Abstract
Patterns of rarity, endemism, and vulnerability are known for four species of yellow-eared bats of the genus Vampyressa: V. melissa, V. voragine, V. elisabethae, and V. sinchi, the last two described based on skull and external morphology. We extracted DNA from the holotypes of V. elisabethae and V. sinchi using strict ancient DNA protocols and sequenced the complete cytochrome-b gene of the mtDNA to investigate the phylogenetic relationships within the genus and employed species-delimitation tests to evaluate the validity of all the currently named species of Vampyressa. The resulting tree topology and our species-delimitation analyses corroborate the validity of V. elisabethae and V. voragine, but places V. sinchi in V. melissa. Based on these results and phenotypic variation, we recognize five valid species in Vampyressa and treat sinchi as a subspecies of a polytypic V. melissa; for which we provide a rediagnosis. Our results show that V. elisabethae is as highly divergent genetically as it is morphologically, and suggest that V. thyone, one of the two species of Vampyressa known to have wide distributions, is a species complex requiring further investigation.
Introduction
Species concepts generally concur that species are independently evolving metapopulation lineages (De Queiroz, Citation2005; Mayden, Citation1999; Simpson, Citation1961). The recognition of species, however, is often imprecise because there is no consensus on where to draw limits along the species-divergence continuum (De Queiroz, Citation2007). Methods for species delimitation can be discovery-based, i.e., without a priori group delimitation; or validation-based, i.e., with putative lineages delimited a priori (Carstens et al., Citation2013). These methods can also incorporate data from a single molecular marker (single-locus methods), cluster-based (e.g., ABGD, Gaussian Clustering), or based on coalescence (e.g., BBP, GMYC; Carstens et al., Citation2013). Congruent results obtained from multiple delimitation methods are generally viewed as indicative of robustness of the taxonomic hypotheses (Carstens et al., Citation2013; Tang et al., Citation2014).
Species recognition, either taxonomically conservative or inflationary, can be problematic (Padial et al., Citation2010; Zachos, Citation2018) for descriptions of their diversity and their conservation. Because many conservation management strategies for imperilled species rely on taxonomic species lists, species recognition should be based on the best data at hand. This is particularly challenging in the case of rare taxa, which are difficult to delimit due to their fragmentary distributions and small number of specimens available (Padial & de la Riva, Citation2006).
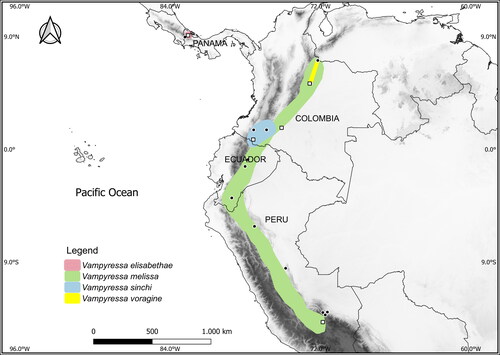
The Neotropical phyllostomid bat genus Vampyressa currently includes six species of delicate yellow-eared fruit-eating bats. The genus includes the two relatively common, widely distributed small species Vampyressa thyone Thomas, Citation1909 and V. pusilla (Wagner, Citation1843); and the four medium-sized species V. elisabethae Tavares et al., Citation2014; V. melissa Thomas, Citation1926; V. voragine Morales-Martínez et al., Citation2021; and V. sinchi Tavares et al., Citation2014, all of which are rare, have restricted distributions (), and are poorly represented in scientific collections (Morales-Martínez et al., Citation2021; Solari et al., Citation2019; Tavares et al., Citation2014). The typical dentition shared by all Vampyressa includes a caniniform second lower premolar and a well-developed entoconid on the second lower molar. The more recently described V. elisabethae, V. sinchi, and V. voragine are known from five, three, and five specimens, respectively, and V. melissa is known from less than 20 (Morales-Martínez et al., Citation2021; Ramírez-Chaves et al., Citation2015; Tavares et al., Citation2014). Vampyressa melissa from the Andes of Colombia, Ecuador, and Peru, and V. sinchi from Colombia () are likely closely related as reflected by their morphological similarity (Tavares et al., Citation2014) and V. voragine also appears similar to these forms. In contrast, V. elisabethae, endemic to Bocas del Toro, Panama (), has a markedly distinctive set of characters (Tavares et al., Citation2014) and does not appear as closely related to these two species, nor is it closely related to V. pusilla and V. thyone.
Fig. 1. Distributions of the four currently recognized large-sized species of Vampyressa. Distribution polygons follow Tavares et al. (Citation2014), Morales-Martínez et al. (Citation2021), and Marsh et al. (Citation2022). Filled circles indicate collecting localities of specimens that have only morphological data available and open squares represent the localities of specimens from which we obtained molecular data.
Molecular data are available for V. pusilla, V. thyone, and the rare Vampyressa melissa and V. voragine. The descriptions of V. elisabethae and V. sinchi were based on their distinctive phenotypes (Tavares et al., Citation2014) as there were no reports of newly captured individuals of either taxon and no freshly collected tissues available for genetic studies (Tavares et al., Citation2014; Ramírez-Chavez et al., Citation2015). Their topological placement in the Vampyressa genetic tree is unknown, and the most inclusive phylogenies included data for only Vampyressa pusilla, V. thyone, and V. melissa (Hoofer et al., Citation2008) and more recently for V. voragine (Morales-Martínez et al., Citation2021).
Here, we expand investigations on the taxonomy and phylogeny of Vampyressa by reporting mtDNA sequences for V. elisabethae and V. sinchi obtained from museum specimens. The tissues analysed were taken from the holotypes of V. sinchi and V. elisabethae, thereby adding objectivity to our taxonomic hypotheses originally based on morphological diagnoses (Chakrabarty, Citation2010). We used phylogenetic analyses and multiple species-limits tests to evaluate relationships within Vampyressa. Our objectives were (1) to investigate species boundaries for all Vampyressa species using new molecular data, with emphasis on testing the validity of V. sinchi and V. elisabethae and (2) to estimate the phylogenetic relationships among the currently valid Vampyressa species to derive a complete species-level tree of the genus.
Material and methods
Molecular sampling
We built a matrix of 50 sequences of the mitochondrial cytochrome-b gene (1140 bp) including new data obtained from the holotypes of V. elisabethae and V. sinchi and data gathered from GenBank of all currently recognized species of Vampyressa: V. melissa (4), V. voragine (2), V. pusilla (3), and V. thyone (10), and from its sister genus and species Mesophylla macconelli Thomas, Citation1901 (13). After carefully comparing the GenBank sequences, we found that the Vampyressa melissa sequences under the accession numbers DQ312427 and FJ154185 refer to the same individual (FMNH 174910) and used only the FJ154185 sequence. We added as outgroup three species of Vampyriscus Thomas, Citation1900: V. bidens (Dobson, Citation1878), V. brocki (Peterson, Citation1968), and V. nymphaea (Thomas, Citation1909), one species of Chiroderma, Chiroderma villosum Peters, Citation1860, and the more distantly related vampyressine Vampyrodes caraccioli (Thomas, Citation1889) (Supplementary Material Table S1).
Table 1. Pairwise mean sequence divergence between species of Mesophylla and Vampyressa under the Kimura 2-parameter model, as implemented in MEGA7 for a total of 31 cytochrome b sequences (1,140 bp). Intraspecific divergence is shown in bold in the diagonal.
DNA extraction from holotypes of V. sinchi and V. elisabethae
Skin clips from museum specimens of V. sinchi (FMNH 114028, holotype) and V. elisabethae (USNM 319283, holotype) were rinsed, and DNA extractions were performed in an isolated ancient DNA facility at the Smithsonian’s Center for Conservation Genomics (CCG), using a standard phenol-chloroform protocol (see McDonough et al., Citation2018) and including a long (up to three days) lysis step. A total of 35 μl of DNA extract was purified and concentrated using 5× magnetic bead purification following Rohland and Reich (Citation2012), then prepared using a KAPA LTP library preparation kit (Kapa Biosystems, Boston, MA) for Illumina platforms with beads following the manufacturer’s protocol, with 1/4 reactions. Dual-indexing PCR (Kircher et al., Citation2012) was performed with Nextera-style indices using KAPA HiFi Hotstart ReadyMix according to manufacturer’s protocol, with 18 cycles for the PCR reaction. The reaction was purified using 1.8× magnetic beads and visualized on a 1.5% agarose gel. Libraries were quantified using fluorometry with a Qubit high-sensitivity assay kit (Thermo Fisher Scientific, Waltham, MA) and pooled in equimolar concentration with other libraries. The final pool was sequenced with paired-end chemistry (read length 143 base pairs) on a single lane of an Illumina HiSeq2500 (Illumina, San Diego, CA).
Sequencing reads were demultiplexed and the forward and reverse reads were merged with PEAR version 0.9.4 (Zhang et al., Citation2014). Adapter sequences were removed using TrimGalore version 0.4.3 (Krueger, Citation2015). We used PRINSEQ–lite version 0.20.4 (Schmieder & Edwards, Citation2011) to remove reads with mean quality scores below 20 (–min qual mean 20), exact duplicates, and 5′ duplicates (–derep 124). Sequencing reads were mapped to the closest mitochondrial genome available using bwa-mem (Li, Citation2013). The cytochrome-b gene was extracted from the mitochondrial genome and used for the final sequence alignment. These newly generated sequences are available at NCBI’s GenBank (accession numbers OM994434 and OM994435).
Species delimitation analyses
Our species delimitation protocols met criteria of congruence among multiple single-locus species delimitation methods for measuring phenotypic distinctiveness among the putative taxa (Padial et al., Citation2010). Our analysis included one distance-based, and four coalescence, tree-based species delimitation methods: the single-threshold Generalized Mixed Yule-Coalescent (sGMYC; Pons et al., Citation2006), the Bayesian implementation of the GMYC (bGMYC; Reid & Carstens, Citation2012), the Bayesian implementation of the Poisson tree processes (bPTP; Zhang et al., Citation2013), and the multi-rate PTP (mPTP; Kapli et al., Citation2017). Tree-based species delimitation methods require either ultrametric (sGMYC and bGMYC) or non-ultrametric trees (bPTP and mPTP) (Pons et al., Citation2006; Tang et al., Citation2014; Zhang et al., Citation2013). To generate the phylogenetic trees, we aligned the sequences using MUSCLE v.3.8.31 (Edgar, Citation2004) and selected the best-fit partitioning schemes and models of nucleotide evolution using PartitionFinder 2 (Lanfear et al., Citation2016), which suggested the K80 + I, HKY + I, and GTR + I models for the first, second, and third codon positions, respectively (Supplementary Material Table S2).
Table 2. Selected diagnostic characters of Vampyressa species.
The PTP models were run on a non-ultrametric tree estimated using MrBayes v. 3.2.3 (Huelsenbeck & Ronquist, Citation2001). For the analysis, two runs of Markov Chain Monte Carlo (MCMC) were run for 40 million generations sampling at every 4,000 generations. We used a burn-in proportion of 25%. The ultrametric tree for the GMYC models was generated using BEAST2 (Bouckaert et al., Citation2014). The analysis was run under a strict clock and a Coalescent Constant Population tree prior. Two MCMC chains were run for 10 million generations sampling at every 1,000 generations. Convergence of the parameters in both MrBayes and BEAST2 analyses was verified via the ESS values (>200), in Tracer v. 1.6. (Rambaut et al., Citation2014).
After obtaining the Maximum Clade Credibility (MCC) trees in the MrBayes and BEAST analyses, we extracted the clade containing specimens of the genera Mesophylla and Vampyressa (35 tips) using the extract.clade function of the R package ‘ape’ (Paradis et al., Citation2004). This subtree was the one used to fit the models. The MrBayes, BEAST, and PartitionFinder analyses were run on the CIPRES online platform (Miller et al., Citation2010).
We used the ‘gmyc’ function of the R package ‘splits’ to fit the sGMYC model (Ezard et al., Citation2009, Fujisawa & Barraclough, Citation2013). The bGMYC analyses were run in the homonymous R package (Reid, Citation2014), using 1,002 phylogenetic trees, 6,000 MCMC analysis, with a burn-in of 1,000 and thinning of 300, resulting in 16,700 samples retained. The bPTP analysis was run on the https://species.h-its.org/ online server, and the mPTP analysis was performed using the online server https://mcmc-mptp.h-its.org/mcmc. For the mPTP, we used the default MCMC and minimum branch length settings, but set different starting delimitations: null model, maximum-likelihood, and random.
The Automatic Barcode Gap Discovery (ABGD), a distance-based method that identifies differences between intraspecific and interspecific pairwise differences in a dataset, was run using the online platform https://bioinfo.mnhn.fr/abi/public/abgd/ (Puillandre et al., Citation2012). Only cytochrome-b sequences of Mesophylla and Vampyressa were used as input. Based on intraspecific divergences reported in previous studies of Mesophylla and Vampyressa (Lim et al., Citation2003; Porter & Baker, Citation2004), we used a Pmin of 0.009 and a Pmax of 0.03, and used the default value of the relative gap width (X = 1.5). We compared results using both JC69 and K80 pairwise distances. Along with species delimitation tests, we calculated intraspecific and interspecific genetic distances under a Kimura 2-parameter (K2P) model (Kimura, Citation1980) as implemented in MEGA v. 7 (Kumar et al., Citation2016).
Results
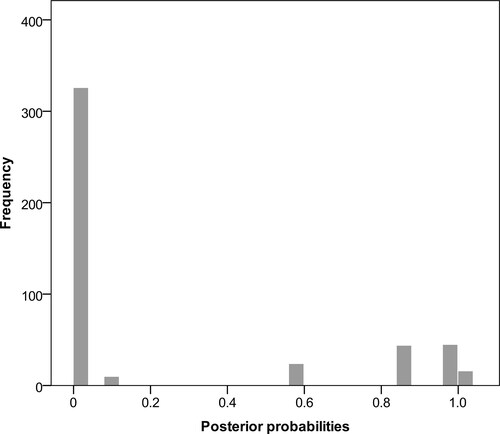
The sGMYC analyses of the MCC tree suggest one species for Mesophylla and six for Vampyressa (CI = 7–8), a result also recovered when using the mean of posterior probability threshold (P = 0.5) under the bGYMC model analysis (). There was a break in the distribution of the pairwise posterior probabilities when P was close to 0.6 () and under this threshold (P = 0.6), the output suggests seven species for Vampyressa (). Under a less restrictive value (P = 0.95), the model outputs a two-Mesophylla and eight-Vampyressa species hypothesis. The bPTP and mPTP models yielded similar results: one species for Mesophylla and six for Vampyressa (). The output from the ABGD analysis also suggests seven species including one Mesophylla for a prior maximum distance (P) of 0.009 ≤ P ≤ 0.010 and, when shifted to 0.011 ≤ P ≤ 0.030, the analysis suggested one Mesophylla and five Vampyressa species, with a monotypic thyone. Average genetic distance between species of Vampyressa was 10.9%, ranging from 4.7%, between the specimens of Vampyressa thyone from Guerrero, Mexico and its sister clade containing the V. thyone (sensu stricto), and 14.5%, between V. elisabethae and V. pusilla ().
Fig. 2. Tree estimated in BEAST based on mitochondrial cytochrome b gene sequences of Vampyressa and Mesophylla. Vertical bars show the comparison of the five species delimitation results as follows: sGMYC, single-threshold Generalized Mixed Yule-Coalescent (GMYC); bGMYC, Bayesian Implementation of the GMYC (using a posterior probability threshold of P = 0.6); bPTP, Bayesian Implementation of the Poisson tree processes (PTP) model; mPTP, multirate Poisson tree processes model; ABGD, Automatic Barcode Gap Discovery (using a prior maximum distance P of 0.011 ≤ P ≤ 0.030). The six putative species are indicated with different colours: Vampyressa thyone (pink), Vampyressa sp. (grey), V. pusilla (orange), V. melissa (green), V. voragine (yellow), V. elisabethae (dark orange), and Mesophylla macconnelli (blue). The ultrametric gene tree shown was estimated in BEAST. Acronyms: FMNH, Field Museum of Natural History, Chicago, IL, USA; ICN, Colección de mamíferos ‘Alberto Cadena García’, Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá, Colombia; MZFC, Mammal Collection of the Zoology Museum, UNAM, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico City, Mexico; TK, TTU, Museum of Texas Tech University, Lubbock, TX, USA; USNM, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
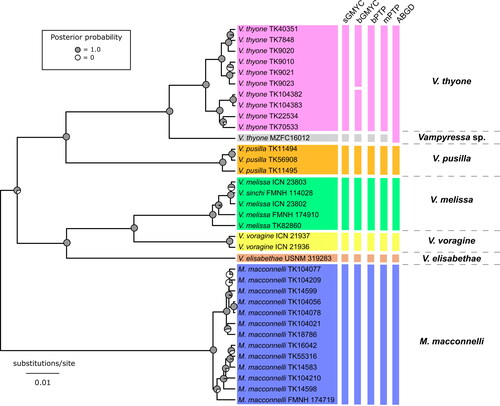
Fig. 3. Histogram of pairwise probabilities of conspecificity for the 35 sequences included in our analyses. Values were obtained from the Bayesian implementation of the Generalized Mixed Yule-Coalescent species delimitation model.
The results of our phylogenetic analyses, combined with those from species delimitation methods, confirmed our determination that Vampyressa is composed of at least five species (). However, the V. thyone specimen from Guerrero, Mexico (MZFC 16012), is positioned as sister to the clade containing all the other Central and South American V. thyone, indicating that it may represent an undescribed sixth species of Vampyressa. The phylogram in Morales-Martínez et al. (Citation2021: ) shows similar results for the Guerrero specimen and other V. thyone.
The Panamanian V. elisabethae was recovered as a highly divergent species from all Vampyressa including its sister group (, ), a clade composed of the sister taxa V. melissa and V. voragine (). One of the two Peruvian specimens of V. melissa (FMNH 174910) appears to be more closely related to the Colombian V. melissa (ICN 21935, ICN 21937) than to the other Peruvian specimen, and the mtDNA sequence of the holotype of the Andean V. sinchi (FMNH 114028) fell into the same clade with those of Colombian and Peruvian V. melissa. We therefore recognize two subspecies of V. melissa based on geographically and phenotypically distinct, but genetically similar populations of V. m. melissa and V. m. sinchi that we redescribe below. We also provide a key to the identification of species and subspecies of Vampyressa.
Systematics
Vampyressa melissa Thomas, Citation1926
Synonyms
See under subspecies.
Type material
The holotype of V. melissa is BMNH 26.5.3.4, a well-preserved skin and skull of an adult female from Puca Tambo (6°9′S, 77°16′W), Moyobamba, San Martín, Peru (1480 m). Measurements and descriptions of the holotype are provided in Tavares et al. (Citation2014).
Rediagnosis
A large Vampyressa with a relatively long and broad skull (forearm length 34.5–41.5 mm; greatest length of the skull 21.2–23.7 mm; zygomatic breadth 12.5–14.8 mm); pelage varying from dark to pale brown; uropatagium hairy on both dorsal and ventral surfaces with a sparse to conspicuous fringe of pale hairs along its posterior border (see subspecies); mesopterygoid fossa either as long as wide or longer than wider (see subspecies); orbitosphenoid foramen small; postglenoid process well developed; inner upper incisors bilobed (see subspecies), and second lower premolar (p4) lacking a well-developed posterior cuspulid.
Synonyms
Vampyressa melissa Thomas, Citation1926: 157, type locality ‘Puca Tambo, Peru, altitude 7100'’, San Martín, Peru.
Vampyressa (Vampyressa) melissa: Peterson, Citation1968: 14; name combination.
Rediagnosis
Medium sized (forearm 34.5–37.7 mm); pelage brownish; fringe of pale hairs on posterior margin of uropatagium dense; upper inner incisors bilobed; mesopterygoid fossa as long as wide (see also Tavares et al., Citation2014).
Distribution
Vampyressa m. melissa occurs at upper elevations (1180–2763 m) in the northern Central Cordillera of Colombia, and in the Andes of Peru and Ecuador ().
Vampyressa m. sinchi Tavares et al., Citation2014
Synonyms
Vampyressa melissa: Lemke et al., Citation1982: 231.
Vampyressa melissa: Alberico et al., Citation2000: 55 (part).
Vampyressa sinchi Tavares et al., Citation2014: 16; type locality ‘Llorente (0°46′40″N, 77°21′50″W; 1,700 m), municipio de Córdoba, departamento de Nariño, Colombia’.
Rediagnosis
The largest Vampyressa (forearm 39.1–41.5 mm) with a pale brown to dark beige pelage; a sparse fringe of pale hairs on the posterior margin of uropatagium, with longer hairs at the midline; dorsal emargination of anterior nares deep; mesopterygoid fossa longer than wide; unevenly bilobed upper inner incisors; stout, robust skull; and stout dentition with well-developed cingula (see also Tavares et al., Citation2014).
Distribution
Vampyressa m. sinchi is distributed at upper elevations (1,620 and 1,900 m) in the southern region of the eastern slope of the Eastern Cordillera (Cordillera Oriental) of Colombia ().
Description and comparisons
Vampyressa melissa is much larger than V. pusilla and V. thyone (Tavares et al., Citation2014) and comparable to the larger members of the Vampyressa group that includes V. elisabethae and V. voragine. Vampyressa m. sinchi is the largest Vampyressa (forearm 39.1–41.5 mm, greatest length of skull 23.3–23.7 mm) with non-overlapping dimensions in relation to all other Vampyressa species (e.g., forearm 34.5–37.7 mm, greatest length of skull 21.2–22.6 mm; Tavares et al., Citation2014). In contrast, V. voragine appears to be smaller among the large Vampyressa in two non-overlapping measurements: the greatest length of skull and length of maxillary toothrow (Morales-Martínez et al., Citation2021). Overall, the pelage colouration of V. m. melissa is brownish, but pale brown to dark beige in V. m. sinchi; creamy brownish in V. voragine (Morales-Martínez et al., Citation2021); and paler, yellowish brown in V. elisabethae. The proximal forearm of V. m. melissa and V. m. sinchi is densely furred along approximately two-thirds of its length, and approximately for half of its length in V. elisabethae. The forearm of V. voragine is also furred, but it is not clear how hair is distributed based on the original description by Morales-Martínez et al. (Citation2021). The fringe on the posterior margin of the uropatagium is pale in V. m. melissa and V. voragine. The uropatagial fringe in V. m. sinchi is sparse with longer hairs at the midline of the membrane; the uropatagium of V. elisabethae lacks a conspicuous fringe on its posterior margin. The third and fifth metacarpals of V. m. melissa, V. m. sinchi, and V. voragine are similar in length, with the fourth metacarpal averaging shorter, whereas V. elisabethae has the third and fifth metacarpals subequal, with the fifth metacarpal longer than the other two.
The mesethmoid plate is thin in V. m. melissa, V. m. sinchi, and V. voragine, and all three also have discrete orbitosphenoid foramina. The mesopterygoid fossa is notably longer than wide in V. sinchi. In contrast, V. elisabethae has a large orbitosphenoid fissure instead of a smaller orbitosphenoid foramen. The nasals of V. m. sinchi are short; as a result, the dorsal emargination of the narial opening is deeper than in any other known Vampyressa. The upper inner incisors of V. sinchi are unevenly bifid, in contrast to V. m. melissa, in which upper inner incisors are more or less evenly bifid. Unevenly bifid upper inner incisors are not unique to V. voragine (contra Morales-Martínez et al., Citation2021). Vampyressa m. sinchi has a conspicuously robust skull and the teeth have more strongly developed cingula and cingulids than found in other Vampyressa. Vampyressa m. sinchi has a longer mesopterygoid fossa than found in other Vampyressa. The postglenoid process in V. m. sinchi projects to the level of the pterygoid process but is less developed in other Vampyressa (Tavares et al., Citation2014).
Key to the species and subspecies of genus Vampyressa
For measurements of Vampyressa, including ranges and sample size, see Tavares et al., Citation2014:11, 15, 18 (Tables 3, 4, 5) and Morales-Martínez et al., Citation2021: 6 ().
1. Size small, forearm shorter than 34.5 mm; greatest length of skull shorter than 21.2 mm, leaflet behind nasal spear present2
1′. Size large, forearm equal to or longer than 34.5 mm, greatest length of skull equal to or longer than 21.2 mm; leaflet behind spear absent 3
2. Noseleaf margins yellowish and outer rim of ear pinnae usually yellow; size smaller, forearm length 29.3–32.3 mm, fifth metacarpal longer than third and fourth; greatest length of skull 17.1–19.2 mmV. thyone
2′. Outer border of ear pinnae and margins of noseleaf faintly paler, usually not yellowish; posterior border of skull flattened in dorsal view; third and fifth metacarpal subequal in length and longer than fourth; forearm length 32.3–34.3 mm; greatest length of skull 19.0–20.6 mmV. pusilla
3. Pelage pale yellowish brown; mesethmoid plate swollen laterally (see Tavares et al., Citation2014: 13; Fig. 5); large sphenorbital fissure present (instead of sphenorbital foramen) (see Tavares et al., Citation2014: 17, 19; Figs 9, 11); coronoid process of mandible low; m3 absent (see Tavares et al., Citation2014: 16; Fig. 7); forearm length 36.2–37.8 mm; greatest length of skull 22.9–23.0 mm; uropatagium lacks fringe of hairs on its posterior borderV. elisabethae
3′. Pelage pale brown or beige; lower anterior margin of nasal opening v-shaped; mesethmoid plate thin; sphenorbital foramen present; coronoid process of the mandible high; peg like m3 present4
4. Margins of ear pinnae greyish or whitish; length of forearm 36.9–37.8 mm; greatest length of skull 20.7–20.3 mm; length maxillary toothrow 6.9–7.0 mmV. voragine
4′. Borders of the ear pinnae yellowish (see Tavares et al., Citation2014: 20; Fig. 10); length of forearm longer than 21 mm5
5. Fringe of pale hairs along posterior border of uropatagium dense; upper inner incisors nearly evenly bilobed (see Tavares et al., Citation2014:13; Figs 5, 9); skull and dentition not robust; length of forearm 34.5–37.7 mm; greatest length of skull 21.2–22.6 mmV. m. melissa
5′. Fringe of hairs along posterior border of uropatagium sparse; upper inner incisors unevenly bilobed (see Tavares et al., Citation2014: 13, Fig. 5); robust skull and robust dentition with well-developed cingula and cingulids (see Tavares et al., Citation2014: 12, 13, 14; Figs 4, 5, 6); length of forearm 39.1–41.5 mm; greatest length of skull 23.3–23.7 mmV. m. sinchi
Discussion
Integrative taxonomy (Dayrat, Citation2005; Will et al., Citation2005) assumes that species limits may be better recognized, and that species will more likely withstand future scrutiny if multiple methods and independent datasets are employed in their assessment. This approach should also reduce biases that result from arbitrary taxonomic decisions and enhance the confidence for taxonomic hypotheses (Dayrat, Citation2005; Padial et al., Citation2010). Our phenotypic and phylogenetic data demonstrate that the current taxonomy of Vampyressa is not congruent with its actual diversity. Vampyressa sinchi and V. elisabethae were unequivocally diagnosed morphologically prior to our investigation. Morphology alone led to the recognition of V. m. sinchi as a separate species, a conclusion not supported by our molecular analysis. Also, not previously obvious to us is that the specimen identified as V. thyone from the Mexican state of Guerrero could represent a new form of Vampyressa, as has been suggested by our phylogenetic analysis.
Based on the Phylogenetic Species Concept and a theoretical framework including diagnosis and monophyly to the recognition of species (dmPSC; Gutiérrez & Garbino, Citation2018), we recognize five species in Vampyressa (V. elisabethae, V. melissa, V. thyone, V. pusilla, V. voragine) and acknowledge that the specimen of V. thyone from Guerrero (Hernández-Canchola et al., Citation2019) may represent a sixth species.
We recognize V. sinchi as a subspecies of Vampyressa melissa, because sinchi represents a phenotypically distinct and geographically restricted form that is not reciprocally monophyletic to the populations referable to V. melissa (see Patten, Citation2015). Therefore, the Colombian Eastern Cordillera Vampyressa melissa shall be known as Vampyressa melissa sinchi. The polytypic V. melissa is represented by two subspecies, V. m. melissa distributed at upper elevation localities in cis-Andean Colombia, Ecuador and Peru, and V. m. sinchi, restricted to the southern region of the eastern Cordillera Oriental of Colombia (Morales-Martínez et al., Citation2021; Tavares et al., Citation2014).
Vampyressa m. sinchi is genetically akin to V. m. melissa according to mitochondrial data available, but it is morphologically distinct. This phenomenon has also been observed in the closely related vampyressine Chiroderma, in the case of Chiroderma d. vizottoi Taddei and Lim Citation2010, previously described as a separate species based on a distinctive phenotype (Garbino et al., Citation2020). Although the hypothesis of an incipient speciation process occurring for these taxa appears attractive, to make well-informed hypotheses is presently difficult. For both polytypic species V. melissa and C. doriae we would have to more broadly understand intraspecific variation, population subdivision, to be able to conduct phylogeographic studies and learn if, and how spatial variation may become structured. Currently. there are not enough individuals sampled for that (Tavares et al., Citation2014; Garbino et al., Citation2020; Morales-Martínez et al., Citation2021), and probably there will not be for some of the Vampyressa (Tavares et al., Citation2014; Ramírez-Chaves et al., Citation2015; Morales-Martínez et al., Citation2021). With the data currently at hand we can only, conservatively, delimitate these taxa using morphological and geographic evidence, in order to more accurately describe, document and protect that subspecific variation found.
The validity of V. elisabethae was corroborated by its high genetic divergence from other species of Vampyressa, as indicated previously by its distinctive morphology. The genetic distance between V. elisabethae and V. melissa is comparable to that found between M. macconnelli and V. pusilla (15.3%). This genetic evidence recommends reexamining the generic status of Vampyressa. We need to obtain additional genetic information for V. elisabethae, including genetic samples from the three paratypes. Vampyressa elisabethae is known from four specimens and has not been collected since the 1970s (Tavares et al., Citation2014). The population status of this taxon is unknown, and it may have become extinct; therefore, we recommend targeted surveys and expeditions to appropriate habitats in Panama in an attempt to determine its status.
Vampyressine bats (subtribe Vampyressina Baker et al., Citation2016) comprise over 30 species of stenodermatines (Phyllostomidae, Stenodermatinae) in the genera Chiroderma Peters, Citation1860; Vampyriscus; Platyrrhinus Saussure, Citation1860; Uroderma Peters, Citation1865; Vampyrodes Thomas, Citation1900; Mesophylla Thomas, Citation1901; and Vampyressa. Although with few exceptions, their intergeneric relationships appear relatively well-resolved, the lack of phylogenetic sampling of other populations does not permit a comprehensive evaluation of their intrageneric diversity and evolutionary history (e.g., Velazco & Patterson, Citation2008). Our data, including the molecular information for the holotypes of the rare taxa, provides a clearer picture of the Vampyressa genetic tree, and is useful for understanding patterns of origin, diversification, and radiation in the tribe Vampyressina. Hoofer and Baker (Citation2006) and Hoofer et al. (Citation2008) performed the first analyses that included molecular data for V. melissa and recovered a sister group relationship of V. melissa to a clade formed by V. pusilla and V. thyone. More recently, Morales-Martínez et al. (Citation2021) obtained cytochrome b data for their new species V. voragine and carried out a phylogenetic analysis on Vampyressa taxa that revealed a sister-relationship between V. voragine and V. melissa. As in previous analyses, this clade proved to be sister to a clade containing V. pusilla and V. thyone. Our data confirm that the Vampyressa phylogenetic tree is divided into two main lineages. One lineage comprises the highland species V. elisabethae as sister to a clade containing V. melissa and V. voragine. The second lineage contains V. pusilla as sister to a possible species complex currently known as V. thyone.
Biogeographically, the sister relationship between V. elisabethae and the V. melissa + V. voragine clade is striking in that all three taxa occur at upper elevations. Vampyressa elisabethae is known only from humid forest habitats between elevations of 1,400 and 1,500 m in Bocas del Toro province in north-western Panama. Vampyressa voragine is known from three cis-Andean localities in the Colombian Departments of Casanare and Norte de Santander and has a similar elevational range (1,400 to 1,500 m). Vampyressa melissa is the most widely distributed, having been collected from a few localities along the cis-Andean Cordillera of Peru, Ecuador, and southern Colombia at elevations between approximately 1,500 m to near 3,000 m. These disjunct distributions suggest colonization from a most recent common ancestor from Central America to the Andes, followed by further differentiation. The diversity within the Andean Vampyressa taxa (V. melissa, V. sinchi, and V. voragine) suggests more recent diversification in South America. Similar diversification and biogeographic patterns between North America and the Andes are found in pairs of species of other vampyressine fruit bats (subtribe Vampyressina) such as between the larger species of Platyrrhinus (see Velazco & Patterson, Citation2008) and the big-eyed bat species Chiroderma salvini and C. scopaeum (Garbino Citation2019, Garbino et al., Citation2020). A comprehensive study of vampyressine biogeography should reveal more evidence of common distributional and evolutionary pathways, and the role of the Andes in their diversification.
Descriptions of new taxa that are apparently rare or poorly represented in collections, require care to avoid taxonomic inflation. Descriptions of rare taxa based on molecular evidence have been hampered historically by the difficulty of assessing intraspecific genetic variation when the sample size consists of only one or a few specimens.
Several of the coalescence-based species delimitation methods are sensitive to undersampling (O’Meara, Citation2010). This shortfall is particularly relevant for species known from a single specimen or that have been collected only once (Lim et al., Citation2012). However, methods such as the Bayesian implementation of the GMYC can accommodate singletons by using sequence data from better sampled species (Reid & Carstens, Citation2012). Cluster-based methods can incorporate singletons because these methods do not require greater evidence of intraspecific variability (Lim et al., Citation2012).
Our study is a robust exercise in reassessing previously proposed species hypotheses based on the best evidence then available. In this context, the use of species delimitation methods that integrate independent data sources within the framework of operational species concepts appears to be an efficient process to reveal species limits problems with small samples of representative taxa (Carstens et al., Citation2013; O’Meara, Citation2010).
In our experience, species of bats known from few specimens and localities, share specialized dietary needs and have restricted habitat requirements, both of which contribute to their rarity and vulnerability. While exact niche peculiarities remain unknown, we do know that the species we report on here have been found in forested montane regions below ∼3,000 m, a zone where humid forests are being converted at a rapid rate throughout Latin America. Therefore, we consider the larger members of the Vampyressa species group to be severely threatened because of habitat loss (Tavares et al., Citation2014; Morales-Martínez et al., Citation2021). Further information is urgently needed to develop effective conservation strategies for these bats.
Associate Editor: Dr Susan Tsang
Supplemental Material S2
Download PDF (59.5 KB)Supplemental Material S1
Download PDF (73.7 KB)Acknowledgements
We are grateful to Bruce Patterson, Field Museum of Natural History, Chicago, IL for permission to sample the holotype of V. sinchi and the Biological Survey Unit, Division of Mammals, National Museum of Natural History, Washington, DC, for permission to sample the holotype of V. elisabethae. We thank Daniel Casali for suggestions on species delimitation methods. VCT had a PNPD CAPES postdoctoral fellowship for part of the period of this research, and GSTG received a pre-doctoral fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES; Code 001).
Disclosure statement
Authors avow no potential conflict of interest.
Supplemental material
Supplemental material for this article can be accessed here: https://doi.org/10.1080/14772000.2022.2117247
References
- Alberico, M., Cadena, A., Hernández-Camacho, J., & Muñoz-Saba, Y. (2000). Mamíferos (Synapsida: Theria) de Colombia. Biota Colombiana, 1, 43–75.
- Baker, R. J., Solari, S., Cirranello, A., & Simmons, N. B. (2016). Acta Chiropterologica 18(1), 1–38.
- Bouckaert, R., Heled, J., Kühnert, D., Vaughan, T., Wu, C., Xie, D., Suchard, M. A., Rambaut, A., & Drummond, A. J. (2014). BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Computational Biology, 10, e1003537–6. https://doi.org/10.1371/journal.pcbi.1003537
- Carstens, B. C., Pelletier, T. A., Reid, N. M., & Satler, J. D. (2013). How to fail at species delimitation. Molecular Ecology, 22, 4369–4383.
- Chakrabarty, P. (2010). Genetypes: A concept to help integrate molecular phylogenetics and taxonomy. Zootaxa, 2632, 67–68. https://doi.org/10.11646/zootaxa.2632.1.4
- Dayrat, B. (2005). Towards integrative taxonomy. Biological Journal of the Linnean Society, 85, 407–415. https://doi.org/10.1111/j.1095-8312.2005.00503.x
- De Queiroz, K. (2007). Species concepts and species delimitation. Systematic Biology, 56, 879–886. https://doi.org/10.1080/10635150701701083
- De Queiroz, K. (2005). A unified concept of species and its consequences for the future of taxonomy [Paper presentation]. Proceedings of the California Academy of Sciences, 56, 196–215.
- Dobson, G. E. (1878). Catalogue of the Chiroptera in the collection of the British Museum (xlii + 567 pp., 29 pls). British Museum (Natural History).
- Edgar, R. C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. https://doi.org/10.1093/nar/gkh340
- Ezard, T., Fujisawa, T., Barraclough, T. G. (2009). Splits: Species’ limits by threshold statistics. https://r-forge.r-project.org/projects/splits/
- Fujisawa, T., & Barraclough, T. G. (2013). Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent Approach: A revised method and evaluation on simulated data sets. Systematic Biology, 62, 707–724. https://doi.org/10.1093/sysbio/syt033
- Garbino, G. S. T., Lim, B. K., & Tavares, V. C. (2020). Systematics of big-eyed bats, genus Chiroderma Peters, 1860 (Chiroptera: Phyllostomidae). Zootaxa, 4846, 1–93. https://doi.org/10.11646/zootaxa.4846.1.1
- Garbino, G. S. T. (2019). Revisão sistemática de Chiroderma Peters, 1860 e filogenia de Vampyressina Baker et al. 2016 (Chiroptera: Phyllostomidae) [Doctoral dissertation]. Program in Zoology, Universidade Federal de Minas Gerais 264 p., 264. pp.
- Gutiérrez, E. E., & Garbino, G. S. T. (2018). Species delimitation based on diagnosis and monophyly, and its importance for advancing mammalian taxonomy. Zoological Research, 39, 1–8.
- Hernández-Canchola, G., Gómez-Jiménez, Y. A., Hernández-Chávez, I., Lucero-Verdugo, S. C., & León-Paniagua, L. (2019). Notes on Vampyressa thyone (Chiroptera: Phyllostomidae): Distribution, genetics and hypopigmentation. Biota Neotropica, 19, e20180621. https://doi.org/10.1590/1676-0611-bn-2018-0621
- Hoofer, S. R., & Baker, R. J. (2006). Molecular systematics of vampyressine bats (Phyllostomidae: Stenodermatinae) with comparison of direct and indirect surveys of mitochondrial DNA variation. Molecular Phylogenetics and Evolution, 39, 424–438. https://doi.org/10.1016/j.ympev.2005.12.001
- Hoofer, S. R., Flanary, W. E., Bull, R. J., & Baker, R. J. (2008). Phylogenetic relationships of vampyressine bats and allies (Phyllostomidae: Stenodermatinae) based on DNA sequences of a nuclear intron (TSHB-I2). Molecular Phylogenetics and Evolution, 47, 870–876.
- Huelsenbeck, J. P., & Ronquist, F. (2001). MRBAYES: Bayesian Inference of phylogenetic trees. Bioinformatics, 33, 754–755.
- Kapli, P., Lutteropp, S., Zhang, J., Kobert, K., Pavlidis, P., Stamatakis, A., & Flouri, T. (2017). Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics (Oxford, England), 33, 1630–1638.
- Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16, 111–120. https://doi.org/10.1007/BF01731581
- Kircher, M., Sawyer, S., & Meyer, M. (2012). Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Research, 40, e3. https://doi.org/10.1093/nar/gkr771
- Krueger, F. (2015). Trim Galore: A wrapper tool around Cutadapt and FastQC to consistently apply quality and adapter trimming to FastQ files, with some extra functionality for MspI-digested RRBS-type (Reduced Representation Bisulite-Seq) libraries. http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/
- Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874.
- Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T., & Calcott, B. (2016). PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution, 34, 772–773.
- Lemke, T., Cadena, A., Pine, R. H., & Hernández-Camacho, J. (1982). Notes on opossums, bats, and rodents new to the fauna of Colombia. Mammalia, 46, 225–234. https://doi.org/10.1515/mamm.1982.46.2.225
- Li, H. (2013). Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXIV:1303.3997, Accessed 22 December 2017.
- Lim, G. S., Balke, M., & Meier, R. (2012). Determining species boundaries in a world full of rarity: Singletons, species delimitation methods. Systematic Biology, 61, 165–169. https://doi.org/10.1093/sysbio/syr030
- Lim, B. K., Pedro, W. A., & Passos, F. C. (2003). Differentiation and species status of the Neotropical yellow-eared bats Vampyressa pusilla and V. thyone (Phyllostomidae) with a molecular phylogeny and review of the genus. Acta Chiropterologica, 5, 15–29. https://doi.org/10.3161/001.005.0102
- Marsh, C. J., Sica, Y. V., Burgin, C. J., Dorman, W. A., Anderson, R. C., Del Toro Mijares, I., Vigneron, J. G., Barve, V., Dombrowik, V. L., Duong, M., Guralnick, R., Hart, J. A., Maypole, J. K., McCall, K., Ranipeta, A., Schuerkmann, A., Torselli, M. A., Lacher, T., Mittermeier, R. A., … Jetz, W. (2022). Expert range maps of global mammal distributions harmonised to three taxonomic authorities. Journal of Biogeography, 49, 979–992. https://doi.org/10.1111/jbi.14330
- Mayden, R. (1999). Consilience and a hierarchy of species concepts: Advances toward closure on the species puzzle. Journal of Nematology, 31, 95–116.
- McDonough, M. M., Parker, L. D., Rotzel McInerney, N., Campana, M. G., & Maldonado, J. E. (2018). Performance of commonly requested destructive museum samples for mammalian genomic studies. Journal of Mammalogy, 99, 789–802. https://doi.org/10.1093/jmammal/gyy080
- Miller, M. A., Pfeiffer, W., & Schwartz, T. (2010 Creating the CIPRES science gateway for inference of large phylogenetic trees [Paper presentation]. 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA., pp. 1–8 https://doi.org/10.1109/GCE.2010.5676129
- Morales-Martínez, D. M., Rodríguez-Posada, M. E., & Ramírez-Chaves, H. E. (2021). A new cryptic species of yellow-eared bat Vampyressa melissa species complex (Chiroptera: Phyllostomidae) from Colombia. Journal of Mammalogy, 102, 90–100. https://doi.org/10.1093/jmammal/gyaa137
- O’Meara, B. C. (2010). New heuristic methods for joint species delimitation and Species Tree Inference. Systematic Biology, 59, 59–73.
- Padial, J. M., & de la Riva, I. (2006). Taxonomic inflation and the stability of species lists: the perils of ostrich’s behavior. Systematic Biology, 55, 859–867.
- Padial, J. M., Miralles, A., de la Riva, I., & Vences, M. (2010). The integrative future of taxonomy. Frontiers in Zoology, 7, 16.
- Paradis, E., Claude, J., & Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289–290. https://doi.org/10.1093/bioinformatics/btg412
- Patten, M. A. (2015). Subspecies and the philosophy of science. The Auk, 132, 481–485. https://doi.org/10.1642/AUK-15-1.1
- Peters, W. (1860). Eine neue Gattung von Flederthieren, Chiroderma villosum, aus Brasilien. In Monatsberichte der Königlichen Preussische Akademie des Wissenschaften zu Berlin (Vol. 1861, pp. 747–755).
- Peters, W. (1865). Abbildungen zu einer Monographie der Chiropteren vor und gab eine Übersicht der von ihmbefolgten systematischen Ordnung der hieher gehörigen Gattungen. In Monatsberichte der Königlichen Preussische Akademie des Wissenschaften zu Berlin (Vol. 1866, pp. 256–258).
- Peterson, R. L. (1968). A new bat of the genus Vampyressa from Guyana, South America, with a brief systematic review of the genus. Life Sciences Contributions, Royal Ontario Museum, 73, 1–17.
- Pons, J., Barraclough, T. G., Gomez-Zurita, J., Cardoso, A., Duran, D. P., Hazell, S., Kamoun, S., Sumlin, W. D., & Vogler, A. P. (2006). Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Systematic Biology, 55, 595–609.
- Porter, C. A., & Baker, R. J. (2004). Systematics of Vampyressa and related genera of phyllostomid bats as determined by cytochrome-b sequences. Journal of Mammalogy, 85, 126–132. https://doi.org/10.1644/BWG-110
- Puillandre, N., Lambert, A., Brouillet, S., & Achaz, G. (2012). ABGD, Automatic barcode gap discovery for primary species delimitation. Molecular Ecology, 21, 1864–1877. https://doi.org/10.1111/j.1365-294X.2011.05239.x
- Rambaut, A., Suchard, M., Xie, D., Drummond, A. (2014). Tracer v1. 6. Tracer (Online 2015, May 29). http://tree.bio.ed.ac.uk/software/tracer/
- Ramírez-Chaves, H., Tavares, V., & Torres-Martínez, M. (2015). Vampyressa melissa. The IUCN Red List of threatened species 2015 e.T22839A22058315.
- Ramírez-Chaves, H., Tavares, V. da C., & Torres-Martinez, M. (2015). Vampyressa melissa. In IUCN 2015. The IUCN red list of threatened species. Version 2015.4.
- Reid, N. M. (2014). bGMYC: A Bayesian MCMC implementation of the general mixed Yule-coalescent model for species delimitation. R Package Version 1.
- Reid, N. M., & Carstens, B. C. (2012). Phylogenetic estimation error can decrease the accuracy of species delimitation: A Bayesian implementation of the general mixed Yule-coalescent model. BMC Evolutionary Biology, 12, 196. https://doi.org/10.1186/1471-2148-12-196
- Rohland, N., & Reich, D. (2012). Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Research, 22, 939–946.
- Saussure, H. de. (1860). Note sur quelques mammifères du Mexique. Revue et Magasin de Zoologie pure et appliquée Series, 2(12), 1–494, 4 pls.
- Schmieder, R., & Edwards, R. (2011). Quality control and preprocessing of metagenomic datasets. Bioinformatics (Oxford, England), 27, 863–864.
- Simpson, G. G. (1961). Principles of animal taxonomy. Columbia University Press.
- Solari, S., Medellín, R., Rodríguez-Herrera, B., Tavares, V., da, C., Garbino, G., Camacho, M. A., Tirira, D., Lim, B., Arroyo-Cabrales, J., Rodríguez-Durán, A., Dumont, E., Burneo, S., Aguirre, L. F., Tschapka, M., & Espinosa, D. (2019). Family Phyllostomidae (New World Leaf-nosed Bats). In D. E. Wilson and R. A. Mittermeier (Eds.), Handbook of the Mammals of the World, Bats (Vol. 9, pp. 444–583). Lynx Edicions.
- Taddei, V. A. & Lim, B. K. (2010) A new species of Chiroderma (Chiroptera, Phyllostomidae) from Northeastern Brazil. Brazilian Journal of Biology, 70(2), 381–386.
- Tang, C. Q., Humphreys, A. M., Fontaneto, D., Barraclough, T. G., & Paradis, E. (2014). Effects of phylogenetic reconstruction method on the robustness of species delimitation using single-locus data. Methods in Ecology and Evolution, 5, 1086–1094.
- Tavares, V. C., Gardner, A. L., Ramírez-Chaves, H. E., & Velazco, P. M. (2014). Systematics of Vampyressa melissa Thomas, 1926 (Chiroptera: Phyllostomidae), with descriptions of two new species of Vampyressa. American Museum Novitates, 3813, 1–26. https://doi.org/10.1206/3813.1
- Thomas, O. (1889). Description of a new stenodermatous bat from Trinidad. Annals and Magazine of Natural History Series 6(4), 167–170.
- Thomas, O. (1900). Descriptions of new Neotropical mammals. Annals and Magazine of Natural History Series 7(5), 269–274.
- Thomas, O. (1901). On a collection of mammals from the Kanuku Mountains, British Guiana. Annals and Magazine of Natural History Series 7(8), 139–154.
- Thomas, O. (1909). Notes on some South-American mammals with descriptions of new species. Annals and Magazine of Natural History Series 8(4), 230–242.
- Thomas, O. (1926). The Goldman-Thomas expedition to Peru. III – on mammals collected by Mr. R.W. Hendee in the Chachapoyas region of north Peru. Annals and Magazine of Natural History 18(9), 156–167. https://doi.org/10.1080/00222932608633489
- Velazco, P. M., & Patterson, B. D. (2008). Phylogenetics and biogeography of the broad-nosed bats, genus Platyrrhinus (Chiroptera: Phyllostomidae). Molecular Phylogenetics and Evolution, 49, 749–759.
- Wagner, J.A. 1843. Diagnosen neuer Arten brasilischer Handflügler. Archiv für Naturgeschichte 9(1), 365–368.
- Will, K. W., Mishler, B. D., & Wheeler, Q. D. (2005). The perils of DNA barcoding and the need for integrative taxonomy. Systematic Biology, 54, 844–851.
- Zachos, F. (2018). Mammals and meaningful taxonomic units: The debate about species concepts and conservation. Mammal Review, 48, 153–159. 10.1111/mam.12121
- Zhang, J., Kapli, P., Pavlidis, P., & Stamatakis, A. (2013). A general species delimitation method with applications to phylogenetic placements. Bioinformatics (Oxford, England), 29, 2869–2876.
- Zhang, J., Kobert, K., Flouri, T., & Stamatakis, A. (2014). PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics (Oxford, England), 30, 614–620.
