Abstract
Large vesicomyid clams (Veneroida: Vesicomyidae: Pliocardiinae) are prominent members of the communities associated with sulphide-rich deep-sea habitats. Taxonomic uncertainties within the Pliocardiinae result from both plasticity in shell morphologies and the common occurrence of cryptic species. Molecular taxonomic studies have now clarified many species-level assignments and provided DNA-barcodes for more than 50 species worldwide. Nonetheless, genus-level assignments remain uncertain, because the existing COI barcode sequences are not sufficient for identifying higher-level groupings. To construct a robust phylogeny for this subfamily, we conducted a combined Bayesian analysis of the COI mitochondrial fragment and five additional independent nuclear gene segments. The phylogenetic results provide a better foundation for assessing genus-level assignments within the subfamily and reveal goals for future taxonomic research. Furthermore, morphological examinations helped to clarify and solidify generic classifications. Calibration of molecular clocks with recently verified fossil data permitted realistic estimates for the origins and evolutionary age of pliocardiins during the Cenozoic Era from a deep-dwelling ancestor.
http://zoobank.org/urn:lsid:zoobank.org:pub:2554122D-96D4-4CBF-BC70-B017998AF64D
Introduction
Relatively large clams of the subfamily Pliocardiinae (family Vesicomyidae) are among the most diverse and conspicuous members of chemosynthetic environments worldwide () living mostly in sulphide-rich sediments at 100–6809 metres depths (Krylova & Sahling, Citation2010). All known representatives of the subfamily (more than 110 species) possess hypertrophied gills that house intracellular symbiotic bacteria (Boss & Turner, Citation1980; Fiala-Médioni & Métivier, Citation1986; Turner, Citation1985). These chemoautotrophic, sulphur-oxidizing gammaproteobacteria provide most or all of the clams' nutrition (Dubilier, Bergin, & Lott, Citation2008; Felbeck, Citation1981; Fisher et al., Citation1988). The symbionts are transmitted vertically to the next generation via the clams' eggs (Cary, Warren, Anderson, & Giovannoni, Citation1993; Endow & Ohta, Citation1990; Hurtado, Mateos, Lutz, & Vrijenhoek, Citation2003; Szafranski, Gaudron, & Duperron, Citation2014), notwithstanding that separate cases of lateral acquisition have been reported (Decker, Olu, Arnaud-Haond, & Duperron, Citation2013; Stewart, Young, Cavanaugh, Citation2008). This symbiotic relationship has resulted in a pattern of co-speciation between the host and symbiont phylogenies (Distel, Felbeck, & Cavanaugh, Citation1994; Peek, Feldman, Lutz, & Vrijenhoek, Citation1998). Over the long term, this obligate relationship has resulted in a significant reduction of bacterial genome sizes and functional capacities (Kuwahara et al., Citation2007; Newton et al., Citation2007; Newton, Girguis, & Cavanaugh, Citation2008). Corresponding physiological and morphological adaptations have co-evolved in the clams (Goffredi & Barry, Citation2002; Le Pennec, Beninger, & Herry, Citation1995).
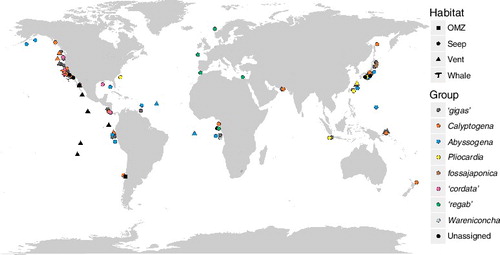
Fig. 1. Pliocardiinae: worldwide distribution (sites of materials examined). Habitats indicated with different symbols and phylogenetic groups differentiated by colours. Overlapping locations were displaced slightly for clarity. Coordinates available in Table S2 (see supplemental material online).
In contrast to Pliocardiinae, the sister subfamily Vesicomyinae includes tiny clams a few mm in size (Krylova & Sahling, Citation2010). The only known genus, Vesicomya Dall 1886, uniting 15 species, occurs worldwide at 400–10,730 m depths in trenches and deep oceanic basins characterized by high inputs of sediment and refractory organic matter (Krylova, Kamenev, Vladychenskaya, & Petrov, Citation2015). Although stable isotope data suggest that chemoautotrophy does not contribute significantly to their nutrition, it remains unclear whether Vesicomya host chemosymbiotic bacteria (Krylova & Sahling, Citation2010, and unpubl. results).
Efforts to discover and explore chemosynthetic environments and the abundance of many pliocardiin clams make them relatively well studied compared with other deep-sea organisms. The landmark discovery of deep-sea hydrothermal vents along the Galápagos Rift (Corliss & Ballard, Citation1977) was quickly followed by a description of ‘Calyptogena’ magnifica Boss & Turner Citation1980, the giant clams that lined basalt crevices (here and elsewhere, single quotation marks are used to denote dubious assignments to genera). Soon after the discovery of vents, pliocardiins were found at cold-water hydrocarbon seeps along the West Florida Escarpment (Hecker, Citation1985; Paull et al., Citation1984). These chemosymbiotic bivalves are now known to occur worldwide in cold seep sediments with hydrogen sulphide concentrations ≥100 µmol (Barry et al., Citation1996; Barry, Kochevar, & Baxter, Citation1997; Fischer et al., Citation2011; Levin, Ziebis, Mendoza, Growney-Cannon, & Walther, Citation2006; Rathburn et al., Citation2009; Sahling et al., Citation2008). Seep sulphides are mostly by-products of anaerobic oxidation of methane (Boetius et al., Citation2000) or other hydrocarbons (Joye et al., Citation2004). Seep fields typically contain multiple patches of clams that range from less than one to several metres in diameter (e.g., Barry et al., Citation1996; Marcon, Ondréas, Sahling, Bohrmann, & Olu, Citation2014; Sahling et al., Citation2008; Suess et al., Citation1998).
The overwhelming majority of pliocardiin species live in cold-water hydrocarbon seeps (summarized herein). To date, the iconic vent clam ‘Calyptogena’ magnifica is the only species known to live mainly on bare basalts near high temperature vents (Kennish & Lutz, Citation1992), but a number of species primarily found at cold seeps also invade sulphide-rich sediments associated with hydrothermal venting (Gebruk, Chevaldonné, Shank, Lutz, & Vrijenhoek, Citation2000; Grehan & Juniper, Citation1996; Levin et al., Citation2012). Some pliocardiin species can occupy sulphide-rich sediments produced by deteriorating whale carcasses (Baco, Smith, Roderick, Peek, & Vrijenhoek, Citation1999; Treude et al., Citation2009) and deposits of fresh organic matter (Cosel & Olu, Citation2009), and one species was recorded from an oxygen minimum zone (Krylova, Sellanes, Valdés, & D'elia, Citation2014). Given the spatial frequency of seeps, whale-falls, and other sites of organic deposition along continental margins, such habitats might have provided a near continuum for pliocardiin dispersal on both contemporary and historical time scales.
Fossil records are not known for the small non-chemosymbiotic Vesicomyinae. The earliest record for a large vesicomyid (putatively a pliocardiin) is from north-east Pacific cold seeps (Goedert & Squires, Citation1990) that date to the middle Eocene, about 40–42 million years ago (Mya) (Goedert, Peckmann, Benham, & Janssen, Citation2013). Hubertschenckia ezoensis, an unequivocal pliocardiin from fossil seeps of the north-western Pacific is 36–38 My old (Amano, Jenkins, Ohara, & Kiel, Citation2014). At least three pliocardiin genera occurred in Late Eocene seeps (Amano & Kiel, Citation2007), and a fairly continuous fossil record persists through the Oligocene and Miocene, 7–30 Mya (Amano & Kiel, Citation2007; Goedert & Squires, Citation1990; Squires & Goedert, Citation1991). If the earliest fossil records are underestimates, a conservative estimate for the origin of pliocardiins is assumed to be about 47 Mya (Amano et al., Citation2014; Kiel, Citation2010). A Cenozoic radiation of pliocardiins corresponds with estimates based on molecular clocks that conservatively placed the origin of pliocardiins at less than 60 Mya (Peek, Gustafson, Lutz, & Vrijenhoek, Citation1997; Vrijenhoek, Citation2013) with major radiations believed to have occurred at 'a regular pace since the Eocene' (Valdés, Sellanes, & Guillermo, Citation2012).
Taxonomic descriptions of Pliocardiinae were based primarily on shell characters that often fail to provide sufficient discrimination among species and genera. It is unsurprising, therefore, that molecular systematic studies of extant species have revealed a number of cryptic species complexes and do not support monophyly of many groups currently treated as genera (Audzijonyte, Krylova, Sahling, & Vrijenhoek, Citation2012; Decker, Olu, Cunha, & Arnaud-Haond, Citation2012; Goffredi, Hurtado, Hallam, & Vrijenhoek, Citation2003; Peek et al., Citation1997, Citation2000; Valdés et al., Citation2012; Vrijenhoek, Schutz, Gustafson, & Lutz, Citation1994). Though molecular traits have generally not been incorporated, recent taxonomic descriptions that included soft anatomical characters have greatly improved the diagnoses of species and genera (Cosel & Olu, Citation2009; Cosel & Salas, Citation2001; Krylova & Cosel, Citation2011; Krylova & Sahling, Citation2006; Krylova, Sahling, & Janssen, Citation2010).
A recent study compared named species defined by morphology (morphospecies) with molecular operational taxonomic units, or MOTUs (sensu Blaxter et al., Citation2005). Audzijonyte et al. (Citation2012) used mitochondrial COI sequences to characterize 44 MOTUs. Except for a few synonymies, many of the morphospecies were well resolved, but 11 undescribed species (MOTUs) were also identified. Consequently, vesicomyid species diversity might be much greater than current estimates inferred from morphospecies criteria alone, a common problem with deep-sea invertebrates (Vrijenhoek, Citation2009). Molecular studies also revealed synonymous species designations that obscured broad trans-oceanic distributions for several clams (Audzijonyte et al., Citation2012; Kojima, Fujikura, & Okutani, Citation2004). Phylogenetic analyses of COI sequences from 51 OTUs suggests a recurrent pattern of speciation, leading from shallow to deep habitats in separate ocean basins (Decker et al., Citation2012). Although these molecular studies have supported some of the morphology-based assignments to genera, examinations of COI sequences alone do not adequately resolve the deeper phylogenetic nodes that might help to define genera.
In this study, we produced a robust multi-gene phylogeny for 49 named vesicomyid species and 15 undescribed MOTUs. We examined new and previously published COI data and added sequences from three nuclear protein-coding genes (Cal, Ant and H3), and two nuclear ribosomal genes (18S and 28S rRNA). Bayesian methods were used to generate a combined phylogeny from the concatenated gene segments, and prior information from the fossil record was incorporated to estimate the ages of evolutionary splits in the combined tree. Factors that might have promoted rapid diversification of pliocardiins during the early to mid-Miocene and Pliocene are discussed. We superimposed the shell and soft body morphological traits on the phylogeny to examine correspondence with current genus-level assignments. We also traced the median depths occupied by each species on the Bayesian phylogeny to test whether pliocardiins followed the general trend of invading deep-sea habitats from the shallows.
Materials and methods
Sample provenance
Sample sources, geographic coordinates, depths, and habitat characteristics are listed in the Electronic Appendix (Table S2, see online supplemental material, which is available from the article's Taylor & Francis Online page at http://dx.doi.org/10.1080/14772000.2016.1252438). The map with species sampling locality information was illustrated in RStudio (RStudio Team, Citation2015) with ggplot2 (Wickham, Citation2009) and ggmap (Kahle & Wickham, Citation2013) (). The depth distributions were illustrated with ggplot2 (Wickham, Citation2009).
DNA sequencing
Specimens used for molecular analyses were deep-frozen (−80°C) or preserved in 95% ethanol. Genomic DNA was extracted and amplified for six genes () following previously described methods (Audzijonyte & Vrijenhoek, Citation2010; Audzijonyte et al., Citation2012). Many of the preserved or frozen specimens obtained from diverse sources were degraded prior to our acquisition. Previously published data were combined with new sequencing results for mitochondrial cytochrome-c-oxidase subunit-I (COI, 4 newly sequenced taxa), nuclear histone-3 (H3, 24 newly sequenced taxa), nuclear ADP/ATP translocase (ANT, 27 newly sequenced taxa), 28S rRNA (25 newly sequenced taxa), 18S rRNA (22 newly sequenced taxa), and nuclear calmodulin (Cal, 18 newly sequenced taxa). GenBank accession numbers for 120 (KX010137-255, KX087164-65) new sequences generated in this study are included along with accession numbers for previously published sequences (Table S1, Figs S1 & S2, see supplemental material online). All of MOTUs could not be amplified for each of the nuclear genes because many of the specimens generated poor PCR products; consequently, we sometimes concatenated nuclear gene sequences from two or more individuals belonging to the same COI-defined MOTU. Multiple replicate individuals for each COI-MOTU were sequenced for each nuclear locus to determine whether variation existed within a MOTU. To ensure correct taxon assignments for the nuclear gene markers, we provide a COI-barcode for each associated nuclear sequence (Table S1, see supplemental material online). All PCR products were diluted in 50 μl PCR grade water then purified using the Multiscreen HTS PCR 96 vacuum manifold system (Millipore Corp. Billerica, MA). Sequencing reactions used the same PCR primers, and sequencing was performed bi-directionally on an ABI3130XL with BigDye Terminator v3.1 chemistry (Life Technologies Corp., Carlsbad, CA). Sequences were aligned and edited in Geneious R9.1.2 (Biomatters, www.geneious.com/).
Table 1. PCR primers, methods and amplicon lengths for six gene-fragments in Vesicomyidae.
Phylogenetic analyses
We used the Bayesian information criterion (BIC) (Akaike, Citation1974) implemented in jModelTest (Posada, Citation2008) to determine the most appropriate evolutionary model for each gene segment. Then, individual trees for each gene-segment were estimated with MrBayes v3.1.2 (Huelsenbeck & Ronquist, Citation2001; Ronquist & Huelsenbeck, Citation2003). Analyses were conducted in two chains for 10–50 × 106 generations, and trees were sampled every 1000 generations after a burn-in of 1000 generations. Each analysis was repeated a minimum of three times, mixing and convergence were accessed with Tracer v1.6 (Rambaut & Drummond, Citation2010) and the resulting trees were visualized with FigTree v1.4.0 (Rambaut, Citation2010).
MrBayes v3.1.2 was also used to estimate a concatenated multi-gene topology. As previously determined, each gene-segment was partitioned and coded for the most appropriate evolutionary model. The resulting topology was examined with BEAST (Drummond & Rambaut, Citation2007) to estimate evolutionary divergence times. We assumed a Yule speciation model with a strict molecular clock. Two fossil ages were used to calibrate evolutionary rates: (1) the first appearance ‘Archivesica’ cf. tschudi during the middle Eocene (Amano & Kiel, Citation2007; Amano et al., Citation2014); and (2) an Oligocene fossil of Calyptogena katallaensis (Kiel & Amano, Citation2010). Analyses were conducted for 106 generations with a 10% burn-in, and repeated 5 times. The results were analysed in Tracer v1.6 (Rambaut & Drummond, Citation2010) to assess convergence. Trees were then thinned and pooled with TreeAnnotator v1.7.5 and plotted with FigTree v1.4.0 (Rambaut, Citation2010).
Morphological analysis
We constructed a morphological dataset that incorporated newly acquired and previously published information (Table S3). Presently, these data comprise the most comprehensive information available for the majority of MOTUs examined in this study. Four conchological and three anatomical features were recorded from adult specimens: (1) maximum shell length; (2) shell shape; (3) extent of reduction of hinge teeth; (4) extent of development of pallial sinus; (5) number of demibranchs; (6) structure of the interlamellar septum in gills; and (7) course of the midgut. We used calipers (± 0.1 mm) to obtain measurements of shell length (L) and height (H). Designation of character states for the hinge teeth followed the vesicomyid literature (Cosel & Salas, Citation2001; Horikoshi, Citation1989; Krylova & Sahling, Citation2006; Okutani, Fujikura, & Kojima, Citation2000). The course of midgut was determined after removal of the left gill and part of visceral mass. Shell lengths were categorized as follows: large (l), L > 200 mm; medium (m), 200 mm ≥ L ≥ 35 mm; and small (s), L < 35 mm. Shell shapes were categorized from height/length (H/L) ratios: elongate (e) shell shape, H/L < 0.3; ovate (o), 0.3 ≤ H/L ≤ 0.7; and round (r), H/L > 0.7. Hinges were categorized as: (1) diminution of anterior ramus of subumbonal cardinal tooth (3a) of the right valve; (2) diminution of posterodorsal cardinal tooth (4b) of the left valve; and (3) the absence of any reduction of hinge teeth. The pallial sinus was categorized as three states: (1) well-developed sinus; (2) sinus present as small indentation; and (3) absence. The number of gill demibranchs varied from 1 to 2 pairs, and the interlamellar septum was categorized as (1) a plate; (2) divided into separate tubes only in its distal parts; and (3) completely tubular. The course of midgut varied from straight (or nearly straight) to Z-shaped.
Trait mapping
We used published and newly obtained minimum and maximum depth records to define the median depth-of-record for each MOTU. Median depths were then binned into four categories: sublittoral (100–299 m); upper bathyal (300–799 m); lower bathyal (800–2999 m); abyssal (3000–6500 m); and hadal (>6500 m) (Table S3). The program Mesquite v2.75 (Maddison & Maddison, Citation2011) used parsimony analyses to trace these records over the concatenated Bayesian phylogeny.
Results
Individual gene-trees
High copy-number mitochondrial DNA improved our success of recovering COI sequences from many of the poorly preserved specimens examined in this study. Incorporation of COI sequences from three morphologically verified Vesicomya species provided the first sound basis for rooting the pliocardiin tree (Fig. S1, see supplemental material online). The 61 pliocardiin MOTUs, defined by a 510 base pair (bp) fragment of unique COI sequences that differed minimally by 3% sequence divergence (Audzijonyte et al., Citation2012), comprised a distinct monophyletic clade. Thirty-eight of these MOTUs fell into three well-defined subclades that were identified in earlier studies: (I) the diverse gigas-group; (II) Calyptogena; and (III) Abyssogena (cf. Audzijonyte et al., Citation2012; Decker et al., Citation2012). Thirteen MOTUs fell into subclades 4–7 that we subsequently discuss after considering the multi-gene analysis. The remaining MOTUs were subsumed in basal polytomies with weaker support.
Individually, the nuclear gene fragments were less informative. We were unsuccessful in amplifying single-copy nuclear genes from many of the poorly preserved samples. However, replicate sequencing efforts of nuclear fragments showed little or no variation (range 0–0.01%) within each COI-defined MOTU. Additionally, very few nuclear sequences from vesicomyids were available in GenBank (Table S1, see supplemental material online). Despite limited taxon coverage, the five nuclear gene-trees reinforced broad patterns evident in the COI tree. For example, three gene trees (H3, based on 351 bp; ANT, based on 463 bp; and 28S rRNA, based on 781 bp) recovered the same four subclades previously defined by COI (Figs S2.1, 2.2, and 2.3, see supplemental material online). Two gene-trees were less informative. The 18S rRNA tree (based on 555 bp) was poorly resolved due to limited variation (Supplementary Fig. S2.4, see supplemental material online). Ambiguous alignments of the short Cal intron sequences (155 bp) provided very few phylogenetically informative sites (Fig. S2.5, see supplemental material online), and the gene-tree recovered only the gigas subclade.
The multi-gene tree
Due to the preponderance of COI sequences and variable nature of this mitochondrial gene, the concatenated phylogeny () topologically resembled the COI-tree. Nonetheless, incorporation of the nuclear gene-segments improved resolution for seven pliocardiin subclades (highlighted colours in ), ranked here in order of species richness: (I) the gigas-group; (II) Calyptogena; (III) Abyssogena; (IV) the cordata-group; (V) the regab-group; (VI) Pliocardia; and (VII) the fossajaponica-group. Relationships among the seven subclades were not well resolved and more informative gene sequences and additional taxon sampling will be required to resolve these relationships.
Fig. 2. Vesicomyidae: fossil-calibrated multi-gene tree with superimposed morphological data (see for details). Dubious generic assignments indicated by single quotes and square brackets. Undescribed species with placeholder epithets, as designated in earlier publications (authors abbreviated in parentheses). Asterisks (*) indicate Posterior Probability (PP) = 1.0. All other PP values are indicated at nodes. Scale-bar = 7 my. Red and blue ovals indicate fossil calibration estimates (see text). Well-supported clades ranked by richness indicated by coloured boxes and roman numerals, including: gigas-group (grey, 1), Calyptogena (orange, 2), Abyssogena (blue, 3), cordata-group (pink, 4), regab-group (green, 5), Pliocardia (yellow, 6), and the fossajaponica-group (tan, 7).
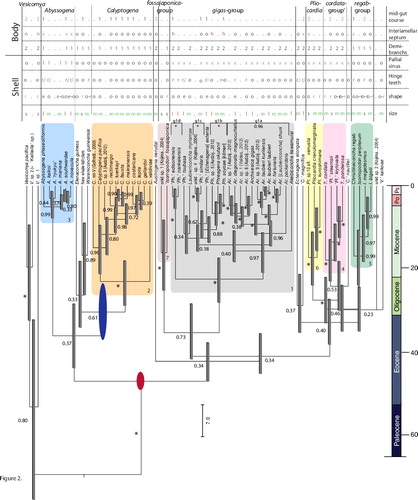
Divergence times
We used the combined phylogeny to estimate a 'time-tree' (Hedges, Dudley, & Kumar, Citation2006) that was calibrated from the limited vesicomyid fossil record. The middle Eocene (∼47 ± 2 Mya) cold seep fossil ‘Archivesica’ cf. tschudi (Amano & Kiel, Citation2007; Amano et al., Citation2014) was used to establish the first verified appearance of a large pliocardiin clam (red oval in ), and the lower Oligocene (∼30 ± 7 Mya) fossil Calyptogena katallaensis (Kiel & Amano, Citation2010) was used to establish first appearance of the genus Calyptogena (blue oval). Accordingly, diversifications of the major pliocardiin subclades appeared to originate during the mid- to late Eocene and Oligocene. Radiations within the subclades originated mostly in the late Oligocene to mid-Miocene. The genus Abyssogena appears to represent the youngest radiation, in the late Miocene to early Pleistocene.
Shell morphology
Shell lengths were small for the representative Vesicomyinae and highly variable among the Pliocardiinae, ranging from 15 mm in Isorropodon megadesmus to 280 mm in Abyssogena novacula. However, lengths appeared to be constrained within the genus Calyptogena (medium) and the fossajaponica-group (short). Shell shapes ranged from subcircular (‘Pl.’ krylovata H/L = 0.88) to extremely elongated (Abyssogena phaseoliformis H/L = 0.19). Shell shapes also appeared to be constrained in Vesicomya (subcircular) and the genus Calyptogena (ovate), whereas elongated clams arose multiple times within Abyssogena and the gigas-group. The expression of hinge teeth varied from complete (three well-developed teeth on each valve and nymphal ridge on the right valve) in Calyptogena pacifica to reduced (two teeth on each valve) in ‘Ectenagena’ elongata. Hinge teeth were conserved in all but the gigas- and regab-groups, where lineages differ in extent of development and relative location of hinge teeth. Position and depth of the pallial sinus was conserved in all groups.
Soft parts morphology
Anatomical characters (Table S3, see supplemental material online) provided somewhat better resolution amongst the major pliocardiin groups. Number of gills was conserved in all groups. Interlamellar septum morphology appeared to be constrained in all but the gigas- and regab-groups. Although there were many missing data, the course of the mid-gut appeared to be constrained for all groups except the regab-group.
Systematic implications
Though taxon sampling was still limited, the combination of molecular and morphological information helped to further delineate several monophyletic genera and informal groupings. Two described extant pliocardiin genera, Waisiuconcha Beets, 1942, and Callogonia Dall, 1889, that were unavailable for the present analyses cannot be placed in the present phylogenetic context. We subsequently consider eight (including the genus Vesicomya) well-supported clades and a small number of unconsolidated taxa.
Genus Vesicomya Dall, 1886
The vesicomyins examined in this study comprised a discrete monophyletic clade in the COI tree, but limited data for the nuclear gene trees weakened their association in the multi-gene analysis. Nonetheless, this small sample of Vesicomya s.s. species were characterized by small sizes, rounded shell shapes, complete sets of hinge teeth, very short siphons with thin siphonal muscle bundles, two pairs of demibranchs without interlamellar septae, and Z-shaped mid-guts. Thus, we considered these traits as plesiomorphic character states for Vesicomyidae (); and we have treated the alternative states as derived features for the Pliocardiinae (Krylova & Sahling, Citation2010; Citation2015; Krylova et al., Citation2014).
Genus Abyssogena Krylova et al. Citation2010
The youngest of the major pliocardiin groupings, Abyssogena is found in the Atlantic and Pacific basins. Shells are medium to large with ovate to elongate shapes, reduced or absent left (4b) and right (3a) hinge teeth, and a small indent in the pallial sinus. Gills are reduced to one pair of demibranchs with an interlamellar septum that consists of separate tubes. The three species examined had straight midguts.
Genus Calyptogena Dall, 1891
Radiating primarily during the Miocene, this genus is broadly distributed in the Pacific, Atlantic and Indian oceans. Shells are medium sized with ovate shapes, unreduced hinge teeth, and no pallial sinus. Gills have one pair of demibranchs with an interlamellar septum and no gill tubes. The midgut is straight.
Genus Pliocardia, Woodring, 1925
Diversifying during the Miocene, available specimens of this well-supported genus have been limited to the western Pacific and western Atlantic oceans. The present OTUs were most closely related to members of cordata-group, which they morphologically resembled in having small- to medium-sized shells with subcircular to ovate shape, unreduced hinge teeth, and the presence of the pallial sinus. Gills have two pairs of demibranchs; one species examined has an interlamellar septum with tubes and Z-shaped midgut.
The gigas-group
Largest of the major pliocardiin subclades, the gigas-group diversified during the late Oligocene and radiated throughout the Miocene and into the Pliocene. The group has a worldwide distribution in the Atlantic, Pacific and Indian oceans. Morphological characteristics were highly variable and often poorly resolved among the available species. Shell sizes are medium to large. Shell shapes vary from ovate to elongate. All examined species are characterized by the presence of pallial sinus. Hinges have complete to reduced set of teeth. Gills have two pairs of demibranchs. Structure of an interlamellar septum vary and can be plate or tubular. The data on midgut course are very fragmentary; in all examined species midguts are straight.
Given their morphological diversity, it is not surprising that five genus names have been applied to members of this group. Nonetheless, assignments of some genus names are paraphyletic (). To remedy this problem, we recommend restriction of the problematic names to monophyletic lineages. The genus Archivesica s.s. Dall, 1908, should be restricted to the cluster of species identified as lineage g1a (PP = 0.96), which includes Ar. [Laubiericononcha] chuni. All g1a members lacked separate tubes in the interlamellar septae. In contrast, the genus Phreagena s.s. Woodring, 1938 should be restricted to the three members of cluster g1b (PP = 1.0); two examined species of this cluster have interlamellar septae divided distally into separate tubes. Two genera include single species on the basis of the material used and lie outside of the other sub-clusters, Laubiericoncha Cosel & Olu, Citation2008 (myriamae) and Akebiconcha Kuroda, 1943 (kawamurai). Laubiericoncha is characterized by a shell with the posterior-most point and the posterodorsal area with two shallow and rounded ridges (Cosel & Olu, Citation2008). A hinge margin with well-developed tightly located radiated teeth distinguishes Ak. kawamurai. This leaves three lineages with dubious paraphyletic assignments to ‘Phreagena’: sub-clusters g1c, g1d + ‘Ph.’ tsubasa and ‘Ph.’ [Ectenagena] extenta. A unique structure in the distal part of lumen of the inhalant siphon that almost entirely closes the aperture of the siphon distinguishes ‘Ph.’ [Ectenagena] extenta. Until more detailed morphological and molecular information is available, we retain the dubious application of ‘Phreagena’ to these problematic OTUs. We suggest that successful radiation of the species was promoted by their ability to exploit diverse microniches on the basis of morphological (Cosel & Olu, Citation2009; Krylova & Cosel, Citation2011) and physiological adaptations (Decker et al., Citation2014; Goffredi & Barry, Citation2002).
The fossajaponica-group
The two fossajaponica-group MOTUs probably warrant recognition as a new genus. Sampled from very deep trenches (4700–6400 m) in the western Pacific, ‘Isorropodon [Calyptogena [Ectenagena]]’ fossajaponica (Okutani et al., Citation2000) was assigned to different genera due to the small ovate shells, a single pair of demibranchs, and no pallial sinus (Krylova & Sahling, Citation2010), but the similarities are convergent. A distinct hinge margin (Oliver & Drewery, Citation2013) and molecular evidence clearly indicate they do not belong with Isorropodon. Morphological data were not available for the unidentified MOTU, ? sp. 1 from the Japan and Kurile trenches (Kojima et al., Citation2004).
The cordata-group
Radiating during the Miocene, the cordata-group members also warrant erection of a new genus. Known from the eastern Pacific and Gulf of Mexico, they appear to be most closely related to Pliocardia. The four members share oval to rounded medium or small size shells, a shallow pallial sinus, a Z-shaped mid-gut and two pairs of demibranchs without separate files in interlamellar septae.
The regab-group
This morphologically diverse group includes three Isorropodon species and the monotypic genus Christineconcha. All four species possess a single pair of demibranchs, lack a pallial sinus, and have a foot shaped with lateral ‘wings’ (Cosel & Salas, Citation2001; Krylova & Cosel, Citation2011) that might be considered synapomorphic. Nevertheless, the large and elongated Christineconcha regab differs from the small-sized and ovate Isorropodon species. Isorropodon also lacked separate tubes in the interlamellar septae, the mantle was fused before the inhalant siphon, and the alimentary tract changed the direction twice rather than had a straight tract as in Christineconcha. Therefore, we retain Isorropodon Sturany, 1896 and Christineconcha Krylova & Cosel, Citation2011 as two closely related but separate genera.
Unconsolidated taxa
Nine species with roots that extend into the Oligocene and Eocene did not fall into well-supported clusters (). The iconic hydrothermal vent specialist, ‘Calyptogena’ magnifica Boss & Turner, Citation1980, represents a new monotypic genus (under description) showing no affinities to other pliocardiins. Similarly, genera Ectenagena Woodring, 1938 (E. elongata), Elenaconcha Cosel & Olu, Citation2009 (E. guiness), and Austrogena Krylova et al., Citation2014 (A. nerudai) were monotypic. Both ‘C.’ magnifica and E. elongata exhibited apomorphies or unique combinations of characters, that corresponded with their unresolved positions in the phylogeny. Genus-level relationships of ‘Vesicomya’ kaikoae Okutani et al., Citation2000 and ‘Calyptogena’ nautilei Okutani & Métivier, 1986 were unresolved and morphological information is unavailable.
Based on COI alone (Fig. S1, see supplemental material online), W. guineensis (Thiele & Jaeckel, Citation1931), the only genetically studied species of the genus Wareniconcha Cosel & Olu, Citation2008, is related to an unusual small clam found near a sunken whale skeleton (Pedro's whale-fall) in the Santa Cruz Basin at 1893 m depth. Although the combined analysis did not strengthen support for this relationship, anatomical similarities exist. Shell morphologies differed, but both species possess one pair of demibranchs without files in the interlamellar septae, and the siphonal muscle is attached to the upper anterior margin of posterior adductor. Calyptogena lepta was previously included with the genus Wareniconcha on the basis of similarity of ovoid shell outline (Krylova & Sahling, Citation2010), but the present molecular data firmly placed lepta within Calyptogena. Wareniconcha and Calyptogena share such characters as the absence of the pallial sinus, the presence of one pair of demibranchs and the plate structure of interlamellar septae. More details on the structure of the siphons are needed to better discriminate between the two genera.
Bathymetric depth
Depths for the taxa examined in this study ranged from ∼100 to 6809 metres (, Table S3). We were not confident about actual depth limits, because many of the OTUs were sampled from only one locality. At best, we could roughly categorize species according to their occupancy of depth zones: sublittoral (100–299), upper bathyal (300–799), lower bathyal (800–2999), abyssal (3000–6500) and hadal >6500 metres. Despite the limited sampling, median depth zones appeared to be constrained for some groups – e.g., all the Abyssogena species were sampled from abyssal depths. In contrast, Calyptogena and the regab-, gigas-, and cordata-groups were sampled from wide depth ranges. The median depths of most Calyptogena species were lower bathyal, but two species extended into the abyssal zone. The minimum depth of Calyptogena species extended from the upper to the lower bathyal zone. Isorropodon bigoti exhibited the shallowest depth record for vesicomyids. Pliocardia appeared to be limited to the upper and lower bathyal zones. The diverse gigas-group also exhibited the greatest variability: minimum depths range from sublittoral to abyssal and maximum depths range from upper bathyal to abyssal. Within subclade (Archivesica + L. chuni) depth ranges varied the greatest, from upper bathyal to abyssal. However, the other gigas group subclades appeared to be more restricted, with maximum depths mainly in the lower bathyal and minimum depths in the upper bathyal zones. Tracing the median depths occupied by each species on the Bayesian phylogeny () identified multiple independent colonizations of abyssal zones.
Fig. 3. Vesicomyidae: median depths traced on Bayesian-estimated multi-gene topology, with known depth ranges detailed above each MOTU. Groups ranked by species richness: (I, grey box) the gigas-group; (II, orange box) Calyptogena; (III, blue box) Abyssogena; (IV, pink box) the cordata-group; (V, green box) the regab-group; (VI, yellow box) Pliocardia; and (VII, tan box) the fossajaponica-group.
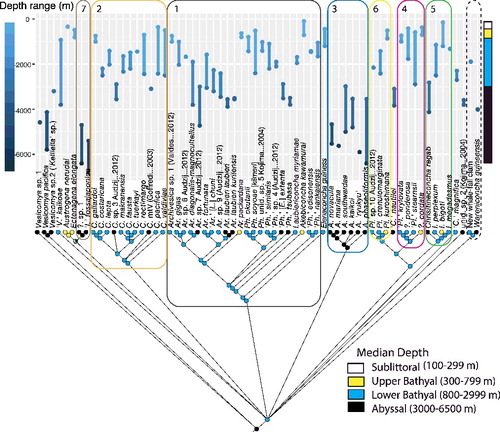
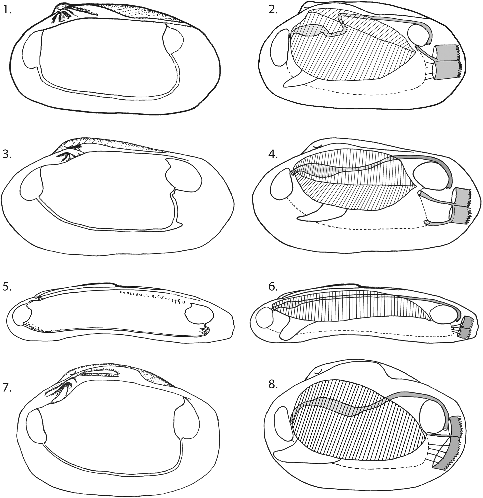
Fig. 4. Schematic drawings of morphological features used in this study including: (1 & 2) Austrogena nerudai, L – 20 mm. Features: two pairs of demibranchs, Z-shaped alimentary gut, the absence of the pallial sinus as a result of attachment of siphonal muscles to the ventral part of the posterior adductor. (3 & 4) Phreagena soyoae, L – 70 mm. Features: two pairs of demibranchs, straight alimentary gut, the presence of a pallial sinus as a result of attachment of well-developed siphonal muscles to the surface of shell anterior to the posterior adductor. (5 & 6) Abyssogena phaseoliformis, L – 200 mm. Features: one pair of demibranchs, straight alimentary gut, and the presence of pallial sinus as a small indentation. (7 & 8) Calyptogena pacifica, L – 55 mm. Features: one pair of demibranchs, straight alimentary gut, and the absence of pallial sinus as a result of attachment of siphonal muscles to the ventral part of the posterior adductor.
Discussion
Previous molecular phylogenetic analyses of symbiotrophic vesicomyids used rather distantly related representatives of different heterodont superfamilies (e.g., veneroids, dreissenoids, cyrenoids, and mactroids) as outgroup taxa (Baco et al., Citation1999; Decker et al., Citation2012; Goffredi et al., Citation2003; Peek et al., Citation1997; Peek et al., Citation2000). We expanded on previous efforts by including new and published COI sequences from pliocardiin and vesicomyin OTUs, by examining DNA sequences from five nuclear gene segments, and by integrating information from the fossil record. We also conducted morphological investigations of several shell and soft-anatomical features. Conchological characters within the Pliocardiinae were highly variable and not often congruent with the molecular groupings. Soft-anatomical characters were more informative and provided better resolution amongst these groups. Together, the molecular and morphological data illuminated relatively robust generic assignments for a majority of the pliocardiins.
Our time-calibrated phylogenetic analysis placed the split between pliocardiins and their non-symbiotrophic vesicomyin relatives during the Late Cretaceous (66–100 Mya) (). This result agrees with Bieler et al. (Citation2014), who generated a bivalve tree-of-life from nine gene fragments and ten fossil calibration points. They estimated a late Cretaceous to Mid-Cenozoic (95% HPD) split between ‘Calyptogena’ magnifica (Pliocardiinae) and ‘Kelliella’ sp. (identified here as Vesicomya sp. 2; or Vesicomyinae, as previously noted by Krylova et al. (Citation2015)). Although a late Cretaceous to early Cenozoic origin of symbiotrophic pliocardiins is not surprising, the implication that they arose from deep-sea-dwelling ancestors stands in contrast to the evolutionary polarities seen in other vent and seep species that typically radiated, or re-radiated, from shallow-water ancestors (Vrijenhoek, Citation2013). We suggest that pliocardiins originated from small-sized Vesicomya-like clams that inhabited bathyal depths. Unlike bathymodiolins, another successful and diverse group of chemosymbiotic bivalves, neither the fossil record nor the molecular evidence suggest that the early evolutionary history of pliocardiins involved a shallow water ancestry that exploited organic substrates or took 'wooden steps to deep-sea vents' (Distel et al., Citation2000; Lorion et al., Citation2013; Samadi et al., Citation2007). Furthermore, the deep-sea ancestors of pliocardiins might have already possessed physiological pre-adaptations that facilitated the invasion of greater abyssal depths, allowing pliocardiins to attain the greatest known species diversity among deep-sea chemosymbiotic bivalves. Their subsequent colonizations of bathyal, abyssal, and sublittoral zones was not unidirectional, however, as various independent lineages have invaded deeper and shallower environments ().
The ancestors of pliocardiins must have acquired sulphur-oxidizing symbionts sometime before the middle Eocene since the first record of a fossilized 'large' vesicomyid is ‘Archivesica’ cf. tschudi, from the Humptulips Formation in western Washington State, USA, about 47 Mya (Amano & Kiel, Citation2007). Morphologically similar to contemporary symbiotrophic pliocardiins (Amano & Kiel, Citation2012), ‘Ar.’ cf. tschudi was found amongst other fossil chemosymbiotic animals in what appears to have been an ancient hydrocarbon seep. Based on our analyses, radiation of the major pliocardiin sub-clades began during the Late Eocene and continued throughout the Oligocene (), which is congruent with an earlier analysis based on COI sequences alone (Valdés et al., Citation2012). The first and major wave of diversification among the chemosymbiotic mussels might have occurred around the same time – in the Middle Eocene–Early Oligocene (Lorion et al., Citation2013). Indeed, most contemporary deep-sea chemosynthetic taxa appear to have originated sometime after the Palaeocene/Eocene thermal maximum (PETM) about 56 Mya (reviewed in Vrijenhoek, Citation2013). Acquisition of chemosynthetic symbiotic bacteria and the radiations of major pliocardiin lineages and bathymodiolin mussels are also coincident with increased sulphate concentrations during the Early Eocene (∼50 Ma) (Kiel, Citation2015). Large-scale extinctions due to anoxic/dysoxic deep-sea environments during the PETM (Jacobs & Lindberg, Citation1998) and increased sulphide availability during the Early Eocene might have created the ecological opportunities that facilitated invasions of chemosynthetic environments by new taxa and promoted re-radiations of pre-existing stem-lineages (Vrijenhoek, Citation2013).
Explaining the high diversity of pliocardiins, even within a single geographic area, such as Monterey Bay, requires a careful examination of potential ecological factors that might have promoted speciation. Evolutionary radiations of pliocardiins also probably were facilitated by bathymetric segregation and niche differentiation. Co-distributed members of the Calyptogena 'pacifica/lepta' species complex often segregate bathymetrically along the western margin of North America (Goffredi et al., Citation2003). Co-habiting species such as Calyptogena pacifica and Phreagena soyoae (cited as ‘Calyptogena’ kilmeri) tend to segregate into distinct microhabitats. The species process sulphides differently and they segregate along sulphide gradients ranging from the centre to the margin of cold seeps in Monterey Bay, California (Barry et al., Citation1997; Goffredi & Barry, Citation2002). Co-habiting species in the Gulf of Guinea, Laubiericoncha chuni buries itself deeply in sediments whereas Christineconcha regab extends far about the sediment (Decker et al., Citation2013). Small changes in the vertical positions affect their capacity to differentially exploit the steep gradients that exist in vent and seep sediments (Barry et al., Citation1996; Barry et al., Citation1997; Decker et al., Citation2013; Goffredi & Barry, Citation2002). Despite local-scale segregation, some pliocardiin species have trans-oceanic distributions including L. chuni, which inhabits the Eastern Atlantic ocean and possibly even as well both sides of the Pacific ocean (Audzijonyte et al., Citation2012). New records of pliocardiins from the northern part of the Bering Sea (Danilin, Citation2013) shows populations bridge the western- and eastern Pacific margins. Their broad ranges might result from the frequent occurrence of hydrocarbon seeps and other reducing habitats along continental margins (Audzijonyte et al., Citation2012).
Conclusions
Pliocardiins represent the most diverse group of chemosymbiotic bivalves living in deep-sea environments. The present evidence supports the hypothesis that they split from small-sized, non-symbiotic, Vesicomya-like predecessors during the Late Cretaceous to the early Cenozoic, and that their acquisition of sulphur-oxidizing intracellular bacterial symbionts allowed them to invade greatly expanded chemosynthetic habitats during the Eocene. We suggest that early stages of the evolution of symbiosis in vesicomyids were not initially linked to organic substrates like sunken-wood. The main trigger for initial diversification probably resulted from the integration of sulphide-oxidizing bacteria that allowed the clams to exploit sulphide-rich deep-sea hydrocarbon seeps. Since the Middle Eocene, pliocardiins radiated into a variety of morphologically and physiologically distinguishable clades, that deserve formal taxonomic recognition. This radiation might have been promoted by morpho-physiological adaptations that allowed the exploitation of narrow and diverse ecological niches in highly gradient seep and vent environments. A large number of monotypic genera of extant fauna combined with fossil data indicate much extinction of pliocardiins. These findings, in combination with evidence for recent speciation in Abyssogena, Archivesica, Phreagena and others, present pliocardiins as a changing and dynamically developing group.
Supplemental data
Supplemental data for this article can be accessed here: http://dx.doi.org/10.1080/14772000.2016.1252438.
Availability of supporting data
All sequence data including alignments are published on GenBank Accession nos. KX010137-255, KX087164-65 (Table S1, Figs S1 & S2, see supplemental material online).
Table S1
Download MS Word (153 KB)Table S2.xls
Download MS Excel (43.5 KB)Tables S3.xls
Download MS Excel (49 KB)Acknowledgements
The project was possible because of the expert help of captains, crews, and submarine pilots of oceanographic vessels, human-occupied vehicles (HOVs), and remotely operated vehicles (ROVs) operated by: the Woods Hole Oceanographic Institute (R/V Atlantis II, R/V Atlantis, HOV Alvin, and ROV Jason II); the Monterey Bay Aquarium Research Institute (R/V Point Lobos, R/V Western Flyer, ROV Ventana, ROV Tiburon, and ROV, Doc Ricketts); the P.P Shirshov Institute of Oceanology RAS (R/V Akademik Mstislav Keldysh, HOV MIR-1 and MIR-2). We thank James Barry (MBARI), Shana Goffredi (Occidental College), Cindy Van Dover (Duke Univ.), Gary Greene (retired, Moss Landing Marine Laboratory), Rudo von Cosel (MNHN), Katrine Linse (BAS), H. Saito (NMNS), Coleen Cavanaugh (Harvard Univ.), Lev Moskalev (retired, IORAN), Sergey Galkin (IORAN), Kazunori Hasegawa (NMNS – National Museum of Nature and Science), and Jun Hashimoto (retired, Japan Marine Science & Technology Center) for contributing essential specimens.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19, 716–723.
- Amano, K., Jenkins, R. G., Ohara, M., & Kiel, S. (2014). Miocene vesicomyid species (Bivalvia) from Wakayama in southern Honshu, Japan. The Nautilus, 128, 9–17.
- Amano, K., & Kiel, S. (2007). Fossil vesicomyid bivalves from the North Pacific region. The Veliger, 49, 270–293.
- Amano, K., & Kiel, S. (2012). Two Neogene vesicomyid species (Bivalvia) from Japan and their biogeographic implications. The Nautilus, 126, 79–85.
- Audzijonyte, A., Krylova, E. M., Sahling, H., & Vrijenhoek, R. C. (2012). Molecular taxonomy reveals broad trans-oceanic distributions and high species diversity of deep-sea clams (Bivalvia: Vesicomyidae: Pliocardiinae) in chemosynthetic environments. Systematics and Biodiversity, 10, 403–415.
- Audzijonyte, A., & Vrijenhoek, R. C. (2010). Three nuclear genes for phylogenetic, SNP and population genetic studies of molluscs and other invertebrates. Molecular Ecology Resources, 10, 200–104.
- Baco, A. R., Smith, C. R., Roderick, G. K., Peek, A. S., & Vrijenhoek, R. C. (1999). The phylogenetic relationships of whale-fall vesicomyid clams based on mitochondrial COI DNA sequences. Marine Ecology Progress Series, 182, 137–147.
- Barry, J. P., Greene, H. G., Orange, D. L., Baxter, C. H., Robison, B. H., Kochevar, R. E., … Mchugh, C. M. (1996). Biologic and geologic characteristics of cold seeps in Monterey Bay, California. Deep-Sea Research I, 43, 1739–1762.
- Barry, J. P., Kochevar, R. E., & Baxter, C. H. (1997). The influence of pore-water chemistry and physiology on the distribution of vesicomyid clams at cold seeps in Monterey Bay: Implications for patterns of chemosynthetic community organization. Limnology and Oceanography, 42, 318–328.
- Bieler, R., Mikkelsen, P. M., Collins, T. M., Glover, E. A., Gonzalez, V. L., Graf, D. L., … Giribet, G. (2014). Investigating the Bivalve Tree of Life – an exemplar-based approach combining molecular and novel morphological characters. Invertebrate Systematics, 28, 32–115.
- Blaxter, M., Mann, J., Chapman, T., Thomas, F., Whitton, C., Floyd, R., & Abebe, E. (2005). Defining operational taxonomic units using DNA barcode data. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 360, 1935–1943.
- Boetius, A., Ravenschlag, K., Schubert, C. J., Rickert, D., Widdel, F., Gieseke, A., … Pfannkuche, O. (2000). A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature, 407, 623–626.
- Boss, K. J., & Turner, R. D. (1980). The giant white clam from the Galapagos rift, Calyptogena magnifica species novum. Malacologia, 20, 161–194.
- Cary, S. C., Warren, W., Anderson, E., & Giovannoni, S. J. (1993). Identification and localization of bacterial endosymbionts in hydrothermal vent taxa with symbiont-specific polymerase chain reaction amplification and in situ hybridization techniques. Molecular Marine Biology and Biotechnology, 2, 51–62.
- Colgan, D. J., Ponder, W. F., & Eggler, P. E. (2000). Gastropod evolutionary rates and phylogenetic relationships assessed using partial 28S rDNA and histone H3 sequences Zoologica Scripta, 29, 29–63.
- Corliss, J. B., & Ballard, R. D. (1977). Oasis of life in the cold abyss. National Geographic Magazine, 152, 441–453.
- Cosel, R. Von, & Salas, C. (2001). Vesicomyidae (Mollusca, Bivalvia) of the genera Vesicomya, Wasiuconcha, Isorropodon and Callogonia in the eastern Atlantic and the Mediterranean. Sarsia, 86, 333–336.
- Cosel, R. V., & Olu, K. (2008). A new genus and new species of Vesicomyidae (Mollusca: Bivalvia) from cold seeps on the Barbados accretionary prism, with comments on other species. Zoosystema, 30, 929–944.
- Cosel, R. V., & Olu, K. (2009). Large Vesicomyidae (Mollusca: Bivalvia) from cold seeps in the Gulf of Guinea off the coasts of Gabon, Congo and northern Angola. Deep-Sea Research II, 56, 2350–2379.
- Danilin, D. D. (2013). Folding mollusks as the potential indicators of regions of hydrothermal activity (in Russian). In Volcanicity and related processes (pp. 291–294). Petropavlovsk-Kamchatsky, Russia: Kamchatka Research Institute of the Fisheries and Oceanography.
- Decker, C., Olu, K., Arnaud-Haond, S., & Duperron, S. (2013). Physical proximity may promote lateral acquisition of bacterial symbionts in vesicomyid clams. Public Library of Science One, 8, e64830–64812.
- Decker, C., Olu, K., Cunha, R. L., & Arnaud-Haond, S. (2012). Phylogeny and diversification patterns among vesicomyid bivalves. Public Library of Science One, 7, e33359.
- Decker, C., Zorn, N., Potier, N., Leize-Wagner, E., Lallier, F. H., Olu, K., & Andersen, A. C. (2014). Globin's structure and function in vesicomyid bivalves from the Gulf of Guinea cold seeps as an adaptation to life in reduced sediments. Physiological and Biochemical Zoology, 87, 855–869.
- Distel, D.L., Baco, A.R., Chuang, E., Morrill, W., Cavanaugh, C., & Smith, C.R. (2000). Marine ecology: Do mussels take wooden steps to deep-sea vents? Nature, 403, 725–726.
- Distel, D. L., Felbeck, H., & Cavanaugh, C. M. (1994). Evidence for phylogenetic congruence among sulfur-oxidizing chemoautotrophic bacterial endosymbionts and their bivalve hosts. Journal of Molecular Evolution, 38, 533–542.
- Drummond, A. J., & Rambaut, A. (2007). BEAST, Bayesian Evolutionary Analysis by Sampling Trees. BioMed Central Evolutionary Biology 7:214. Retrieved from: https://bmcevolbiol.biomedcentral.com/articles/10.1186/1471-2148-7-214 (accessed 19 October 2016).
- Dubilier, N., Bergin, C., & Lott, C. (2008). Symbiotic diversity in marine animals: The art of harnessing chemosynthesis. Nature Reviews Microbiology, 6, 725–740.
- Endow, K., & Ohta, S. (1990). Occurrence of bacteria in the primary oocytes of vesicomyid clam Calyptogena soyoae. Marine Ecology Progress Series, 64, 309–311.
- Felbeck, H. (1981). Chemoautotrophic potential of the hydrothermal vent tube worm, Riftia pachyptila Jones (Vestimentifera). Science, 213, 336–338.
- Fiala-Médioni, A., & Métivier, C. (1986). Ultrastructure of the gill of the hydrothermal vent bivalve Calyptogena magnifica, with a discussion of its nutrition. Marine Biology, 90, 215–222.
- Fischer, D., Sahling, H., Nöthen, K., Bohrmann, G., Zabel, M., & Kasten, S. (2011). Interaction between hydrocarbon seepage, chemosynthetic communities and bottom water redox at cold seeps of the Makran accretionary prism: Insights from habitat-specific pore water sampling and modeling. Biogeosciences Discussions, 8, 9763–9811.
- Fisher, C. R., Childress, J. J., Arp, A. J., Brooks, J. M., Distel, D. L., Dugans, J. A.,, … Soto, T. (1988). Variation in the hydrothermal vent clam, Calyptogena magnifica, at the Rose Garden vent on the Galapagos spreading center. Deep-Sea Research II, 35, 1811–1831.
- Gebruk, A. V., Chevaldonné, P., Shank, T., Lutz, R. A., & Vrijenhoek, R. C. (2000). Deep-sea hydrothermal vent communities of the Logatchev area (14°45′N, Mid-Atlantic Ridge): Diverse biotypes and high biomass. Journal of the Marine Biological Association of the United Kingdom, 80, 383–393.
- Giribet, G., Carranza, S., Baguna, J., Riutort, M., & Ribera, C. (1996). First molecular evidence for the existence of a Tardigrada + Arthropoda clade. Molecular Biology and Evolution, 13, 76–84.
- Goedert, J. L., Peckmann, J., Benham, S. R., & Janssen, A. W. (2013). First record of the Eocene pteropod Heliconoides nitens (Gastropoda: Thecosomata: Limacinidae) from the Pacific Basin. Bulletin of the Biological Society of Washington, 126, 72–82.
- Goedert, J. L., & Squires, R. L. (1990). Eocene deep-sea communities in localized limestones formed by subduction-related methane seeps, southwestern Washington. Geology, 18, 1182–1185.
- Goffredi, S. K., & Barry, J. P. (2002). Species-specific variation in sulfide physiology between closely-related Vesicomyid clams. Marine Ecology Progress Series, 225, 227–238.
- Goffredi, S. K., Hurtado, L. A., Hallam, S., & Vrijenhoek, R. C. (2003). Evolutionary relationships of deep-sea vent and seep clams (Mollusca: Vesicomyidae) of the ‘pacifica/lepta’ species complex. Marine Biology, 142, 311–320.
- Grehan, A. J., & Juniper, S. K. (1996). Clam distribution and subsurface hydrothermal processes at Chowder Hill (Middle Valley), Juan de Fuca Ridge. Marine Ecology Progress Series, 130, 105–115.
- Hecker, B. (1985). Fauna from a cold sulfur-seep in the Gulf of Mexico: Comparison with hydrothermal vent communities and evolutionary implications. Bulletin of the Biological Society of Washington, 6, 465–473.
- Hedges, S. B., Dudley, J., & Kumar, S. (2006). TimeTree: A public knowledge-base of divergence times among organisms. Bioinformatics, 22, 2971–2972.
- Horikoshi, M. (1989). inge structures, their variations and changes during growth, of some Japanese deep-sea, giant white clams, Calyptogena, collected during the ‘KAIKO’ Project. Palaeogeography, Palaeoclimatology, Palaeoecology, 71, 137–160.
- Huelsenbeck, J. P., & Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17, 754–755.
- Hurtado, L. A., Mateos, M., Lutz, R. A., & Vrijenhoek, R. C. (2003). Coupling of bacterial endosymbiont and host mitochondrial genomes in the hydrothermal vent clam Calyptogena magnifica. Applied and Environmental Microbiology, 69, 2058–2064.
- Jacobs, D. K., & Lindberg, D. R. (1998). Oxygen and evolutionary patterns in the sea: Onshore/offshore trends and recent recruitment of deep-sea faunas. Proceedings of the National Academy of Sciences of the United States of America, 95, 9396–9401.
- Joye, S. B., Boetius, A., Orcutt, B. N., Montoya, J. P., Schulz, H. N., Erickson, M. J., & Lugo, S. K. (2004). The anaerobic oxidation of methane and sulfate reduction in sediments from Gulf of Mexico cold seeps. Chemical Geology, 205, 219–238.
- Kahle, D., & Wickham, H. (2013). Spatial Visualization with ggplot2. The R Journal, 5, 144–161.
- Kennish, M. J., & Lutz, R. A. (1992). The hydrothermal vent clam, Calyptogena magnifica (Boss and Turner, 1980): A review of existing literature. Reviews in Aquatic Sciences, 6, 29–66.
- Kiel, S. (2010). The fossil record of vent and seep mollusks. In S. Kiel (Ed.). The Vent and Seep Biota, Topics in Geobiology 33 (pp. 255–277). Dordrecht, Netherlands: Springer.
- Kiel, S. (2015). Did shifting seawater sulfate concentrations drive the evolution of deep-sea methane-seep ecosystems? Proceedings of the Royal Society of London, B, 282, 20142908.
- Kiel, S., & Amano, K. (2010). Oligocene and Miocene vesicomyid bivalves from the Katalla district, Southern Alaska. The Veliger, 51, 76.
- Kojima, S., Fujikura, K., & Okutani, T. (2004). Multiple trans-Pacific migrations of deep-sea vent/seep-endemic bivalves of the family Vesicomyidae. Molecular Phylogenetics and Evolution, 32, 396–406.
- Krylova, E. M., & Cosel, R. V. (2011). A new genus of large Vesicomyidae (Mollusca, Bivalvia, Vesicomyidae, Pliocardiinae) from the Congo margin, with the first record of the subfamily Pliocardiinae in the Bay of Biscay (northeastern Atlantic). Zoosystema, 33, 83–99.
- Krylova, E. M., Kamenev, G. M., Vladychenskaya, I. P., & Petrov, N. B. (2015). Vesicomyinae (Bivalvia: Vesicomyidae) of the Kuril–Kamchatka Trench and adjacent abyssal regions. Deep-Sea Research II, 111, 198–209.
- Krylova, E. M., & Sahling, H. (2006). Recent bivalve molluscs of the genus Calyptogena (Vesicomyidae). Journal of Molluscan Studies, 72, 359–395.
- Krylova, E. M., & Sahling, H. (2010). Vesicomyidae (Bivalvia): Current taxonomy and distribution. Public Library of Science One, 5, e9957.
- Krylova, E. M., Sahling, H., & Janssen, R. (2010). Abyssogena: A new genus of the family vesicomyidae (Bivalvia) from deep-water vents and seeps. Journal of Molluscan Studies, 76, 107–132.
- Krylova, E. M., Sellanes, J., Valdés, F., & D'elia, G. (2014). Austrogena: A new genus of chemosymbiotic bivalves (Bivalvia; Vesicomyidae; Pliocardiinae) from the oxygen minimum zone off central Chile described through morphological and molcular analyses. Systematics and Biodiversity, 12, 225–246.
- Kuwahara, H., Yoshida, T., Takaki, Y., Shimamura, S., Nishi, S., Harada, M.,, … Maruyama, T. (2007). Reduced genome of the thioautotrophic intracellular symbiont in a deep-sea clam, Calyptogena okutanii. Current Biology, 17, 881–886.
- Le Pennec, M., Beninger, P. G., & Herry, A. (1995). Feeding and digestive adaptations of bivalve molluscs to sulphide-rich habitats. Comparative Biochemistry and Physiology Part A: Physiology, 111, 183–189.
- Lenaers, G., Maroteaux, L., Michot, B., & Herzog, M. (1989). Dinoflagellates in evolution. A molecular phylogenetic analysis of large subunit ribosomal RNA. Journal of Molecular Evolution, 29, 40–51.
- Levin, L. A., Orphan, V. J., Rouse, G. W., Rathburn, A. E., Ussler, W., Cook, G. S., … Strickrott, B. (2012). A hydrothermal seep on the Costa Rica margin: Middle ground in a continuum of reducing ecosystems. Proceedings of the Royal Society of London. Series B: Biological Sciences, 279, rspb20120205–20122588.
- Levin, L. A., Ziebis, W., Mendoza, G. F., Growney-Cannon, V., & Walther, S. (2006). Recruitment response of methane-seep macrofauna to sulfide-rich sediments: An in situ experiment. Journal of Experimental marine Biology and Ecology, 330, 132–150.
- Lorion, J., Kiel, S., Faure, B., Kawato, M., Ho, S.Y.W., Marshall, B., … Fujiwara, Y. (2013). Adaptive radiation of chemosymbiotic deep-sea mussels. Proceedings of the Royal Society of London. Series B: Biological Sciences, 280, 20131243.
- Maddison, W. P., & Maddison, D. R. (2011). Mesquite: A modular system for evolutionary analysis, Version 2.75. Tucson, AZ: Mesquite Project Team, University of Arizona. Retrieved from: http://mesquiteproject.org (accessed 19 October 2016).
- Marcon, Y., Ondréas, H., Sahling, H., Bohrmann, G., & Olu, K. (2014). Fluid flow regimes and growth of a giant pockmark. Geology, 42, 63–66.
- Newton, I. L. G., Girguis, P. R., & Cavanaugh, C. M. (2008). Comparative genomics of vesicomyid clam (Bivalvia: Mollusca) chemosynthetic symbionts. BioMed Central Genomics, 9, 585.
- Newton, I. L. G., Woyke, T., Auchtung, T. A., Dilly, G. F., Dutton, R. J., Fisher, M. C., … Cavanaugh, C. M. (2007). The Calyptogena magnifica chemoautotrophic symbiont genome. Science, 315, 998–1000.
- Okutani, T., Fujikura, K., & Kojima, S. (2000). New taxa and review of vesicomyid bivalves collected from the Northwest Pacific by deep sea research systems of Japan Marine Science & Technology Center. Venus (Japanese Journal of Malacology), 59, 83–101.
- Oliver, P. G., & Drewery, J. (2013). New species of chemosymbiotic clams (Bivalvia: Vesicomyidae and Thyasiridae) from a putative ‘seep’ in the Hatton–Rockall Basin, north-east Atlantic. Journal of the Marine Biological Association of the United Kingdom, 94, 389–403.
- Paull, C. K., Hecker, B., Commeau, R., Freeman-Lynde, R. P., Neumann, C., Corso, W. P., … Curray, J. (1984). Biological communities at the Florida Escarpment resemble hydrothermal vent taxa. Science, 226, 965–967.
- Peek, A., Gustafson, R., Lutz, R., & Vrijenhoek, R. (1997). Evolutionary relationships of deep-sea hydrothermal vent and cold-water seep clams (Bivalvia: Vesicomyidae): Results from the mitochondrial cytochrome oxidase subunit I. Marine Biology, 130, 151–161.
- Peek, A. S., Feldman, R. A., Lutz, R. A., & Vrijenhoek, R. C. (1998). Cospeciation of chemoautotrophic bacteria and deep-sea clams. Proceedings of the National Academy of Sciences of the United States of America, 95, 9962–9966.
- Peek, A. S., Gaut, B. S., Feldman, R. A., Barry, J. P., Kochevar, R. E., Lutz, R. A., & Vrijenhoek, R. C. (2000). Neutral and nonneutral mitochondrial genetic variation in deep sea clams from the family Vesicomyidae. Journal of Molecular Evolution, 50, 141–153.
- Posada, D. (2008). jModelTest: Phylogenetic Model Averaging. Molecular Biology and Evolution, 25, 1253–1256.
- Rambaut, A. (2010). FigTree. Edinburgh: University of Edinburgh. Retrieved from: http://tree.bio.ed.ac.uk/software/figtree/ (accessed 19 October 2016).
- Rambaut, A., & Drummond, A. J. (2010). Tracer. Edinburgh: University of Edinburgh. Retrieved from: http://tree.bio.ed.ac.uk/software/tracer/ (accessed 19 October 2016).
- Rathburn, A. E., Levin, L. A., Tryon, M., Gieskes, J. M., Martin, J. B., Pérez, M. E., … Ziebis, W. (2009). Geological and biological heterogeneity of the Aleutian margin (1965–4822m). Progress in Oceanography, 80, 22–50.
- Ronquist, F., & Huelsenbeck, J. P. (2003). MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
- Rstudio Team (2015). RStudio: Integrated Development for R. RStudio Inc. Retrieved from: http://www.rstudio.com/ (accessed 19 October 2016).
- Sahling, H., Masson, D.G., Ranero, C.R., Hühnerbach, V., Weinrebe, W., Klaucke, I., … Suess, E., (2008). Fluid seepage at the continental margin offshore Costa Rica and southern Nicaragua. Geochemistry, Geophysics, Geosystems, 9, Q05S05.
- Samadi, S., Quemere, E., Lorion, J., Tillier, A., Von Cosel, R., Lopez, P., … Boisselier-Dubayle, M.-C. (2007). Molecular phylogeny in mytilids supports the wooden steps to deep-sea vents hypothesis. Comptes Rendus Biologies, 330, 446–456.
- Squires, R. L., & Goedert, J. L. (1991). New late Eocene mollusks from localized limestone deposits formed by subduction-related methane seeps, southwestern Washington. Journal of Paleontology, 65, 412–416.
- Stewart, F.J., Young, C.R., & Cavanaugh, C.M. (2008). Lateral symbiont acquisition in a maternally transmitted chemosynthetic clam endosymbiosis. Molecular Biology and Evolution, 25, 673–687.
- Suess, E., Bohrmann, G., Huene, R., Linke, P., Wallmann, K., Lammers, S., … Orange, D. (1998). Fluid venting in the eastern Aleutian Subduction Zone. Journal of Geophysical Research: Solid Earth, 103, 2597–2614.
- Szafranski, K. M., Gaudron, S. M., & Duperron, S. (2014). Direct evidence for maternal inheritance of bacterial symbionts in small deep-sea clams (Bivalvia: Vesicomyidae). Naturwissenschaften, 101, 373–383.
- Thiele, J., & Jaeckel, S. (1931). Muscheln der deutschen Tiefsee- Expedition. Wissenschaftliche Ergebnisse der deutschen Tiefsee Expedition 1898–1899, 21, 160–268.
- Treude, T., Smith, C., Wenzhöfer, F., Carney, E., Bernardino, A. F., Hannides, A. K., … Boetius, A. (2009). Biogeochemistry of a deep-sea whale fall: Sulfate reduction, sulfide efflux and methanogenesis. Marine Ecology Progress Series, 382, 1–21.
- Turner, R. D. (1985). Notes on mollusks of deep-sea vents and reducing sediments. Advances in Marine Biology, Special Edition No. 1, 23–34.
- Valdés, F., Sellanes, J., & Guillermo, D. E. (2012). Phylogenetic position of Vesicomyid clams from a methane seep off Central Chile (∼36°S) with a molecular timescale for the diversification of the Vesicomyidae. Zoological Studies, 51, 1154–1164.
- Vrijenhoek, R. C. (2009). Cryptic species, phenotypic plasticity, and complex life histories: Assessing deep-sea faunal diversity with molecular markers. Deep-Sea Research II, 56, 1713–1723.
- Vrijenhoek, R. C. (2013). On the instability and evolutionary age of deep-sea chemosynthetic communities. Deep-Sea Research II, 92, 189–200.
- Vrijenhoek, R. C., Schutz, S. J., Gustafson, R. G., & Lutz, R. A. (1994). Cryptic species of deep-sea clams (Mollusca, Bivalvia, Vesicomyidae) in hydrothermal vent and cold-seep environments. Deep-Sea Research II, 41, 1171–1189.
- Wickham, H. (2009). ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag.Associate Editor: Ana Riesgo
