata Statistical Software version 7.0. Odds ratio (OR) adjusted for confounders or multiple logistic regression were employed to discuss the correlation between epilepsy exposure and adverse pregnancy outcomes. Therefore, the effect sizes for our systematic review were OR and 95% confidence interval (CI). Q test was employed to analyze the heterogeneity among the included results, and I2 was used to quantify the level of heterogeneity. If I2 < 50%, the fixed effect model is used for meta-analysis, otherwise if I2 > 50%, the random effects model is adopted for meta-analysis. We also conducted subgroup analysis, sensitivity analysis, and meta-regression analysis to explore potential sources of heterogeneity. A funnel plot provided evidence of publication bias and statistical tests such as Egger’s and Begg’s tests were used to assess publication bias. If publication bias is detected, shear and complement methods are used to explore the impact of publication bias on the meta-analysis. In this study, statistical significance was determined as p < 0.05.
3. Results
3.1. Study selection
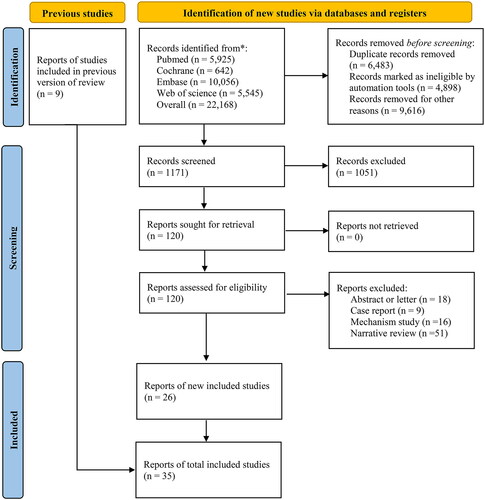
We successfully collected 22,168 records. After removing 6,483 duplicates, 4,898 ineligible records based on automatic selection, and 9,616 ineligible studies based on manual selection, the full texts of the remaining 1,171 citations were undergoing further evaluation. Upon screening the titles and abstracts, 1,145 publications were eliminated for the following reasons: 1,051 were noncompliant with inclusion and exclusion criteria, 16 were mechanism studies, 51 were narrative reviews, 9 were case reports, and 18 were abstracts or letters. Finally, 26 studies [Citation2–4,Citation7,Citation10–31] met our inclusion criteria. Additionally, we obtained 9 studies [Citation32–40] in the research of Luz Viale et al. [Citation8]. The literature screening process is presented in .
3.2. Study characteristics
Overall, this review consists of a total of 35 studies that involve 142,577 mothers with epilepsy and 34,381,373 mothers without epilepsy. The included systematic reviews or meta-analyses were published between 1992 and 2022. The study location was spread across nations in which 8 studies [Citation10,Citation11,Citation13,Citation22,Citation23,Citation30,Citation31,Citation33] were conducted in America, 6 studies [Citation4,Citation12,Citation17–19,Citation34] were carried out in Norway, 3 studies [Citation21,Citation28,Citation32] were performed in Israel, and 2 studies [Citation14,Citation16] were based in China. All of the 35 studies provided rates of adverse outcomes for women with epilepsy compared with those without epilepsy. Of these primary studies, 2 studies were cross-sectional studies [Citation14,Citation22], 10 were case-control studies [Citation3,Citation16,Citation18,Citation26,Citation32,Citation34,Citation37,Citation39,Citation41], 13 were retrospective cohort studies [Citation3,Citation4,Citation7,Citation12,Citation13,Citation15,Citation17,Citation23,Citation27,Citation28,Citation30,Citation33,Citation36]and 10 were prospective studies [Citation10,Citation11,Citation19–21,Citation24,Citation25,Citation31,Citation35,Citation38]. Data were acquired from hospital-based cohorts in 18 studies [Citation2,Citation3,Citation7,Citation16,Citation21,Citation24–28,Citation33,Citation35–41] and from registry data in 16 [Citation4,Citation10–15,Citation17,Citation19,Citation20,Citation22,Citation23,Citation30–32,Citation34,Citation40] studies.
Maternal outcomes were evaluated in 28 studies [Citation3,Citation4,Citation7,Citation10,Citation12,Citation13,Citation15–25,Citation27,Citation28,Citation30–32,Citation34,Citation37–41]. These studies have explored the relationship between PWWE and the following maternal outcomes: cesarean section, preeclampsia/eclampsia, hypertensive disorders of pregnancy, induction of labor, postpartum hemorrhage, diabetes mellitus, vaginal bleeding, spontaneous abortion, and anemia. There were no differences between the two groups for operative vaginal delivery, placental complications, breech presentation, and premature rupture of membranes.
Fetal and neonatal outcomes were assessed in 33 studies [Citation2–4,Citation7,Citation10–28,Citation30,Citation32,Citation33,Citation35–39,Citation41]. The studies examined the association between fetuses whose mothers were exposed to epilepsy and adverse outcomes including preterm birth, congenital malformations, fetal distress, small for gestational age, low birth weight (< 2500 g), fetal growth restriction (IUGR< 10th percentile), neonatal intensive care unit admission, newborn mortality, APGAR (≤ 7), and meconium-stained amniotic fluid. The basic features of the included studies are summarized in Table S2.
3.3. Assessment of study quality
Newcastle-Ottawa Scale (NOS) was employed to evaluate the methodological quality of the included studies, risk of bias in the selection and comparability of cohorts, and outcome measures. In the case-control studies, the main limitations were related to the selection of control cases and whether the study adequately controlled for confounding factors. For cohort studies, the primary concern was whether the studies sufficiently controlled for confounding factors. Finally, 28 (80%) [Citation2–4,Citation7,Citation10–15,Citation17–21,Citation23,Citation24,Citation26–28,Citation30–36,Citation38] of the 35 included studies were assessed to be high-quality, 6 (17%) [Citation16,Citation25,Citation37,Citation39–41] were moderate-quality, and only one (3%) was low-quality [Citation22]. The detailed results of the quality assessment are presented in the Table S3.
3.4. Meta-analysis
3.4.1. Adverse maternal outcomes
3.4.1.1. Synthesized results
Pregnant women with epilepsy were more prone to experiencing several complications than those without epilepsy. Several complications that showed a higher prevalence among pregnant women with epilepsy included cesarean section (OR = 1.56, 95% CI: 1.36 ∼ 1.79), preeclampsia/eclampsia (OR = 1.34, 95% CI: 1.21 ∼ 1.48), gestational hypertension (OR = 1.26, 95% CI: 1.10 ∼ 1.45), induction of labor (OR = 1.33, 95% CI: 1.20 ∼ 1.48), postpartum hemorrhage (OR = 1.21, 95% CI: 1.17 ∼ 1.25), gestational diabetes (OR = 1.12, 95% CI: 1.08 ∼ 1.15), vaginal bleeding (OR = 1.18, 95% CI: 1.02 ∼ 1.37), spontaneous abortion (OR = 1.78, 95% CI: 1.11 ∼ 2.85), anemia (OR = 4.11, 95% CI: 1.14 ∼ 14.78), and delivery hospitalization stays of 6 + days (OR = 2.36, 95% CI: 1.85 ∼ 3.01) (, supplementary Figure 1). However, no significant differences were identified between the two groups for operative vaginal delivery, placental complications, breech presentation, or premature rupture of membranes.
Table 1. Relationship between maternal epilepsy diagnosis and adverse maternal outcomes.
3.4.1.2. Subgroup analysis
We undertook subgroup analyses based on the conditions at the time of cesarean section, the severity of preeclampsia/eclampsia, the onset of vaginal bleeding during pregnancy, and the type of operative vaginal delivery (). Pregnant women with epilepsy exhibited significantly increased risks of cesarean section, regardless of whether it was planned, elective, or emergency (OR = 1.69, 95% CI: 1.47 ∼ 1.95; OR = 1.74, 95% CI: 1.39 ∼ 2.18; OR = 1.13, 95% CI: 1.07 ∼ 1.20; respectively). Furthermore, pregnant women with epilepsy showed higher risks of preeclampsia/eclampsia at all severity levels (mild and severe levels) (OR = 1.38, 95% CI: 1.20 ∼ 1.58; OR = 1.73, 95% CI: 1.00 ∼ 3.02; respectively) than women without epilepsy. Moreover, there was a higher rate of vaginal bleeding during pregnancy in the epileptic group than in the nonepileptic group. However, there were no increased risks of operative vaginal delivery, whether it involved forceps or vacuum deliveries in the epilepsy group.
3.4.1.3. Reporting biases
We conducted publication bias analysis for outcomes with nine or more reported articles. Funnel plots demonstrated asymmetry across studies that reported adverse maternal outcomes in induced of labor (Supplementary Figure 2).
3.4.2. Adverse fetal outcomes
3.4.2.1. Synthesized results
Infants of PWWE had a modestly increased risk of preterm birth (OR = 1.35, 95% CI: 1.24 ∼ 1.47), small for gestational age (OR = 1.26, 95% CI: 1.19 ∼ 1.34), low birth weight (<2500 g) (OR = 1.39, 95% CI: 1.20 ∼ 1.62), and fetal growth restriction (IUGR < 10th percentile) (OR = 1.37, 95% CI: 1.02 ∼ 1.84). Furthermore, there was an increased risk of congenital malformations (OR = 1.93, 95% CI: 1.47 ∼ 2.56), fetal distress (OR = 1.62, 95% CI: 1.20 ∼ 2.19), Neonatal Intensive Care Unit admission (OR = 2.50, 95% CI: 1.68 ∼ 3.70) were observed. No increased risks of newborn mortality or low infant Apgar score are present in the epilepsy group (, Supplementary Figure 3).
Table 2. Relationship between maternal epilepsy diagnosis and adverse fetal and neonatal outcomes.
3.4.2.2. Subgroup analysis
Subgroup analyses did not show increased risks of the ORs of infant 1-min and 5-min Apgar score ≤ 7, fetal death, neonatal death, and perinatal deaths. Nevertheless, the ORs of stillbirth slightly increased according to seven studies (OR = 1.28, 95% CI: 1.19 ∼ 1.38) ().
3.4.2.3. Reporting biases
Publication bias analysis for outcomes with 9 or more reported articles was conducted. Funnel plots are shown in Supplementary Figure 4. We observed funnel plot asymmetry for the adverse fetal outcomes in fetal distress and low infant Apgar score.
4. Discussion
This meta-analysis specifically examined issues during pregnancy, labor, and the neonatal period among PWWE. By including more studies, this study offers a more accurate quantitative estimate of women with epilepsy and obstetric or fetal issues. Our findings revealed increased risks of various maternal and fetal outcomes such as cesarean section, hypertensive disorders, induction of labor, antepartum hemorrhage, postpartum hemorrhage, spontaneous abortion, hypertensive disorders of pregnancy, preterm birth, and fetal growth restriction in PWWE compared to unaffected women. These findings are supported by previous research from Luz Viale et al. [Citation8]. Furthermore, we identified a greater risk of preeclampsia/eclampsia, diabetes mellitus, bleeding in the vagina, congenital malformations, fetal distress, small for gestational age, and low birth weight (< 2500 g) in PWWE.
Hypertensive pregnancy complications include multiple diagnoses such as gestational hypertension, pre-eclampsia, early onset pre-eclampsia, eclampsia etc., which with different risks and implications for fetal and maternal outcomes. Our study identified a significant increase in preeclampsia/eclampsia in PWWE compared with women without epilepsy. A recent study showed an increased risk of mild pre-eclampsia in PWWE, but the specified newer ASMs, lamotrigine and levetiracetam, used in monotherapy did not predispose for this complication [Citation42]. Cardiovascular risk factors (BMI >30 kg/m2, hypertension and diabetes etc.) were relevant for hypertensive complications in WWE. Additionally, some ASMs have been associated with metabolic changes and increased obesity, which in turn contribute to the development of gestational diabetes [Citation43]. The mechanism for the increased risk of hypertensive complications in PWWE could therefore be a combination of epilepsy and the comorbidity of ASMs.
Moreover, our analysis demonstrated an increased incidence of adverse fetal outcomes, including preterm delivery, low birth weight (LBW), and small for gestational age (SGA) among PWWE. However, the mechanisms between epilepsy and adverse fetal outcomes are not yet fully understood. It is hypothesized that trauma caused by unexpected seizures may complicate pregnancies, which results in ruptured fetal membranes and susceptibility to infection as well as premature delivery. Trauma, even minor injuries have been linked to an increased risk of adverse pregnancy outcomes such as spontaneous abortion, premature labor, preterm premature rupture of the membranes and uterine rupture [Citation44]. In an in vivo study, reduced uterine perfusion pressure in a pregnant rat can recapitulate the physiological characteristics of preeclampsia in a pregnant woman, including hypertension, proteinuria, and fetal growth restriction, with decreased litter size and pup weight [Citation45]. These findings suggest interconnectedness between seizures, preeclampsia, and some adverse fetal outcomes. Neonates born preterm, LBW, and SGA are prone to diseases during infancy and later life, prompting a comprehensive study to elucidate the mechanisms.
An increase in cesarean delivery (CD) is observed among women with epilepsy. This trend can be attributed to multiple factors including fetal growth restriction, fetal distress, hypertensive disorders of pregnancy, and seizures in pregnancy. We also found a similar increased risk of induced labor and spontaneous abortion in PWWE. Furthermore, cesarean section may prolong hospitalization and increase the chance of being exposed to nosocomial infection. It is important to note that epilepsy does not warrant CD or labor induction except in cases where a seizure occurs during the second stage of labor or when the patient is unable to cooperate during vaginal delivery. Nevertheless, some women with epilepsy may choose to undergo CD or induction due to concerns about the potential impact of seizure disorder on the baby or if there are ultrasound findings suggesting congenital anomalies. High rates of CD among PWWE may be attributed to uncertainty and a misperception of increased complication risks.
Postpartum hemorrhage is a significant cause of maternal mortality globally and is characterized by excessive bleeding from the placental implantation site, trauma to the genital tract, and adjacent structures, or a combination of both. In the case of women with epilepsy, the high rate of CD contributes to the increased risk of postpartum hemorrhage. Further studies are required to determine if postpartum hemorrhage is correlated to the mode of delivery (CD or vaginal deliveries). On the other hand, our updated meta-analysis did not indicate a significant increase in the risk of forceps or vacuum-assisted deliveries.
Children born to women with epilepsy encounter a higher risk of intrauterine growth restriction and congenital malformations. Several causative factors could contribute to this condition, including epilepsy itself, exposure to ASMs, seizures, genetic factors, any underlying conditions, and environmental factors. A recent study [Citation10] reported that severe adverse events including fetal loss and major congenital malformations were more prevalent in PWWE compared to healthy women (7.9% vs 1.9% respectively). ASMs have been reported to be used in 30%–70% of PWWE [Citation42]. Therefore, it is necessary to evaluate the safety of fetal exposure to ASMs so that when treated with ASMs during pregnancy, a balance can be struck between adequate control of the risk of seizures and the potential adverse effects of fetal exposure to ASMs. Most studies have shown that certain older ASMs (e.g. valproate, phenobarbital) have teratogenic effects [Citation46–48]. A cohort study demonstrated that the risk of congenital malformations of lamotrigine, carbamazepine, valproate, and phenobarbital was dose-dependent [Citation49]. In addition to the risk of fetal malformations, older ASMs can cause poor cognitive outcomes. Valproic acid carries the greatest risk, followed by phenobarbital and phenytoin [Citation50], while these data are reassuring for infant neurodevelopment and the risk of congenital malformations after in-utero exposure to certain new monotherapy of ASMs, such as lamotrigine or levetiracetam [Citation51–53]. However, a recent study has brought about concern due to the association found between levetiracetam exposure and an elevated risk of ADHD and anxiety disorders [Citation54]. Perampanel, which has been approved for clinical use in the United States since 2012, is a once-daily ASM prescribed for focal-onset seizures and generalized tonic–clonic seizures. Nevertheless, there is a lack of sufficient research on pregnancy outcomes in women treated with perampanel. Vazquez et al. [Citation55] found adverse pregnancy outcomes after perampanel exposure. Of the 96 pregnancies treated with perampanel, six cases had spontaneous miscarriages, two cases had incomplete spontaneous miscarriages, one case had premature delivery, and one had stillbirth due to Fallot’s tetralogy. Emerging evidence suggests that topiramate may have adverse effects on neurodevelopment [Citation54,Citation56]. A systematic review demonstrated that there was insufficient evidence or a lack of evidence to determine the long-term effects of clonazepam, gabapentin, pregabalin, zonisamide, and lacosamide [Citation57]. Interestingly, Neda Razaz et al. [Citation15] found that the increased risk of maternal and fetal outcomes could be attributed to pathological factors linked to epilepsy rather than the direct effect of ASMs. These epilepsy-related factors may be linked to various comorbidities of epilepsy, such as autoimmune disorders. An optimal approach for epilepsy involves striking a balance between epilepsy control and minimizing adverse effects.
Given the antifolate effect of certain ASMs are known to cause low folic acid concentrations [Citation58,Citation59], it is often recommended that women with epilepsy should be supplemented with high-dose folic acid before and during pregnancy [Citation60]. Several studies have indicated that folic acid supplementation during the perinatal period plays a protective role in mitigating ASM-associated adverse neurodevelopment in children of women with epilepsy [Citation50,Citation61–64]. To some extent, folic acid exposure in utero was not associated with poor neurodevelopment in children of women with epilepsy using ASM [Citation65]. Moreover, the utilization of periconceptional folate in pregnant women with epilepsy who are taking ASMs is linked to better cognitive development [Citation61]. A cohort study offered Class III evidence that periconceptional folic acid supplementation reduces the risk of preterm birth for PWWE using ASMs [Citation66]. Nevertheless, there is growing concern that excess folic acid intake during pregnancy may have potential adverse effects on neurodevelopment in children. Numerous published studies employing high dosage folate (5 mg per day) have already shown no benefit from high doses of folic acid (5 mg per day) in reducing fetal malformation [Citation67–70]. Frank et al. also found no beneficial effect of the folic acid dose range studied on valproate-associated fetal structural malformation rates, including spina bifida, at least over the folic acid dose range studied [Citation71]. High doses of folic acid during pregnancy increase the risk of cancer in offspring [Citation72,Citation73]. In conclusion, there is currently debate about the effectiveness of folic acid in the peri-conceptional period. Therefore, further research is needed on the optimal dosage of folic acid supplementation for WWE. More efforts should be taken to monitor folic acid concentrations during pregnancy and conduct long-term, multi-center follow-ups to explore the effect of folic acid deficiency on offspring neurodevelopment.
Other limitations of our study should also be recognized. First, we did not compare outcomes between women with epilepsy who received ASMs and those who did not, preventing us from assessing the contribution of treatment or lack thereof to these outcomes. Second, we encountered difficulties in evaluating the impact of seizure frequency during pregnancy, which could be a significant factor among PWWE. The impact of other possible confounders, such as dosage of ASMs exposure, ASMs serum levels, or exposure to other potential teratogens, needs to be assessed in future studies.
Author contributions
Conceptualization: Huimin Kuang, Lin Zhang; Data curation: Huimin Kuang, Lei Wei; Formal analysis: Huimin Kuang; Funding acquisition:Yuan Wu; Investigation: Yixun Li, Lei Wei; Methodology: Yixun Li, Lin Zhang; Project administration: Yixun Li; Resources: Yixun Li; Software: Yuling Lu, Yuan Wu; Supervision: Yuling Lu, Yuan Wu; Validation: Yuling Lu, Yuan Wu; Visualization: Lin Zhang, Yuan Wu; Writing -original draft: Huimin Kuang, Yixun Li, Yuling Lu, Lin Zhang, Lei Wei, Yuan Wu; Writing-review & editing: Huimin Kuang, Yixun Li, Yuling Lu, Lin Zhang, Lei Wei, Yuan Wu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download Zip (3.5 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Epilepsy: a public health imperative. Geneva: World Health Organization; 2019.
- Richmond JR, Krishnamoorthy P, Andermann E, et al. Epilepsy and pregnancy: an obstetric perspective. Am J Obstet Gynecol. 2004;190(2):1–13. doi:10.1016/j.ajog.2003.09.020.
- Kaur TP, Sahu L, Rathore AM, et al. Obstetric outcomes in pregnant women with seizure disorder: a hospital-based, longitudinal study. Turk J Obstet Gynecol. 2020;17(3):161–169. doi:10.4274/tjod.galenos.2020.87300.
- Borthen I, Eide MG, Veiby G, et al. Complications during pregnancy in women with epilepsy: population-based cohort study. BJOG. 2009;116(13):1736–1742. doi:10.1111/j.1471-0528.2009.02354.x.
- Tomson T, Battino D, Bromley R, et al. Executive summary: management of epilepsy in pregnancy: a report from the international league against epilepsy task force on women and pregnancy. Epilepsia. 2019;60(12):2343–2345. doi:10.1111/epi.16395.
- Mazzone PP, Hogg KM, Weir CJ, et al. Comparison of perinatal outcomes for women with and without epilepsy: a systematic review and meta-analysis. JAMA Neurol. 2023;80(5):484–494. doi:10.1001/jamaneurol.2023.0148.
- Işıkalan MM, Gündoğan KM, Acar A. Peripartum hemorrhage and other obstetric and neonatal outcomes in pregnant women with epilepsy: a single-center study. Epilepsy Res. 2021;171:106566. doi:10.1016/j.eplepsyres.2021.106566.
- Viale L, Allotey J, Cheong-See F, et al. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386(10006):1845–1852. doi:10.1016/s0140-6736(15)00045-8.
- Wells GA, Wells, G, Shea B, et al. editors. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014.
- Meador KJ, Pennell PB, May RC, et al. Fetal loss and malformations in the MONEAD study of pregnant women with epilepsy. Neurology. 2020;94(14):E1502–E1511. doi:10.1212/WNL.0000000000008687.
- Van Marter LJ, Pennell PB, Brown C, et al. Neonatal outcomes in the MONEAD study of pregnant women with epilepsy. J Pediatr. 2021;X:7. doi:10.1016/j.ympdx.2021.100073.
- Danielsson KC, Gilhus NE, Borthen I, et al. Maternal complications in pregnancy and childbirth for women with epilepsy: time trends in a nationwide cohort. PLoS One. 2019;14(11):e0225334. doi:10.1371/journal.pone.0225334.
- Decker BM, Thibault D, Davis KA, et al. A nationwide analysis of maternal morbidity and acute postpartum readmissions in women with epilepsy. Epilepsy Behav. 2021;117:107874. doi:10.1016/j.yebeh.2021.107874.
- Chen YH, Chiou HY, Lin HC, et al. Affect of seizures during gestation on pregnancy outcomes in women with epilepsy. Arch Neurol. 2009;66(8):979–984. doi:10.1001/archneurol.2009.142.
- Razaz N, Tomson T, Wikström A-K, et al. Association between pregnancy and perinatal outcomes among women with epilepsy. JAMA Neurol. 2017;74(8):983–991. doi:10.1001/jamaneurol.2017.1310.
- Huang CY, Dai YM, Feng LM, et al. Clinical characteristics and outcomes in pregnant women with epilepsy. Epilepsy Behav. 2020;112:107433. doi:10.1016/j.yebeh.2020.107433.
- Borthen I, Eide MG, Daltveit AK, et al. Delivery outcome of women with epilepsy: a population-based cohort study. BJOG. 2010;117(12):1537–1543. doi:10.1111/j.1471-0528.2010.02694.x.
- Borthen I, Eide MG, Daltveit AK, et al. Obstetric outcome in women with epilepsy: a hospital-based, retrospective study. BJOG. 2011;118(8):956–965. doi:10.1111/j.1471-0528.2011.03004.x.
- Farmen AH, Grundt JH, Nakling JO, et al. Increased rate of acute caesarean sections in women with epilepsy: results from the Oppland perinatal database in Norway. Eur J Neurol. 2019;26(4):617–623. doi:10.1111/ene.13865.
- Thomas SV, Sindhu K, Ajaykumar B, et al. Maternal and obstetric outcome of women with epilepsy. Seizure. 2009;18(3):163–166. doi:10.1016/j.seizure.2008.08.010.
- Sarusi MM, Wainstock T, Sheiner E, et al. Maternal epilepsy- perinatal outcome and long-term neurological morbidity of the offspring: a population-based cohort study. Arch Gynecol Obstet. 2022;305(1):55–62. doi:10.1007/s00404-021-06114-7.
- Gyamfi-Bannerman C, Huang Y, Bateman BT, et al. Maternal morbidity and mortality associated with epilepsy. J Mater Fetal Neonatal Med. 2021;35(25):7917–7923. doi:10.1080/14767058.2021.1938528.
- MacDonald SC, Bateman BT, McElrath TF, et al. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981–988. doi:10.1001/jamaneurol.2015.1017.
- Melikova S, Bagirova H, Magalov S. The impact of maternal epilepsy on delivery and neonatal outcomes. Childs Nerv Syst. 2020;36(4):775–782. doi:10.1007/s00381-019-04435-2.
- Hiilesmaa VK, Bardy A, Teramo K. Obstetric outcome in women with epilepsy. Am J Obstet Gynecol. 1985;152(5):499–504. doi:10.1016/0002-9378(85)90615-5.
- Sabers A, aRogvi-Hansen B, Dam M, et al. Pregnancy and epilepsy: a retrospective study of 151 pregnancies. Acta Neurol Scand. 1998;97(3):164–170. doi:10.1111/j.1600-0404.1998.tb00631.x.
- Soontornpun A, Choovanichvong T, Tongsong T. Pregnancy outcomes among women with epilepsy: a retrospective cohort study. Epilepsy Behav. 2018;82:52–56. doi:10.1016/j.yebeh.2018.03.001.
- Salman L, Shmueli A, Ashwal E, et al. The impact of maternal epilepsy on perinatal outcome in singleton gestations. J Mater Fetal Neonatal Med. 2018;31(24):3283–3286. doi:10.1080/14767058.2017.1368483.
- Vanya M, Árva-Nagy N, Szili K, et al. Effects of maternal epilepsy and antiepileptic therapy in women during pregnancy. Ideggyogyaszati Szemle. 2015;68(3-4):105–112.
- Mueller BA, Cheng-Hakimian A, Crane DA, et al. Morbidity and rehospitalization postpartum among women with epilepsy and their infants: a population-based study. Epilepsy Behav. 2022;136:108943. doi:10.1016/j.yebeh.2022.108943.
- McElrath TF, Druzin ML, Van Marter LJ, et al. The obstetrical care and delivery experience of women with epilepsy in the MODEAD study. Am J Perinatol. 2022;41(7):935–943. doi:10.1055/a-1788-4791.
- Katz O, Levy A, Wiznitzer A, et al. Pregnancy and perinatal outcome in epileptic women: a population-based study. J Mater Fetal Neonatal Med. 2006;19(1):21–25. doi:10.1080/14767050500434096.
- McPherson JA, Harper LM, Odibo AO, et al. Maternal seizure disorder and risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2013;208(5):378.e371–378.e375. doi:10.1016/j.ajog.2013.01.048.
- Veiby G, Daltveit AK, Engelsen BA, et al. Pregnancy, delivery, and outcome for the child in maternal epilepsy. Epilepsia. 2009;50(9):2130–2139. doi:10.1111/j.1528-1167.2009.02147.x.
- Steegers-Theunissen RPM, Renier WO, Borm GF, et al. Factors influencing the risk of abnormal pregnancy outcome in epileptic women: a multi-Centre prospective study. Epilepsy Res. 1994;18(3):261–269. doi:10.1016/0920-1211(94)90046-9.
- Hvas CL, Henriksen TB, Ostergaard JR, et al. Epilepsy and pregnancy: effect of antiepileptic drugs and lifestyle on birthweight. BJOG. 2000;107(7):896–902. doi:10.1111/j.1471-0528.2000.tb11089.x.
- Laskowska M, Leszczyńska-Gorzelak B, Oleszczuk J. Pregnancy in women with epilepsy. Gynecol Obstet Invest. 2001;51(2):99–102. doi:10.1159/000052902.
- Tanganelli P, Regesta G. Epilepsy, pregnancy, and major birth anomalies: an italian prospective, controlled study. Neurology. 1992;42(4 Suppl 5):89–93.
- Saleh AM, Abotalib ZM, Al-Ibrahim AA, et al. Comparison of maternal and fetal outcomes, in epileptic and non-epileptic women. Saudi Med J. 2008;29(2):261–266.
- Goel P, Devi L, Saha PK, et al. Maternal and perinatal outcome in pregnancy with epilepsy. Internet J Gynecol Obstetr. 2005;5. doi:10.5580/102b.
- Artama M, Gissler M, Malm H, et al. Effects of maternal epilepsy and antiepileptic drug use during pregnancy on perinatal health in offspring: nationwide, retrospective cohort study in Finland. Drug Saf. 2013;36(5):359–369. doi:10.1007/s40264-013-0052-8.
- Danielsson KC, Borthen I, Morken NH, et al. Hypertensive pregnancy complications in women with epilepsy and antiepileptic drugs: a population-based cohort study of first pregnancies in Norway. BMJ Open. 2018;8(4):e020998. doi:10.1136/bmjopen-2017-020998.
- Bider-Canfield Z, Martinez MP, Wang X, et al. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatr Obes. 2017;12(2):171–178. doi:10.1111/ijpo.12125.
- Schiff MA, Holt VL, Daling JR. Maternal and infant outcomes after injury during pregnancy in Washington state from 1989 to 1997. J Trauma. 2002;53(5):939–945. doi:10.1097/00005373-200211000-00021.
- Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41(3):457–462. doi:10.1161/01.Hyp.0000053448.95913.3d.
- Bromley R, Adab N, Bluett-Duncan M, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2023;8(8):Cd010224. doi:10.1002/14651858.CD010224.pub3.
- Tomson T, Battino D, Perucca E. Teratogenicity of antiepileptic drugs. Curr Opin Neurol. 2019;32(2):246–252. doi:10.1097/wco.0000000000000659.
- Bromley R, Weston J, Adab N, et al. Treatment for epilepsy in pregnancy: neurodevelopmental outcomes in the child. Cochrane Database Syst Rev. 2014;2014(10):Cd010236. doi:10.1002/14651858.CD010236.pub2.
- Tomson T, Battino D, Bonizzoni E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530–538. doi:10.1016/s1474-4422(18)30107-8.
- Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244–252. doi:10.1016/s1474-4422(12)70323-x.
- Pennell PB, Karanam A, Meador KJ, et al. Antiseizure medication concentrations during pregnancy: results from the maternal outcomes and neurodevelopmental effects of antiepileptic drugs (MONEAD) study. JAMA Neurol. 2022;79(4):370–379. doi:10.1001/jamaneurol.2021.5487.
- Knight R, Wittkowski A, Bromley RL. Neurodevelopmental outcomes in children exposed to newer antiseizure medications: a systematic review. Epilepsia. 2021;62(8):1765–1779. doi:10.1111/epi.16953.
- Veroniki AA, Cogo E, Rios P, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 2017;15(1):95. doi:10.1186/s12916-017-0845-1.
- Dreier JW, Bjørk MH, Alvestad S, et al. Prenatal exposure to antiseizure medication and incidence of childhood- and Adolescence-Onset psychiatric disorders. JAMA Neurol. 2023;80(6):568–577. doi:10.1001/jamaneurol.2023.0674.
- Vazquez B, Tomson T, Dobrinsky C, et al. Perampanel and pregnancy. Epilepsia. 2021;62(3):698–708. doi:10.1111/epi.16821.
- Bjørk MH, Zoega H, Leinonen MK, et al. Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. 2022;79(7):672–681. doi:10.1001/jamaneurol.2022.1269.
- Honybun E, Cockle E, Malpas CB, et al. Neurodevelopmental and functional outcomes following in utero exposure to antiseizure medication: a systematic review. Neurology. 2024;102(8):e209175. doi:10.1212/wnl.0000000000209175.
- Belcastro V, Striano P. Antiepileptic drugs, hyperhomocysteinemia and B-vitamins supplementation in patients with epilepsy. Epilepsy Res. 2012;102(1-2):1–7. doi:10.1016/j.eplepsyres.2012.07.003.
- Linnebank M, Moskau S, Semmler A, et al. Antiepileptic drugs interact with folate and vitamin B12 serum levels. Ann Neurol. 2011;69(2):352–359. doi:10.1002/ana.22229.
- Tomson T, Battino D, Bromley R, et al. Global survey of guidelines for the management of epilepsy in pregnancy: a report from the international league against epilepsy task force on women and pregnancy. Epilepsia Open. 2020;5(3):366–370. doi:10.1002/epi4.12420.
- Meador KJ, Pennell PB, May RC, et al. Effects of periconceptional folate on cognition in children of women with epilepsy: NEAD study. Neurology. 2020;94(7):e729–e740. doi:10.1212/wnl.0000000000008757.
- Bjørk M, Riedel B, Spigset O, et al. Association of folic acid supplementation during pregnancy with the risk of autistic traits in children exposed to antiepileptic drugs in utero. JAMA Neurol. 2018;75(2):160–168. doi:10.1001/jamaneurol.2017.3897.
- Husebye ESN, Gilhus NE, Riedel B, et al. Verbal abilities in children of mothers with epilepsy: association to maternal folate status. Neurology. 2018;91(9):e811–e821. doi:10.1212/wnl.0000000000006073.
- Gorski JR, Lebofsky M, Rozman K. Corticosterone decreases toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in hypophysectomized rats. J Toxicol Environ Health. 1988;25(3):349–360. doi:10.1080/15287398809531214.
- Husebye ESN, Wendel AWK, Gilhus NE, et al. Plasma unmetabolized folic acid in pregnancy and risk of autistic traits and language impairment in antiseizure medication-exposed children of women with epilepsy. Am J Clin Nutr. 2022;115(5):1432–1440. doi:10.1093/ajcn/nqab436.
- Alvestad S, Husebye ESN, Christensen J, et al. Folic acid and risk of preterm birth, preeclampsia, and fetal growth restriction among women with epilepsy: a prospective cohort study. Neurology. 2022;99(6):e605–e615. doi:10.1212/wnl.0000000000200669.
- Morrow JI, Hunt SJ, Russell AJ, et al. Folic acid use and major congenital malformations in offspring of women with epilepsy: a prospective study from the UK epilepsy and pregnancy register. J Neurol Neurosurg Psychiatry. 2009;80(5):506–511. doi:10.1136/jnnp.2008.156109.
- Mawer G, Briggs M, Baker GA, et al. Pregnancy with epilepsy: obstetric and neonatal outcome of a controlled study. Seizure. 2010;19(2):112–119. doi:10.1016/j.seizure.2009.11.008.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a Population-Based cohort study. PLoS One. 2015;10(7):e0131130. doi:10.1371/journal.pone.0131130.
- Thomas SV, Jose M, Divakaran S, et al. Malformation risk of antiepileptic drug exposure during pregnancy in women with epilepsy: results from a pregnancy registry in South India. Epilepsia. 2017;58(2):274–281. doi:10.1111/epi.13632.
- Vajda FJE, O’Brien TJ, Graham JE, et al. Folic acid dose, valproate, and fetal malformations. Epilepsy Behav. 2021;114(Pt A):107569. doi:10.1016/j.yebeh.2020.107569.
- Stephen LJ, Harden C, Tomson T, et al. Management of epilepsy in women. Lancet Neurol. 2019;18(5):481–491. doi:10.1016/s1474-4422(18)30495-2.
- Vegrim HM, Dreier JW, Alvestad S, et al. Cancer risk in children of mothers with epilepsy and high-dose folic acid use during pregnancy. JAMA Neurol. 2022;79(11):1130–1138. doi:10.1001/jamaneurol.2022.2977.