Abstract
The purpose of the present study was to evaluate the salivary Chromogranin-A (CgA) response to the psychological stress induced by a cognitive test battery. The subjects were 14 healthy volunteers administered the cognitive test battery CogScreen Aeromedical Edition (CogScreen-AE). CogScreen-AE is a test of reaction time and fundamental cognitive ability in the assessment of aeroplane pilots. The subjects were given five batteries of the test (1st ∼ 5th) on separate days with 2 week intervals. Saliva samples were collected at 20 min before the test (BASE), immediately before the test (PRE), in the middle of the test (MID), and 5 min after the test (POS) for each subject. The concentration of CgA was determined by enzyme-linked immunosorbent assay. ANOVA revealed a significant by time interaction (BASE, PRE, MID and POS) without a significant effect of battery interaction (1st, 2nd, 3rd, 4th and 5th). The CgA concentration increased at PRE (2.46 ± 0.24 pmol·mg protein− 1) from BASE (1.19 ± 0.10 pmol·mg protein− 1). CgA level remained increased in the MID (2.90 ± 0.26 pmol·mg protein− 1) and remained high in the POS samples (2.81 ± 0.23 pmol·mg protein− 1). Salivary CgA remained at basal levels during a control study over the same time course without exposure to CogScreen-AE. The changes in salivary CgA secretion as a result of exposure to a cognitive task may indicate psychological stress in humans.
Introduction
Considerable attention of many researchers including physiologists and psychologists has been focused on two of the classical stress hormones, catecholamine (CA) (Euler and Lundberg Citation1954; Krahenbuhl et al. Citation1978; Iyer et al. Citation1994; Leino et al. Citation1995; Kobayashi Citation1996; Leino et al. Citation1999; Otsuka et al. Citation2006) and cortisol (Tarui and Nakamura Citation1991; Kirschbaum and Hellhammer Citation1994; Leino et al. Citation1995; Kobayashi Citation1996; Farrace et al. Citation1996; Leino et al. Citation1999) as physiological and/or psychological markers of various stresses. Recently, interest has developed in measuring salivary chromogranin-A (CgA) concentration as a method of evaluating psychological stress (mental workload or psychological tension) (Nakane et al. Citation1998, Citation2002; Ng et al. Citation2003; Noto et al. Citation2005; Obara and Iwama Citation2005). Others have reported that human saliva CgA measurement is clinically promising as a psychological stress marker, and they also reported that CgA in humans is produced by the submandibular glands and secreted into saliva (Saruta et al. Citation2005). However, it is still unclear whether there are changes in salivary CgA levels during exposure to a cognitive test battery as a psychological stress in healthy humans. Moreover, normal levels of salivary CgA and/or its response time to stress are not well understood.
CgA is a major soluble protein in adrenal chromaffin cells and adrenergic neurons (Blaschko et al. Citation1967; Smith and Winkler Citation1967; Smith and Kirshner Citation1967; Winkler and Fischer-Colbrie Citation1992). It is also known that CgA is involved in the intracellular storage of CA, and is secreted with CA into human blood (Smith and Kirshner Citation1967; Winkler and Fischer-Colbrie Citation1992). Moreover, Taupenot et al. (Citation2003) also reported that CgA is a valuable indicator of sympathoadrenal activity. Salivary CgA has been proposed as a marker of sympathetic nervous activity involving the sympathetic-adrenomedullary system (Nakane et al. Citation1998), and might be used as an alternative to measurement of plasma CA concentration (Kanno et al. Citation1999). Moreover, salivary CgA secretion may be an index for activated sympatho-adrenal activity in the stress response (Kanno et al. Citation1998; Nakane et al. Citation1998; Kanno et al. Citation1999; Yanaihara et al. Citation1999). Additionally, salivary CgA changes more rapidly and more sensitively to psychological stressors than doses salivary cortisol, which is a well-known stress hormone reflecting activity of the hypothalamic-pituitary-adrenal axis (Nakane et al. Citation1998). Generally, evaluation of biochemical markers in saliva is a useful method for objectively assessing physiological and/or psychological stress. The collection of saliva is a convenient sampling method because it is noninvasive and relatively non-stressful (Kirschbaum and Hellhammer Citation1994). Evidently, however, there has been no study which has investigated whether salivary CgA secretion is affected by exposure to a cognitive test battery as a psychological stress. The purpose of the present study was to evaluate the salivary CgA response to the psychological stress induced by exposure to the cognitive test battery CogScreen Aeromedical Edition (CogScreen-AE). This test battery has been used extensively by defense research laboratories (Callister et al. Citation1996; de Quervain et al. Citation1998; Taylor et al. Citation2000). CogScreen-AE is a test of reaction time and fundamental cognitive ability that, in general, performs well. It is well constructed, easy to administer, and commercially available (Callister et al. Citation1996). CogScreen-AE is a computer-delivered and scored cognitive-screening instrument designed to rapidly assess deficits or changes in attention, immediate and short-term memory, visual perceptual functions, sequencing functions, logical problem solving, calculation skills, reaction time, simultaneous information processing abilities, and executive functions (Kay Citation1995). It is used as a psychological test battery in the medical evaluation of pilots with known or suspected neurological and/or psychiatric conditions. The present study was designed to define the time course of changes of salivary CgA as a marker of sympathetic nervous system activity in humans exposed to psychological stress caused by performing a cognitive task.
Materials and methods
Subjects
Healthy adult volunteers (nine males and five females) were subjects in this study. The subjects did not have periodontal or salivary gland disease. The mean age of the 14 subjects was 37.2 years (maximum 56 years, minimum 22 years, standard deviation 10.4). None was on any medication. The intake of drink containing caffeine and smoking were prohibited from rising in the morning to the end of the test. The study protocol was approved by the Ethical Committee of Aeromedical Laboratory (Aeromed Lab), Japan Air Self-Defense Force (JASDF). All subjects were fully briefed on the scope of the experiment, and informed consent was obtained from all subjects before experiment. The subjects had previously never experienced a study using a cognitive test battery.
Experimental protocol
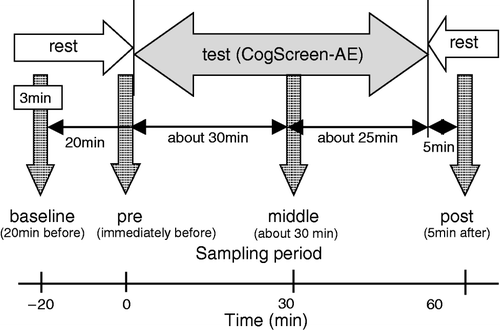
All experiments were conducted in a quiet laboratory where room temperature was maintained between 20 and 23°C. All subjects performed the cognitive test battery CogScreen-AE (CogScreen, LLC, Washington DC, USA) over about 50 min in the morning between 8 and 11 a.m. On days without announcing planned CogScreen-AE test battery exposure, control saliva samples were collected for the same subjects at similar time points as during the CogScreen-AE test, and under the same conditions. The CogScreen-AE tasks include backward digit span, mathematical tasks, visual sequence comparison, symbol digit coding, matching-to sample, manikin (mental rotation task), divided attention task, auditory sequence comparison, pathfinder (visual sequencing and scanning task), shifting attention, and dual task. Accordingly, the CogScreen-AE test battery is suitable and/or sufficient for used as a psychological stressor. In its full form, CogScreen-AE measures 65 variables derived from 11 subtests; details can be found in the test manual (Kay Citation1995). CogScreen-AE consists of a series of computerized cognitive tasks, each self-contained and presented with instructions and a practice segment, and uses a desktop IBM computer (PC; PS/V Model 2410, IBM Corporation, USA) running CogScreen-AE software (Version 1.10). A light pen system (PXL-780 Light Pen Interface, Fast Point Technologies, Inc., USA) was used as the response input device to reduce the potential advantage of having prior computer and keyboard experience. All subjects rested by sitting on a chair in front of the PC on the desk for 20 min before the test. During this rest period, the subjects might be asked to answer questions about physical and mental condition that morning. The subjects were given five trials of the cognitive battery on separate days with 2 week intervals to prevent any effects of repeated trials at short intervals.
Saliva sampling and chromogranin-A analyses
Saliva samples were collected at approximately 20 min before the test as baseline measurement (BASE), immediately before the test (PRE), in the middle of the test (MID), and 5 min after the test (POS) for each subject (). Saliva samples were obtained using a saliva collector, which the subjects placed under their tongue for 5 min, and then deposited in a sample tube (Salivette, Sarstedt, Germany). The tubes were stored on ice until centrifugation (for 10 min at 3000 rpm). The collected saliva was stored at − 80°C until analysis. The concentration of salivary CgA was determined by enzyme-linked immunosorbent assay (ELISA) using the YK070 Human CgA EIA Kit (Yanaihara Institute Inc., Japan). Salivary protein concentration was determined using Advanced Protein Assay Reagent (Cytoskeleton, Inc., USA). The CgA concentration was corrected by protein concentration and data are presented as pmol per mg protein (Nakane et al. Citation1998).
Statistical analyses
Results are expressed as the mean ± standard error of the mean (SEM) at each sampling period for all subjects. The ratios (PRE/BASE, MID/BASE, and POS/BASE ratio) of the salivary CgA concentrations during the experiment to the baseline concentration were calculated. ANOVA with repeated measures was performed to detect time-related and trial-related differences. Tukey's test was used for multiple comparisons. A p-value of < 0.05 was considered statistically significant.
Results
shows the salivary CgA levels and ratios relative to baseline values during the experiment for each exposure to the CogScreen-AE battery. CgA concentrations were significantly changed by the administration of the test. and show the mean concentrations of CgA at each sampling period for all five exposures to the CogScreen-AE battery in all subjects () and in males and females separately (). Control levels for CgA measured at matched sample times during control experiments are also shown in . There was no significant change in salivary CgA levels during the control experiments.
TABLE I. Salivary CgA concentrations for baseline (BASE), immediately before the CogScreen-AE test (PRE), the middle of the test (MID), and 5 min after the test (POS) (n=14). Data are concentrations (upper), and ratios (lower) relative to BASE.
Figure 2 Changes in salivary CgA concentrations in all subjects during the cognitive test battery and control experiments without anticipation of exposure to the test battery. Values are group means ± standard error of the mean. **:p < 0.01, significant difference from baseline level. N = 14.
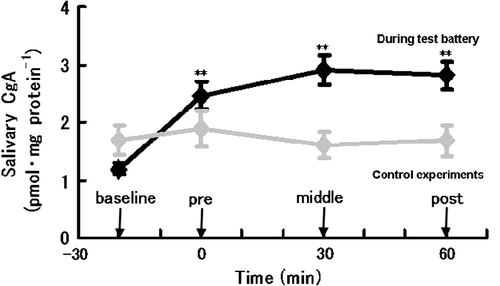
Figure 3 Changes in salivary CgA concentrations during the cognitive test in men and women. Values are group means ± standard error of the mean. N = nine men; five women. *:p < 0.05, **:p < 0.01, significant difference from baseline level.
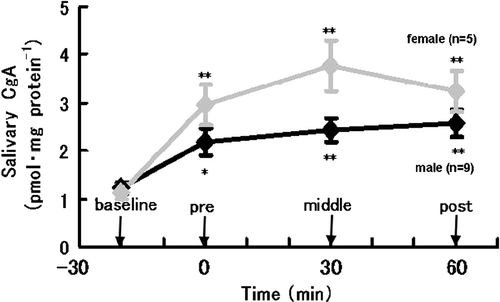
ANOVA for the two repeated-measurement factors, trial number (1st, 2nd, 3rd, 4th and 5th trial) and time during trial (BASE, PRE, MID and POS), revealed a significant by time interaction (F(3; 279) = 13.2588, p < 0.0001) without a significant effect of trial interaction. Tukey's test showed significantly elevated CgA levels during the experiment (PRE, MID and POS) from the baseline level. The CgA concentration was already increased immediately before the test (PRE) from the baseline level (p < 0.01). Compared with the baseline the levels of salivary CgA remained increased at the mid-point of the test (MID) and 5 min after the test (POS) (p < 0.01). Both males and females showed a significant increase in the concentration of CgA just before (PRE) and during the test battery (). An apparent trend toward a sex related difference in the levels of salivary CgA, particularly during the CogScreen-AE test, failed to reach statistical significance, though the sample size was small ().
Discussion
Recent studies in humans indicated that salivary CgA changes sensitively in response to psychological stress. Such stressors include psychosomatic stress (Nakane et al. Citation1998), computer operation psychological stress (Nakane et al. Citation2002), academic assessment stress (Ng et al. Citation2003), and psychological tension stress before surgery or anesthesia (Obara and Iwama Citation2005). Ng et al. (Citation2003), however, reported that no significant differences were noted between the pre- and post-test saliva samples for CgA levels with academic assessment stress among dental undergraduates. However, the presumably mild to moderate stress of the mid-term test used in this study might explain this negative result. Noto et al. (Citation2005) examined the relationship between State-Trait Anxiety Inventory score (STAI-s) in subjects exposed to the 15 min mental arithmetic task stress and salivary CgA. The STAI-s was not significantly correlated to salivary CgA. They concluded that salivary CgA was not a good marker of stress in their study. They also described that a mental arithmetic task may change autonomic tone, but this change may not be strong enough to increase salivary CgA. Otherwise, Dimsdale et al. (Citation1989) suggest that within the normal physiological range, as exemplified by small postural changes and mental stress (orthostatic challenge and mental arithmetic), plasma CgA level is stable or else slow to respond. Basal CgA levels were unrelated to other manifestations of sympathetic nervous system activity. Moreover, others have reported that the measurement of salivary CgA may not be a good parameter to evaluate the activity of the sympathetic nervous system (Obara and Iwama Citation2005).
In contrast, in the present study, salivary CgA concentrations were significantly changed by the administration of the cognitive test battery CogScreen-AE: CgA increased immediately before the cognitive test (PRE), and remained increased during the experiment (MID and POS). In a study of psychosomatic stress, Nakane et al. (Citation1998) reported a significant elevation in salivary CgA immediately before an oral presentation in a group of male volunteers. None of the subjects in our study had experienced the CogScreen-AE before, nor had they been subjects in an experiment like this study. The increase in salivary CgA immediately before the cognitive battery (PRE; ) was presumably a result of psychological stress caused by anticipation of the test, similar to that before an oral presentation (Nakane et al. Citation1998). Nakane et al. (Citation2002) reported that salivary CgA level increased more than three-fold during computer operation performed by subjects who did not have touch-typing skills. The observed 1.68–3.35-fold increase (PRE, MID and POS; ) in salivary CgA levels during exposure to the CogScreen-AE test battery is presumed to reflect psychological stress before and during the test. On the other hand, in our experiments with stressful high Gz exposure on a centrifuge (Sekiguchi et al. Citation1986), we found levels of salivary CgA for student pilots were increased before the physical stress, and not further changed after the Gz exposure (unpublished). These results suggest that the increased salivary CgA with CogScreen-AE tasks or anticipation of high Gz exposure reflects psychological stress.
Apparently, few data are available concerning the normal levels of salivary CgA and its response time in healthy humans. Control (normal) level in salivary CgA observed in healthy subjects in this study was 1.71 ± 0.15 pmol/mg protein (n = 11, seven males and four females; ). As expected, there was no significant time of day effect on salivary CgA in control conditions. Furthermore, there was no difference in the control levels between males and females. The observed change in salivary CgA change was relatively rapid, from just before to within 20 min after the start of the CogScreen-AE test battery ( and ). The rapidity of the salivary CgA response to an expected emotional stress requires further study.
In summary, the results of the present study clearly demonstrated that psychological stress associated with the CogScreen-AE test battery is associated with significant increases in salivary CgA levels. Thus changes in salivary CgA secretion may indicate psychological stress in humans exposed to a cognitive task. In this study, a trend towards a sex related difference of the levels of salivary CgA with psychological stress, particularly during the test battery, was apparent, thought this failed to reach statistical significance. Further study of sex differences in the salivary CgA response to emotional stress is required.
Acknowledgements
The authors wish to thank Asao Kobayashi, Ph.D., Chief of Pharmacochemistry Section, Second Division, Aeromed Lab, JASDF, for kindly advice on planning of this study, and also thank Eri Makita, Pharmacochemistry Section, for her technical support. The views expresses in this paper are those of the authors and do not reflect the official policy or position of JASDF.
References
- Blaschko H, Comline RS, Schneider FH, Silver M, Smith AD. Secretion of a chromaffine granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature 1967; 215: 58–59
- Callister JD, King RE, Retzlaff PD. Cognitive assessment of USAF pilot training candidates. Aviat Space Environ Med 1996; 67: 1124–1129
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 1998; 394: 787–790
- Dimsdale JE, O'Connor D, Ziegler M, Mills P. Does chromogranin A respond do short-term mild physiologic challenge?. Neuropsychopharmacology 1989; 2: 237–240
- Euler US, Lundberg U. Effect of flying on the epinephrine excretion in air force personnel. J Appl Physiol 1954; 6: 551–555
- Farrace S, Biselli R, Urbani L, Ferlini C, De Angelis C. Evaluation of stress induced by flight activity by measuring the hormonal response. Biofeedback Self Regul 1996; 21: 217–228
- Iyer EM, Banerjee PK, Sengupla AK, Baboo NS. Neuroendocrine responses of flight cadets during midterm tests and of fighter pilots during tail chase sorties. Aviat Space Environ Med 1994; 65: 232–236
- Kanno T, Asada N, Yanase H, Iwanaga T, Ozaki T, Nishikawa Y, Iguchi K, Mochizuki T, Hoshino M, Yanaihara N. Salivary secretion of highly concentrated chromogranin A in response to noradrenaline and acetylcholine in isolated and perfused rat submandibular glands. Exp Physiol 1999; 84: 1073–1083
- Kanno T, Asada N, Yanase H, Iwanaga T, Nishikawa Y, Hoshino M, Yanaihara N. Autonomic control of submandibular chromogranin A secretion in the anaesthetized rat. Biomed Res 1998; 19: 411–444
- Kay GK. CogScreen aeromedical edition, professional manual. Psychological Assessment Resources INC, Odessa 1995
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendcrine research: Recent developments and applications. Psychoneuroendocrinology 1994; 19: 313–333
- Kobayashi A. Trace element and hormonal responses during flight aptitude test. Aviat Space Environ Med 1996; 67: 333–337
- Krahenbuhl GS, Marett JR, Reid GB. Task-specific simulator pretraining and in-flight stress of student pilots. Aviat Space Environ Med 1978; 49: 1107–1110
- Leino T, Leppäluoto J, Huttunen P, Ruokonen A, Kuronen P. Neuroendocrine responses to real and simulated BA Hawk MK 51 flight. Aviat Space Environ Med 1995; 66: 108–113
- Leino TK, Leppäluoto J, Ruokonen A, Kuronen P. Neuroendocrine responses to psychological workload of instrument flying in student pilots. Aviat Space Environ Med 1999; 70: 565–570
- Nakane H, Asami O, Yamada Y, Ohira H. Effect of negative air ions on computer operation, anxiety and salivary chromogranin A-like immunoreactivity. Int J Psychophysiol 2002; 46: 85–89
- Nakane H, Asami O, Yamada Y, Harada T, Matsui N, Kanno T, Yanaihara N. Salivary chromogranin A as an index of psychosomatic stress response. Biomed Res 1998; 19: 401–406
- Ng V, Koh D, Mok BYY, Chia S, Lim L. Salivary biomarkers associated with academic assessment stress among dental undergraduates. J Dent Educ 2003; 67: 1091–1094
- Noto Y, Sato T, Kudo M, Kurata K, Hirota K. The relationship between salivary biomarkers and State-Trait Anxiety Inventory score under mental arithmetic stress: A pilot study. Anesth Analg 2005; 101: 1873–1876
- Obara S, Iwama H. Assessment of psychological tension after premedication by measurement of salivary chromogranin A. J Clin Anesth 2005; 17: 554–557
- Otsuka Y, Onozawa A, Miyamoto Y. Hormonal responses of pilots to training flights: The effects of experience on apparent stress. Aviat Space Environ Med 2006; 77: 410–414
- Saruta J, Tsukinoki K, Sasaguri K, Ishii H, Yasuda M, Osamura YR, Watanabe Y, Sato S. Expression and localization of chromogranin A gene and protein in human submandibular gland. Cells Tissues Organs 2005; 180: 237–244
- Sekiguchi C, Iwane M, Oshibuchi M. Anti-G training of Japanese Air Self Defense Force fighter pilots. Aviat Space Environ Med 1986; 57: 1029–1034
- Smith AD, Winkler H. Purification and properties of an acidic protein from chromaffin granules of bovine adrenal medulla. Biochem J 1967; 103: 483–492
- Smith WJ, Kirshner N. A specific soluble protein from the catecholamine storage vesicles of bovine adrenal medulla. I. Purification and chemical characterization. Mol Pharmacol 1967; 3: 52–62
- Tarui H, Nakamura A. Hormonal responses of pilots flying high-performance aircraft during seven repetitive flight missions. Aviat Space Environ Med 1991; 62: 1127–1131
- Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med 2003; 348: 1134–1149
- Taylor JL, O'Hara R, Mumenthaler MS, Yesavage JA. Relationship of CogScreen-AE to flight simulator performance and pilot age. Aviat Space Environ Med 2000; 71: 373–380
- Winkler H, Fischer-Colbrie R. The chromogranins A and B: The first 25 years and future perspectives. Neuroscience 1992; 49: 497–528
- Yanaihara H, Hata M, Nishikawa Y, Hoshino M, Yanaihara N, Murai M. Application of region-specific immunoassay for human chromogranin A: Substantial clue for detection and measurement of chromogranin A in human plasma. Regul Pept 1999; 80: 83–90