Abstract
Etisine crabs are some of the most abundant cryptobionts in Indo-West Pacific coral reef systems. Despite their ecological importance and abundance in museum collections, several recent systematic studies have indicated family- to subspecies-level taxonomic problems. One such case involves the former chlorodielline genus Soliella Lasley, Klaus & Ng, Citation2015 (treated here as part of Etisinae), which currently comprises two valid species and three available names that have been in flux in recent literature. The validity of these taxa has only been cursorily discussed. To resolve species limits and distributions, a thorough morphological examination of hundreds of specimens was conducted, including scanning electron microscopy of male gonopods, along with analysis of sequence data of the mitochondrial marker cytochrome c oxidase subunit I (COI) from 84 exemplars across the distribution of the genus. The status of two species that have Indian Ocean versus Pacific Ocean distributions with overlap in the Indo-Australian Archipelago and adjacent regions is confirmed. While external morphology is not reliable for identification, a few discrete, although slight, differences in gonopod morphology were found, and these results are consistent with a ‘pseudocryptic species’ designation. Speciation conforms to a previously published etisine model of allopatric differentiation followed by subsequent divergence of gonopod morphology upon secondary sympatry. This pattern, the biogeography of the two species and the concept of ‘pseudocryptic species’ are discussed.
http://zoobank.org/urn:lsid:zoobank.org:pub:ABEC01C7-EA5B-4BB6-87E0-405FE3895D95
Introduction
The Indo-West Pacific (IWP) is the world’s largest marine biogeographic region, characterized by many wide-ranging species that mostly disperse via long-lived, planktonic larvae (Briggs & Bowen, Citation2012; Forest & Guinot, Citation1961; Kay, Citation1984; Myers, Citation1994). Recent studies have indicated that many species previously thought to range across the IWP comprise mosaics of allo- or parapatric, cryptic lineages (e.g., Drew & Barber, Citation2009; Malay & Paulay, Citation2010; Meyer et al., Citation2005; Titus et al., Citation2018). One such study of the brachyuran crab clade ‘Chlorodiellinae’ (now Etisinae) found that while some species have IWP-wide distributions with little genetic structuring, others are complexes of deeply divergent allopatric lineages (Lasley et al., Citation2023). That study also uncovered a strong correlation between genetic distance (time), sympatry, and the divergence of genital morphology, highlighting the important roles of both allopatric genetic differentiation and genital divergence in the speciation process. Here the differentiation in one of these genera is examined in greater detail.
Members of the xanthid subfamily Etisinae Ortmann, Citation1893 are some of the most abundant crustacean cryptofauna in IWP coral reefs (Monteforte, Citation1987; Peyrot-Clausade, Citation1977, Citation1979, Citation1989). Despite their ecological importance, abundance and prevalence in museum collections, the taxonomy of this group has proved challenging and needs attention. The molecular phylogenetic study of the superfamily Xanthoidea Macleay, Citation1838, by Mendoza et al. (Citation2022) greatly expanded the Etisinae, merging it with the subfamily Chlorodiellinae Ng and Holthuis, Citation2007 (sensu Ng et al., Citation2008). Mendoza et al. (Citation2022) further included three xanthine genera, Leptodius A. Milne-Edwards, Citation1863, Macromedaeus Ward, Citation1942, and Neoxanthops Guinot, Citation1968. Although a formal morphological diagnosis for this grouping has yet to be proposed, its members commonly share spoon-tipped chelae and, to a lesser extent, a dactylopropodal lock and calcareous dactylar spines on the ambulatory legs. Nevertheless, most chlorodielline genera have been recovered in a subclade within Xanthidae with high support, and this lineage has been the subject of recent systematic studies (Lai et al., Citation2011; Lasley et al., Citation2013, Citation2015, Citation2022, Citation2023; Mendoza et al., Citation2022). Lasley et al. (Citation2015) revised the genus-level taxonomy of ‘Chlorodiellinae’, particularly of Pilodius Dana, Citation1851, which was shown to be polyphyletic, and described two new genera: Luniella Lasley, Klaus & Ng, Citation2015 and Soliella Lasley, Klaus & Ng, Citation2015.
Three nominal species could be attributed to Soliella: Pilodius flavus Rathbun, Citation1893, Chlorodopsis melanospinis Rathbun, Citation1911, and Chlorodopsis hawaiiensis Edmondson, Citation1962 (see Clark & Galil, Citation1993; Ng et al., Citation2008). In their revision of the genus Pilodius, Clark and Galil (Citation1993) considered all three to pertain to P. flavus. Lasley et al. (Citation2015) also recognized S. melanospinis as valid based on morphology of the male gonopod (G1) and sequence data from two specimens but did not evaluate C. hawaiiensis. The differences between the species’ G1s remain unclear, as do external morphological differences, historical literature, and geographic distributions of these species.
To stabilize the taxonomy of Soliella and investigate speciation in the genus, we conducted genus-level phylogenetic analyses using the DNA barcoding gene COI, and morphological examination, including scanning electron microscopy of male genital structures (first gonopod or ‘G1’), of hundreds of specimens. Historical records were also reviewed especially to assess the geographic distributions of the two species.
Material and methods
Specimens for morphological and molecular analyses were obtained from the following institutions: Zoological Reference Collection of the Lee Kong Chian Natural History Museum, National University of Singapore, Singapore (ZRC); Florida Museum of Natural History, University of Florida, Gainesville, Florida, USA (UF); US National Museum of Natural History, Smithsonian Institution, Washington DC, USA (USNM); and American Museum of Natural History, New York, New York, USA (AMNH). Historical literature and material examined are covered in SM1.
Morphological examination was conducted using a dissecting microscope (Leica MZ16, Leica Biosystems, Wetzlar, Germany) and a scanning electron microscope (SEM) (Leica Stereoscan 440 at the USNM Imaging Laboratory). The right first and second male gonopods (G1, G2) were removed for examination unless they were damaged, in which case the left one was removed. G1s were prepared for SEM as described by Felgenhauer (Citation1987) and Lasley et al. (Citation2022). Geographic ranges were compiled from locality information from material examined and literature. These data were checked against locality information associated with COI sequences when possible. Occurrence maps were generated with the R package ggplot2 (Wickham, Citation2016).
COI sequence data were obtained from Lasley et al. (Citation2023), including sequences of Pilodius maotieni Serène, Citation1971, Luniella spinipes (Heller, Citation1860), and Cyclodius granulatus (Targioni Tozzetti, Citation1877) as outgroups (Lasley et al., Citation2015) (Supplemental Table S1). Maximum likelihood trees were generated using RAxML-HPC BlackBox 8.2.12 (Stamatakis, Citation2014) in the computer cluster of CIPRES (CyberInfrastructure for phylogenetic RESearch project) (http://www.phylo.org; Miller et al., Citation2011). The GTRGamma + I model of nucleotide substitution was selected and the analysis was conducted with 1000 bootstrap replicates. A Neighbour-Joining analysis using the Tamura–Nei genetic distance model was also performed in Geneious 8.1.9 with 1000 bootstrap replicates. Between group mean p-distance between species was calculated using Mega version 11.0.13.
Nomenclature and terminology follow Dana (Citation1851), Serène (Citation1984), Ng et al. (Citation2008), and Davie et al. (Citation2015). Measurements provided (in millimetres) are of the maximum carapace width and length, respectively. The following abbreviations are used: G1, male first gonopod; G2, male second gonopod; stn., station; and coll., collected by. Works by Raoul Serène’s Vietnamese assistant, Nguyen Van Luom, have erroneously been referred to using one of his given names, ‘Luom’, rather than his surname ‘Nguyen’, in previous studies. Here the name is used in full, ‘Nguyen Van Luom’, e.g., Serène and Nguyen Van Luom (Citation1958), for clarity (Waterman, Citation1953).
Results
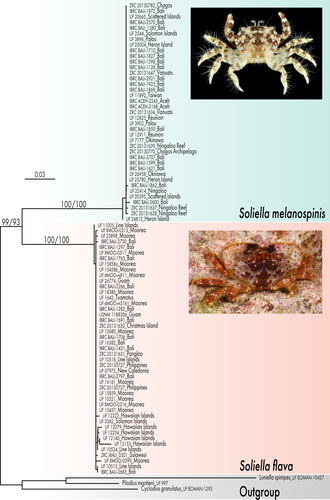
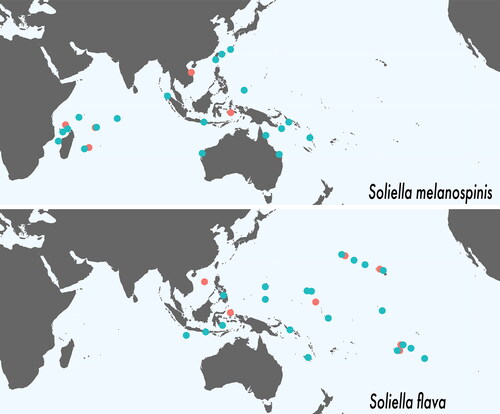
Specimens grouped into two species based on G1 morphology and these corresponded to two reciprocally monophyletic COI clades separated by 11.3% p-distance (). The S. melanospinis clade comprises individuals from the Western Indian Ocean to the Indo-Australian Archipelago and adjacent areas: the Scattered Islands, Reunion Island, Chagos Archipelago, Indonesia (Aceh and Bali), Ningaloo Reef (W. Australia), Taiwan, Okinawa, Palau, Heron Island (Great Barrier Reef), the Solomon Islands, and Vanuatu. The S. flava clade comprises individuals from the Indo-Australian Archipelago and adjacent areas to the Eastern Pacific Barrier: Christmas Island (Indian Ocean), the Philippines, Indonesia (Bali and Sulawesi), Guam, New Caledonia, Line Islands, Society Islands, Tuamotu Islands, and Hawaiian Islands ().
Taxonomy
Superfamily Xanthoidea MacLeay, Citation1838
Family Xanthidae MacLeay, Citation1838
Subfamily Etisinae Ortmann, Citation1893
Soliella Lasley, Klaus & Ng, Citation2015
Chlorodopsis, Rathbun, Citation1911: 226.–Balss, Citation1938: 58.–Serène & Nguyen Van Luom, Citation1959: 88; 1959: 336.
Pilodius, Balss, Citation1938: 56.–Forest & Guinot, Citation1961: 81.–Serène, Citation1984: 233.–Clark & Galil, Citation1993: 1121.–Ng et al., Citation2008: 197.
Soliella Lasley et al., Citation2015: 173
Diagnosis
Carapace transversely subhexagonal, dorsal surface granular, covered with short and long, light-coloured setae, regions well defined. Front sinuous, quadrilobate; submedian lobes broadly arched, separated by median, narrow, U-shaped notch, margins granular or spinose; lateral lobes distinct, narrow. Anterolateral margin with four lobes, each tipped with emergent, anteriorly directed spine surrounded by smaller accessory spines. Basal antennal article with distolateral extension reaching approximately halfway into orbital hiatus. Male thoracic sternum relatively broad; suture 3/4 distinct near lateral margins, interrupted medially; median line present on sternite 4 as short suture or shallow depression midway between anterior border of sternite and sterno-pleonal cavity, interrupted in exposed posterior part, reappearing in sterno-pleonal cavity on posterior surface of sternite 4; tubercle of press-button locking mechanism located on anterior half of sternite 5. External, superior surfaces of chelipeds spinose, granular, with numerous long, simple, yellow setae. Ambulatory legs relatively stout; dactylopropodal lock present, well developed; tip of dactylus terminating in long, curved, chitinous claw and two subdistal, small, calcareous spines. Pleon relatively long, slender, tip of telson reaching beyond imaginary transverse line connecting sternal condyles of P1 coxae; pleonites 3–5 functionally fused, with distinct furrows delineating 3/4 and 4/5; telson subtriangular, basal width slightly greater than median length. G1 narrow, sinuous but not drastically curved; distal tip tubular or spatulate with numerous subdistal, proximally directed, spiniform setae. G2 ca. one-third length of G1, sigmoidally curved, terminal segment ca. one-fourth length of subterminal segment. Penis emerging at anterior portion of sternal condyle of P5 coxa.
Remarks
Lasley et al. (Citation2015) provided a diagnosis of the genus and compared it with four genera that were, along with Soliella, previously classified in the subfamily Chlorodiellinae (= Etisinae in part): Chlorodiella Rathbun, Citation1897, Cyclodius Dana, Citation1851, Pilodius, and Luniella. Soliella differs from these genera, most notably, in the morphology of its G1 () (Serène Citation1984, figs 144–158, 163–165, 167–172, 173–177). Soliella also differs from all species in these genera with the exceptions of Luniella pubescens (Dana, Citation1852), Luniella scabricula (Dana, Citation1852), and Cyclodius paumotensis (Rathbun, Citation1907), by the presence of long and short, light-coloured setae on the carapace. Traditional characters, such as the shape and disposition of the basal antennal article and the form of the subterminal (bifid) spine of the ambulatory leg dactylus, that have been used to differentiate these genera are problematic and are not shared with the closest relatives of Soliella: Cyclodius and Pilodius (Ng & Yang, Citation1998; Clark & Ng, Citation1999; Lasley et al., Citation2015). The length of the distolateral extension of the basal antennal article varies in some genera (e.g., Pilodius) and with age (Lasley et al., Citation2015; Serène, Citation1984: 233, footnote by Crosnier). In Soliella, the basal antennal article has a distolateral extension that reaches approximately halfway the into orbital hiatus (vs no extension in Cyclodius and usually reaching the orbital hiatus in Pilodius). Soliella has small, calcareous subterminal spines on the flexor margin of the ambulatory leg dactylus, while the presence and length of subterminal spines vary in Pilodius and Cyclodius (Lasley et al., Citation2015). Relationships between Soliella and more distantly related genera that were previously assigned to Chlorodiellinae were reviewed in Lasley et al. (Citation2015), e.g., Tweedieia Ward, Citation1935, Vellodius Ng & Yang, Citation1998, and Sulcodius Clark & Ng, Citation1999. All other etisine genera have been treated in Serène (Citation1984). In view of the results from recent molecular phylogenetic studies on Xanthidae (Lai et al., Citation2011; Lasley et al., Citation2015; Mendoza et al., Citation2022), however, the diagnoses for the different genera in an expanded Etisinae will need to be re-evaluated and emended, with greater focus on thoracic sternal characters and other such non-traditional but informative characters.
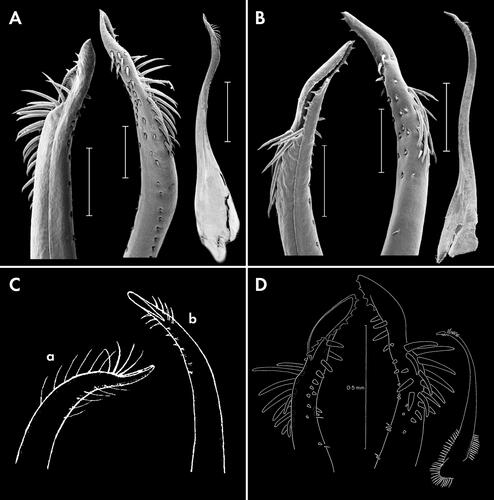
Fig. 3. First male gonopods (G1) of Soliella species; A, Soliella flava (Rathbun, Citation1893), G1, internal detail (scale = 200 μm), external detail (scale = 200 μm), and external full (scale = 1 mm) (UF 12254); B, Soliella melanospinis (Rathbun, Citation1911), G1, internal detail (scale = 200 μm), external detail (scale = 200 μm), and external full (scale = 1 mm) (ZRC 2013.1647); C, S. flava G1 (a) after Edmondson (Citation1962: 21d) as Chlorodopsis hawaiiensis, (b) after Edmondson (Citation1962: fig. 22b) as Pilodius flavus; D, S. flava G1 after Clark & Galil (Citation1993: ) as Pilodius flavus.
Soliella flava (Rathbun, Citation1893)
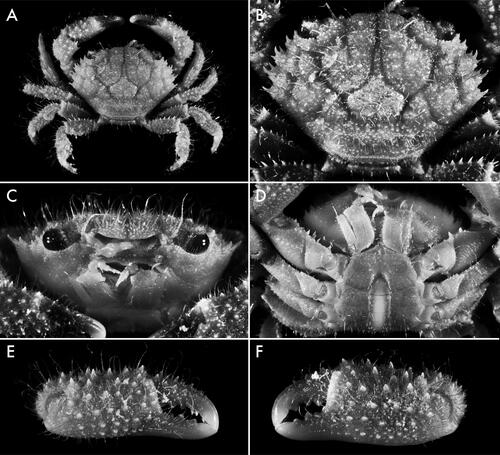
Fig. 4. Soliella flava (Rathbun, Citation1893), holotype female, 9 × 6 (USNM 17317), Hawaiian Islands, dorsal view.
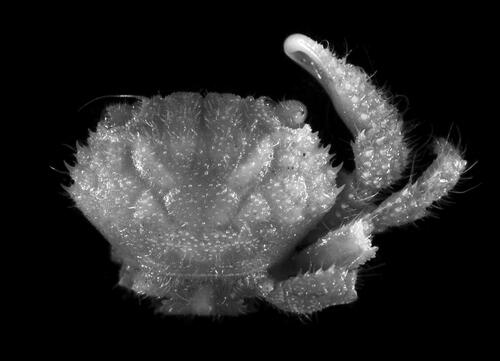
Fig. 5. Soliella flava (Rathbun, Citation1893), male, 10.2 × 6.9 (USNM 1181377), Marshall Islands; A, dorsal view; B, carapace, dorsal view; C, frontal view; D, thoracic sternum; E, minor chela, external view; F, major chela, external view.
Pilodius flavus Rathbun, Citation1893: 239; 1906: 860, fig. 21.–Edmondson, Citation1925: 43; Citation1933: 249; Citation1962: 275, fig. 22a, b.–Balss, Citation1938: 57.–Miyake, Citation1939: 215.–Forest & Guinot, Citation1961: 95.–Serène, Citation1968: 80; Citation1984: 235, 239 [key].–Peyrot-Clausade, Citation1989: 111.–Clark & Galil, Citation1993: 1130 (in part), figs 4A–G, 32B, 40D, 41 A.–DeFelice et al., Citation1998: 16; Citation2002: 30, 72.–Coles et al., Citation2002a: 271 (list); Citation2002b: 141, 194; Citation2008: 63 (list)–Ng et al., Citation2008: 197 (list).–Castro, Citation2011: 92. –Mendoza et al., Citation2014: 278.
Chlorodopsis flava, Serène & Nguyen Van Luom, Citation1959: 330, figs 2C, 5F, pl. 1 fig. B, pl. 3 fig. B.
Chlorodopsis hawaiiensis Edmondson, Citation1962: 273, fig. 21a–e.
Soliella flava, Lasley et al., Citation2015: 174, suppl. figs S1D, S3C, D, S5F.
Chlorodopsis melanodactylus, Miers, Citation1884: 531 (in part, from Etoile Island). Not Pilodius melanodactylus A. Milne-Edwards, Citation1873. [fide Clark & Galil, Citation1993].
Pilodius pubescens, De Man, Citation1902: 619. Not Pilodius pubescens Dana, Citation1852 [fide Balss, Citation1938].
?Pilodius pubescens, Nobili, Citation1907: 395. Not Pilodius pubescens Dana, Citation1852 [fide Balss, Citation1938].
Diagnosis
Carapace () transversely subhexagonal, ca. 1.5 times as broad as long; surface covered in short, stout and few long, light-coloured setae; regions well defined, separated by distinct, smooth furrows; 1 F indistinct; 2 F distinct; 1 M separated from 2 F and inner branch of 3 M by shallow furrow; 2 M entire or feebly divided anteriorly, 3 M entire; 4 M indistinct; 1 L indistinct; 1 L and 2 L partially confluent; 3 L-6L distinct; 1 P with defined anterior and posterior borders, lateral borders diffuse; 2 P with transverse row of granules. Submedian lobes of front () broadly convex, margin lined with granules, separated by median V- or U-shaped notch; lateral lobes triangular, granulate, separated from submedian lobes by deep, triangular notch, separated from orbits/supraorbital margin by rounded, L-shaped notch. Supraorbital margin lined dorsolaterally with short spines or conical granules; infraorbital margin lined with conical granules. Anterolateral margin with four spinose lobes. Anterolateral angle of basal antennal article slightly expanded, entering less than halfway into orbital hiatus. Pterygostomial region minutely granulate, with plumose setae diagonally from posterior to lateral surface. Male thoracic sternum () relatively broad, minutely granulate, with few long, scattered setae; tubercle of press-button locking mechanism located on anterior half of sternite 5; suture 3/4 distinct near lateral margins, interrupted medially; median line present on sternite 4 as short suture midway between anterior border of sternite and sterno-pleonal cavity, interrupted in exposed posterior part, reappearing in sterno-pleonal cavity on posterior surface of sternite 4, absent at level of sternites 5 and 6, present and complete at level of sternites 7 and 8. Chelipeds () subequal, covered with long, simple, light-coloured setae, spinose; merus stout. Ambulatory legs () stout, setose; setae long, simple, light-coloured; extensor margin of merus lined with long spines; dactylopropodal lock present, well developed; tip of dactylus terminating in long, curved, chitinous claw and two subdistal, small, calcareous spines. Male pleon () moderately stout, with few long posterior setae; pleonites 3–5 functionally fused, with distinct furrows delineating 3/4 and 4/5; pleonite 6 subquadrate, ca. as broad as long; telson subtriangular, ca. as broad as long. G1 () slender, sinuous, distal 1/4 curved ventrally; apex pointing anteroventrally with ca. 20 subdistal, perpendicular to proximally-directed, stout, spiniform setae on the anterior surface; apical lobe almost tubular, opening facing anteriorly. G2 ca. one-third length of G1, sigmoidally curved, terminal segment ca. one-fourth length of subterminal segment.
Female morphology
Females are similar to males, except in having nearly equal chelipeds and in sexual characters. Sternopleonal cavity wide, with the median line obscured completely by the pleon; sutures 2/3, 6/7, and 7/8 complete; suture 3/4 indicated only near lateral margin; sutures 4/5 and 5/6 interrupted medially. Vulvae crescent-shaped, positioned on sternite 6 near suture 5/6. Pleon long and wide relative to male; tip of telson reaching imaginary line between midpoint of cheliped coxae; all pleonites freely articulated.
Type status
The female holotype (USNM17317) from the Hawaiian Islands was examined for this study (SM1).
Remarks
Soliella flava and S. melanospinis are difficult to differentiate based on external morphology. Rathbun (Citation1893, Citation1911) described both species. In her description of S. melanospinis, Rathbun (Citation1911) stated that S. flava has a less deeply areolated carapace, a dorsum devoid of spines, and an upper margin of the orbit (supraorbital margin) without spines (vs. less deeply areolated regions, a spinose dorsum, and upper margin of the orbit in P. melanospinis). Serène (Citation1984) stated that the spination on the supraorbital margin was a good character for differentiation, but that the difference in the areolation of the carapace was difficult to assess. He also stated that the G1s are similar, although he had provided figures of the two in his previous publications with Nguyen Van Luom (Serène & Nguyen Van Luom, Citation1958: pl. 4 fig. f; 1959: figs 2C, 2 bis M).
Edmondson (Citation1962) described Chlorodopsis hawaiiensis without comparison with S. flava or S. melanospinis. He also provided illustrations of their G1s. Clark & Galil (Citation1993) synonymized S. melanospinis and S. hawaiiensis with S. flava. However, Lasley et al. (Citation2015, Citation2023) recovered two distinct, divergent species-level clades in Soliella in their molecular phylogenetic analyses, while there are three different G1 morphotypes illustrated in the literature. Edmondson (Citation1962: figs 21d, 22b) provided figures of the ladle-like G1 of Chlorodopsis hawaiiensis and the tubular G1 of S. flava, illustrating them with distinct morphologies albeit in a simplistic, even schematic, style (). Rathbun’s (Citation1893) Hawaiian holotype of S. flava is female. Examination of many Hawaiian specimens (SM1), however, including those previously identified as Chlorodopsis hawaiiensis and S. flava makes it clear that the G1s show only slight variation that had been exaggerated in the figures of Edmondson (Citation1962). These gonopod morphotypes fall within the S. flava COI clade in the present analysis. The third G1 morphotype was illustrated by Clark & Galil (Citation1993: fig. 4D–G) as S. flava, although their specimen is a paratype of S. melanospinis (). This is the same morphotype as those illustrated by Serène and Nguyen Van Luom (Citation1959: fig 2M) and Serène (Citation1984: fig. 146), but in these studies, they are identified as S. melanospinis.
In summary, the external morphological characters of Rathbun (Citation1911) are difficult to appreciate, but G1 morphology and phylogenetic analyses indicate that there are clearly two species. Although the depth of the furrows separating the carapace regions and spination of the supraorbital margin vary, S. flava specimens do generally have less defined carapace regions and a supraorbital margin with shorter spines or conical granules (vs less relatively deeply defined regions and supraorbital margin with larger spines in S. melanospinis). These characters, however, display too much variation, especially in small individuals, to be used without caution. The form of the G1 appears to be the only reliable morphological character for identification. Soliella flava has a G1 pointing anteroventrally with an apical lobe opening anteriorly and ca. 20 spiniform subdistal setae on the anterior surface (vs apex pointing ventrally with an apical lobe that is longitudinally hollowed with a sinuous anterior margin and ca. 12 subdistal setae; ). The two G1 morphotypes correspond with the well-supported clades in the phylogenetic analyses.
Distribution
Soliella flava is reported from Christmas Island (Indian Ocean) and the Indo-Australian Archipelago to the Hawaiian Islands and French Polynesia ().
Soliella melanospinis (Rathbun, Citation1911)
, 6
Chlorodopsis melanospinis Rathbun, Citation1911: 226, pl. 18 fig. 11.—Balss, Citation1938: 62.—Serène & Nguyen Van Luom, Citation1958: 108, pl. 1 fig. D, pl. 3 fig. b, pl. 4 fig. c; 1959: 302, fig. 2 bis M.
Pilodius melanospinis, Guinot, Citation1964: 67; Citation1967: 268.—Serène, Citation1968: 80; Citation1984: 242, figs 143e, 146, pl. 33 fig. E.
Pilodius flavus, Clark & Galil, Citation1993: 1130 (in part).—Ng et al., Citation2008: 197 (list).
Chlorodopsis pilumnoides, Laurie, Citation1906: 406 (from Ceylon = Sri Lanka). Not Pilodius pilumnoides (White, 1848) [fide Clark & Galil, Citation1993].
Diagnosis
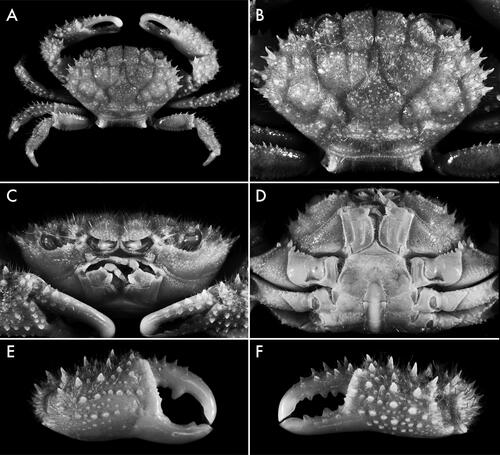
Carapace () transversely hexagonal, ca. 1.5 as broad as long; surface covered with short, stout light-coloured setae and few long, light-coloured setae; regions well defined, separated by wide, smooth, relatively deep furrows; 1 F indistinct; 2 F distinct; 1 M separated from 2 F and inner branch of 3 M by shallow furrow; 2 M entire or feebly divided anteriorly, 3 M entire; 4 M indistinct; 1 L indistinct; 1 L and 2 L partially confluent; 3 L–6L distinct; 1 P with defined anterior and posterior borders, lateral borders diffuse; 2 P with transverse row of granules. Submedian lobes of front () broadly convex, margin lined with granules, separated by median V- or U-shaped notch; lateral lobes triangular, separated from submedian lobes by deep, triangular notch, separated from orbits/supraorbital margin by rounded, L-shaped notch. Supraorbital margin generally lined dorsolaterally with relatively long spines or conical granules; infraorbital margin lined with conical granules. Anterolateral margin with four spinose lobes. Anterolateral angle of basal antennal segment slightly expanded, entering less than halfway into orbital hiatus. Pterygostomial region minutely granulate, with plumose setae diagonally from posterior to lateral surface. Male thoracic sternum () relatively broad, minutely granulate, with few long, scattered setae; tubercle of press-button locking mechanism located on anterior half of sternite 5; suture 3/4 distinct near lateral margins, interrupted medially; median line present on sternite 4 as short suture midway between anterior border of sternite and sterno-pleonal cavity, interrupted in exposed posterior part, reappearing in sterno-pleonal cavity on posterior surface of sternite 4, absent at level of sternites 5 and 6, present and complete at level of sternites 7 and 8. Chelipeds () subequal, covered with long, simple, light-coloured setae, spinose; merus stout. Ambulatory legs () stout, setose; setae long, simple, light-coloured; extensor margin of merus lined with long spines; dactylopropodal lock present, well developed; tip of dactylus terminating in long, curved, chitinous claw and two subdistal, small, calcareous spines. Male pleon () moderately stout, few long posterior setae; pleonites 3–5 functionally fused, with distinct furrows delineating 3/4 and 4/5; pleonite 6 subquadrate, ca. broad as long; telson subtriangular ca. as broad as long. G1 () slender, sinuous, distal 1/4 curved ventrally; apex pointing ventrally with ca. 12 subdistal, perpendicular to proximally directed, stout, spiniform setae on the anterior surface; apical lobe almost spatulate, longitudinally hollowed with sinuous anterior margin. G2 ca. one-third length of G1, sigmoidally curved, terminal segment ca. one-fourth length of subterminal segment.
Fig. 6. Soliella melanospinis (Rathbun, Citation1911), holotype male, 17.0 × 11.4 (USNM 41268), Saya del Malha Bank; A, dorsal view; B, carapace, dorsal view; C, frontal view; D, thoracic sternum; E, major chela, external view; F, minor chela, external view.
Female morphology
Females are similar to males, except in having nearly equal chelipeds and in sexual characters. These characters are the same as those outlined for Soliella flava females (see above).
Remarks
See Remarks for Soliella flava.
Distribution
Soliella melanospinis occurs from the Western Indian Ocean to the Indo-Australian Archipelago and adjacent areas including Taiwan, Japan, Palau, the Solomon Islands, and Vanuatu ().
Type status
The male holotype (USNM 41268) from Saya del Malha Bank, Western Indian Ocean, was examined for this study (SM1).
Key to the species of Soliella
G1 ultimately pointing anteroventrally with apical lobe opening anteriorly. Carapace regions relatively less defined. Supraorbital margin with relatively low spines or conical granulesS. flava
G1 apex pointing ventrally with an apical lobe that is longitudinally hollowed with a sinuous anterior margin. Carapace regions relatively well defined. Supraorbital margin generally with longer spinesS. melanospinis
Discussion
The synonymy of S. melanospinis with S. flava by Clark & Galil (Citation1993) reflects the morphological similarity between the two species. The examination here further demonstrates this similarity: there are no external features that can reliably distinguish these two species. The two species, however, have discrete, although relatively slight, differences in G1 morphology (compare with G1 figures of chlorodiellines in Serène Citation1984 and Lasley et al., Citation2023). Therefore, the term ‘pseudocryptic’ is used because: (1) molecular data guided the discovery of the species distinctions, and (2) there are minor but reliable morphological differences in a previously lumped species. The agreement between reciprocal monophyly in COI (here) and other markers (Lasley et al., Citation2023) with discrete differences in genital structures substantiates the use of the term ‘species’, especially considering their sympatric ranges and that genital divergence in arthropods is commonly used to infer reproductive isolation (Eberhard, Citation1985).
Lasley et al. (Citation2023) showed a correlation between secondary sympatry and divergence of G1s among species in the clade Chlorodiellinae, which includes Soliella. The implication is that these crabs differentiate first in allopatry (or technically parapatry if some degree of homogenizing gene flow was present) and secondary contact is accompanied, or allowed, by G1 divergence. For analysis in the study, lineages were categorized as (a) sharing a G1 morphology with its sibling lineage or (b) possessing a unique G1; and geographic distribution was categorized as allopatric, narrowly sympatric, or sympatric. ‘Narrowly sympatric’ sibling lineages were defined as those having less than 10% overlap in total distribution. Soliella melanospinis and S. flava were coded as having unique G1s and sympatric distributions (). This differs from many sibling lineages in Chlorodiellinae that show less genetic divergence, are allopatric, and share the same G1 morphology. Although the ranges of the two Soliella broadly overlap in the West Pacific, S. melanospinis is the sole species through most of the Indian Ocean while only S. flava is known from remote Oceania in the central Pacific. Allopatric divergence between the Indian and Pacific Ocean basins is the most prevalent geographic differentiation in IWP marine taxa (Barber et al., Citation2000; Malay & Paulay, Citation2010, Ahti et al., Citation2016). The distribution of Soliella is suggestive of a similar history of allopatric divergence, followed by secondary range overlap in the West Pacific, likely allowed or facilitated by genital divergence, but accompanied by little other morphological differentiation.
Our limited COI dataset indicates panmixia in both species – not uncommon in marine organisms with long larval durations. Population genomic data could, however, indicate fine-scale divergence and/or directionality of gene flow. These data could shed more light on the geographic origins of, and processes that govern speciation in, these species.
Associate Editor: Dr Polly Hayes
Supplemental Material - Table S1
Download MS Excel (13.3 KB)Supplemental Material - SM1_LiteratureMaterialExamined.docx
Download MS Word (18.8 KB)Acknowledgements
We are grateful to numerous people who have aided in sequencing and other lab work, and the many more people who have helped in the field or provided specimens. We also thank Mandy Bemis and John Slapcinsky (UF) for their efforts in curation. We thank Peter Ng for his taxonomic expertise and input, which have been invaluable in unravelling chlorodielline systematics.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental material
Supplemental material for this article can be accessed here: https://doi.org/10.1080/14772000.2023.2249896.
Additional information
Funding
References
- Ahti, P. A., Coleman, R. R., DiBattista, J. D., Berumen, M. L., Rocha, L. A., & Bowen, B. (2016). Phylogeography of Indo‐Pacific reef fishes: Sister wrasses Coris gaimard and C. cuvieri in the Red Sea, Indian Ocean and Pacific Ocean. Journal of Biogeography, 43, 1103–1115. https://doi.org/10.1111/jbi.12712
- Balss, H. (1938). Die Dekapoda Brachyura von Dr. Sixten Bocks Pazifik-Expedition 1917–1918. Göteborges Kungliga Vetenskaps- och Vitterhets-Samhälles Handlingar. Ser. B, 5, 1–85.
- Barber, P. H., Palumbi, S. R., Erdmann, M. V., & Moosa, M. K. (2000). A marine Wallace’s line? Nature, 406, 692–693. https://doi.org/10.1038/35021135
- Briggs, J. C., & Bowen, B. W. (2012). A realignment of marine biogeographic provinces with particular reference to fish distributions. Journal of Biogeography, 39, 12–30. https://doi.org/10.1111/j.1365-2699.2011.02613.x
- Castro, P. (2011). Catalog of the anomuran and brachyuran crabs (Crustacea: Decapoda: Anomura, Brachyura) of the Hawaiian Islands. Zootaxa, 2947, 1. https://doi.org/10.11646/zootaxa.2947.1.1
- Clark, P. F., & Galil, B. S. (1993). A revision of the xanthid genus Pilodius Dana, 1851 (Crustacea: Brachyura: Xanthoidea). Journal of Natural History, 27, 1119–1206. https://doi.org/10.1080/00222939300770691
- Clark, P. F., & Ng, P. K. L. (1999). The identity of Chlorodius miliaris A. Milne Edwards, 1873, and the establishment of a new genus of Chlorodiinae (Crustacea, Decapoda, Brachyura, Xanthoidea, Xanthidae) from New Caledonia. Zoosystema, 21, 353–365.
- Coles, S. L., DeFelice, R. C., & Eldredge, L. G. (2002a). Nonindigenous marine species in Kane‘ohe Bay, O‘ahu, Hawai‘i. (Final report prepared for the David and Lucile Packard Foundation and the State of Hawaìi, Department of Land and Natural Resources, Division of Aquatic Resources. Bishop Museum Technical Report, 24, i–vii, 1–353 pp.), BishopMuseum.
- Coles, S. L., DeFelice, R. C., & Eldredge, L. G. (2002b). Nonindigenous marine species at Waikīkī and Hawai‘i Kai, O‘ahu, Hawai‘i. (Final Report prepared for the David and Lucile Packard Foundation and the State of Hawaìi Department of Land and Natural Resources, Division of Aquatic Resources. Bishop Museum Technical Report, 25, i–vi, 1–245), BishopMuseum.
- Coles, S. L., Giuseffi, L., & Hutchinson, M. (2008). Assessment of species composition, diversity and biomass in marine habitats and subhabitats around offshore islets in the main Hawaiian Islands. (Final report prepared for the Hawaii Coral Reef Initiative and the National Fish and Wildlife Foundation. Bishop Museum Technical Report, 39, i–vi, 1–72), Bishop Museum.
- Dana, J. D. (1851). On the classification of the Cancroidea. American Journal of Science and Arts, Series, 2, 12, 121–131.
- Dana, J. D. (1852). Conspectus Crustaceorum, & c. Conspectus of the Crustacea of the exploring expedition under Capt. Wilkes, U.S.N., including the Crustacea Cancroidea Corystoidea. Proceedings Academy of Natural Sciences of Philadelphia, 6, 73–86.
- Davie, P. J., Guinot, D., & Ng, P. K. L. (2015). Phylogeny of Brachyura. In P. Castro, P. J. F. Davie, D. Guinot, F. R. Schram & J. C. Von Vaupel Klein (Eds.), Decapoda: Brachyura, Treatise on Zoology – Anatomy, taxonomy, biology. The Crustacea, Complementary to the volumes translated from the French of the Traité de Zoologie [founded by P.-P. Grassé (†)] 9C-II. :639–1221 (pp. 921–979). Brill.
- DeFelice, R. C., Coles, S. L., Muir, D., & Eldredge, L. G. (1998). Investigation of the marine communities of Midway Harbor and adjacent lagoon, Midway Atoll, Northwestern Hawaiian Islands. (Report Prepared for U.S. Fish and Wildlife Service, Pacific Islands Area Office, Honolulu, 30 pp.). Hawaiian Biological Survey, Bishop Museum.
- DeFelice, R. C., Minton, D., & Godwin, S. (2002). Records of shallow-water marine invertebrates from French Frigate shoals, Northwestern Hawaiian Islands, with a note on nonindigenous species (Bishop Museum Technical Report, 23, 1–78), Bishop Museum.
- Drew, J., & Barber, P. H. (2009). Sequential cladogenesis of the reef fish Pomacentrus moluccensis (Pomacentridae) supports the peripheral origin of marine biodiversity in the Indo-Australian archipelago. Molecular Phylogenetics and Evolution, 53, 335–339. https://doi.org/10.1016/j.ympev.2009.04.014
- Eberhard, W. G. (1985). Sexual selection and animal genitalia. Harvard University Press.
- Edmondson, C. H. (1925). Marine zoology of tropical central Pacific. Crustacea. Bulletin of the Bernice P. Bishop Museum, 27, 3–62.
- Edmondson, C. H. (1933). Reef and shore fauna of Hawaii, Special Publications (22 [1st ed.], ii -i- 3-295, figs 1–163). Bernice Pauahi Bishop Museum.
- Edmondson, C. H. (1962). Xanthidae of Hawaii. Occasional Papers of Bernice P. Bishop Museum, 22, 215–309.
- Felgenhauer, B. E. (1987). Techniques for preparing crustaceans for scanning electron microscopy. Journal of Crustacean Biology, 7, 71–76. https://doi.org/10.1163/193724087X00054
- Forest, J., & Guinot, D. (1961). Crustacés Décapodes Brachyoures de Tahiti et des Tuamotu. In: Expédition française sur les récifs coralliens de la Nouvelle-Calédonie. Volume préliminaire. Editions de la Fondation Singer Polignac. 9-11, 1-195, pls. 1-18.
- Guinot, D. (1964). Crustaces Decapodes Brachyoures (Xanthidae) des campagnes de la Calypso en Mer Rouge (1952), dans le Golfe Persique et a I'Ile Aldabra (1954), Memoires du Museum national d‘Histoire naturelle (Paris), nouvelle, serie, A (Zoologie), 32(1), 1–108 + i-iii (pls. 1–12).
- Guinot, D. (1967). La faune carcinologique (Crustacea Brachyura) de l‘Ocean Indien occidental et de la Mer Rouge. Catalogue, remarques biogeographiques et bibliographic. In: Reunion de Specialistes C.S.A. sur les Crustaces, Zanzibar 1964. Memoires de I'lnstitut Fondamental d‘Afrique Noire (Ifan-Dakar), 1966(77), 237–352.
- Guinot, D. (1968). Recherches préliminaires sur les groupements naturels chez les Crustacés Décapodes Brachyoures. IV. Observations sur quelques genres de Xanthidae. Bulletin du Muséum national d’Histoire naturelle, Paris, 2e série, 39, 695–727.
- Heller, C. (1860). Beiträge zur Crustaceen-Fauna des rothen Meeres. Erster Theil. – Sitzungsberichte der mathematisch-naturwissenschaftlichen Classe der kaiserlichen Akademie der Wissenschaften, 43 (pls. I-IV), 297–374.
- Kay, E. A. (1984). Patterns of speciation in the Indo-west Pacific. In P. H. Raven, F. J Radovsky, & S. H. Sohmer (Eds.), Bio-geography of the tropical Pacific (pp. 15–31). Association of Systematic Collections and B. P. Bishop Museum. x + 221 pp.
- Lai, J. C. Y., Mendoza, J. C. E., Guinot, D., Clark, P. F., & Ng, P. K. L. (2011). Xanthidae MacLeay, 1838 (Decapoda: Brachyura: Xanthoidea) systematics: A multi-gene approach with support from adult and zoeal morphology. Zoologischer Anzeiger – A Journal of Comparative Zoology, 250, 407–448. https://doi.org/10.1016/j.jcz.2011.07.002
- Lasley, R. M., Lai, J. C., & Thoma, B. P. (2013). A new genus for Chlorodiella longimana (H. Milne Edwards) supported by morphology and molecular data, with a preliminary phylogeny of the Chlorodiellinae (Crustacea: Decapoda: Xanthidae). Invertebrate Systematics, 27, 379–390. https://doi.org/10.1071/IS12075
- Lasley, R. M., Jr, Klaus, S., & Ng, P. K. L. (2015). Phylogenetic relationships of the ubiquitous coral reef crab subfamily Chlorodiellinae (Decapoda, Brachyura, Xanthidae). Zoologica Scripta, 44, 165–178. https://doi.org/10.1111/zsc.12094
- Lasley, R. M., Jr, Smith, A., Paulay, G., & Ng, P. K. L. (2022). Revision of the coral reef crab genus Tweedieia Ward, 1935 (Decapoda: Brachyura: Xanthidae). Journal of Crustacean Biology, 42 ruab081. https://doi.org/10.1093/jcbiol/ruab081
- Lasley, R. M., Evans, N., Paulay, G., Michonneau, F., Windsor, A., Ng, P., Irwansyah, K. L. (2023). Allopatric mosaics in the Indo-West Pacific crab subfamily Chlorodiellinae reveal correlated patterns of sympatry, genetic divergence, and genitalic disparity.Molecular Phylogenetics and Evolution, 181, 107710. https://doi.org/10.1016/j.ympev.2023.107710
- Laurie, R. D. (1906). Report on the Brachyura collected by Prof. Herdman, at Ceylon, in 1902. In: Herdman, W.A. (Ed), Report to the government of Ceylon on the pearl oyster fisheries of the gulf of Manaar, Part V, Supplementary Report No. 40 (349–432, pls. 1, 2).
- MacLeay, W. S. (1838). On the brachyurous decapod Crustacea brought from the Cape by Dr. Smith, In Smith A., Illustrations of the zoology of South Africa; consisting chiefly of figures and descriptions of the objects of natural history Collected during an expedition into the interior of South Africa, in the years 1834, 1835, and 1836; fitted out by ‘The Cape of Good Hope Association for Exploring Central Africa’: Together with a summary of African zoology, and an Inquiry into the geographical ranges of species in that quarter of the globe. Invertebratae (pp. 53–71). Smith, Elder & Co., pls. 2, 3.
- Malay, M. C. M. D., & Paulay, G. (2010). Peripatric speciation drives diversification and distributional pattern of reef hermit crabs (Decapoda: Diogenidae: Calcinus). Evolution; International Journal of Organic Evolution, 64, 634–662. https://doi.org/10.1111/j.1558-5646.2009.00848.x
- Man, J. G. D. (1902). Die von Herrn Professor Kukenthal im Indischen Archipel gesammelten Dekapoden und Stomatopoden, in W. Kukenthal, Ergebnisse einer Zoologischen Forschungsreise in den Molukken und Borneo, im Auftrage der Senckenbergischen naturforschenden Gesellschaft von Dr. Willy Kukenthal, ordentl. Professor der Zoologie an der Universitat Breslau. Abhandlungen hrsg. von der Senckenbergischen Naturforschenden Gesellschaft, (Frankfurt A.M.), 25 (III), 467–929 (pls. 19-27).
- Mendoza, J. C. E., Lasley, R. M., Jr., & Ng, P. K. L. (2014). New rock crab records from Christmas and Cocos (Keeling) Islands, Eastern Indian Ocean. Raffles Bulletin of Zoology, 2014, 274–300.
- Mendoza, J. C., Chan, K. O., Lai, J. C., Thoma, B. P., Clark, P. F., Guinot, D., Felder, D. L., & Ng, P. K. L. (2022). A comprehensive molecular phylogeny of the brachyuran crab superfamily Xanthoidea provides novel insights into its systematics and evolutionary history. Molecular Phylogenetics and Evolution, 177, 107627. https://doi.org/10.1016/j.ympev.2022.107627
- Meyer, C. P., Geller, J. B., & Paulay, G. (2005). Fine scale endemism on coral reefs: Archipelagic differentiation in turbinid gastropods. Evolution, 59, 113–125. https://doi.org/10.1111/j.0014-3820.2005.tb00899.x
- Miers, E. J. (1884). Crustacea. (Report on the zoological collections made in the Indo-Pacific Ocean during the voyage of H.M.S. Alert 1881–1882. Part I. The collections from Melanesia. Part 11. The collections from Western Indian Ocean) (pp. 178-322, 513-575, pls. 18-32, 46-51). British Museum (Natural History).
- Miller, M. A., Pfeiffer, W., Schwartz, T. (2011). The CIPRES science gateway: A community resource for phylogenetic analyses. In Proceedings of the 2011 TeraGrid Conference: extreme Digital Discovery, pp. 1–8.
- Milne-Edwards, A. (1863). Monographie des Crustacés Fossiles de la Famille des Canceriens. Annales des Sciences Naturelles, 4e série 20, 273–324. pls. 5–12.
- Milne-Edwards, A. (1873). Recherches sur la faune carcinologique de la Nouvelle-Caledonie, Deuxieme Partie, Nouvelles Archives du Museum d‘Histoire naturelle (Paris), 9, 155–332 (pls 4-18).
- Miyake, S. (1939). Notes on Crustacea Brachyura collected by Prof. Teiso Esaki’s Micronesia Expeditions 1937–1938 together with check list of Micronesian Brachyura. Record of Oceanographic Works in Japan. National Research Council (Tokyo), 10, 168–247. (pls. 12-17 [1-6])
- Monteforte, M. (1987). The decapod Reptantia and stomatopod crustaceans of a typical high island coral reef complex in French Polynesia (Tiahura, Moorea Island): zonation, community composition and trophic structure. Atoll Research Bulletin, 309, 1–37. https://doi.org/10.5479/si.00775630.309.1
- Myers, A. A. (1994). Biogeographic patterns in shallow-water marine systems and the controlling process at different scales. In P. S. Giller, A. G. Hildrew, & D. G. Raffaelli (Eds.), Aquatic ecology, scale, pattern and process british ecological society symposium (pp. 547–574). Blackwell Scientific.
- Ng, P. K. L., & Yang, S.-L. (1998). Description of a new genus for the xanthid crab Pilodius etisoides Takeda and Miyake, 1968 (Crustacea: Decapoda: Brachyura: Xanthoidea). Journal of Natural History, 32, 1685–1696. https://doi.org/10.1080/00222939800771201
- Ng, P. K. L., & Holthuis, L. B. (2007). Case 3394. Etisus H. Milne Edwards, 1834 and Chlorodiella Rathbun, 1897 (Crustacea, Decapoda, Brachyura): Proposed conservation of the generic names by suppression of the generic name Clorodius A. G. Desmarest, 1823. Bulletin of Zoological Nomenclature, 64, 19–24.
- Ng, P. K. L., Guinot, D., & Davie, P. J. F. (2008). Systema Brachyurorum: Part I. An annotated checklist of extant brachyuran crabs of the world. The Raffles Bulletin of Zoology, 2008(Supplement 17), 1–286.
- Nobili, G. (1907). Ricerche sui Crostacei della Polinesia. Decapodi, Stomatopodi, Anisopodi e Isopodi. Memorie della (Reale) Accademia delle Scienze di Torino, 57(2), 351–430 (pls. 1–3).
- Ortmann, A. (1893). Die Decapoden-Krebse des Strassburger Museums, mit besonderer Berücksichtigung der von Herrn Dr. D¨oderlein bei Japan und bei den Liu-Kiu-Inseln gesammelten und zur Zeit im Strassburger Museum aufbewahrten Formen. VII. Theil. Abtheilung: Brachyura (Brachyura genuina Boas) II. Unterabtheilung: Cancroidea, 2. Section: Cancrinea, 1. Gruppe: Cyclometopa. Zoologische Jahrbücher, 7, 23–88. pl. 17. https://doi.org/10.5962/bhl.part.24064
- Peyrot-Clausade, M. (1977). Decapodes brachyoures et anomoures (à l’exception des Paguridae) de la cryptofaune de Tiahura Ile de Moorea. Extrait Des Cahiers du Pacifique, n°, 20, 211–222.
- Peyrot-Clausade, M. (1979). Cryptofaune annelidienne et carcinologique des récifs de l’ile de la Réunion et de l’ile Maurice. The Mauritius Institute Bulletin, 8, 1–41.
- Peyrot-Clausade, M. (1989). Crab cryptofauna (Brachyura and Anomura) of Tikehau, Tuamotu Archipelago, French Polynesia. Coral Reefs, 8, 109–117. https://doi.org/10.1007/BF00338266
- Rathbun, M. J. (1893). Scientific results of explorations by the U.S. Fish Commission steamer Albatross. No. XXIV.—Descriptions of new genera and species of crabs from the west coast of North America and the Sandwich Islands. Proceedings of the United States National Museum, 16, 223–260. https://doi.org/10.5479/si.00963801.933.223
- Rathbun, M. J. (1897). A revision of the nomenclature of the Brachyura. Proceedings of the Biological Society of Washington, 11, 153–167.
- Rathbun, M. J. (1907). Reports on the scientific results of the expedition to the tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission steamer “Albatross,” from August, 1899, to March, 1900, Commander Jefferson F. Moser, U.S.N., commanding. IX. Reports on the scientific results of the expedition to the eastern tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission steamer “Albatross,” from October, 1904, to March, 1905, Lieut.-Commander L.M. Garrett, U.S.N., commanding. X: The Brachyura. Memoirs of the Museum of Comparative Zoology at Harvard College, 35, 25–74.
- Rathbun, M. J. (1911). No XI.–Marine Brachyura. In: The Percy Sladen Trust Expedition to the Indian Ocean in 1905 under the leadership of Mr. J. Stanley Gardiner, Volume III. Transactions of the Linnaean Society of London, (2)14(2), 191–261, pls. 15–20.
- Serène, R. (1968). The Brachyura of the Indo-West Pacific region. Prodromus for a Check List of the (non-planctonic) Marine Fauna of South East Asia, Special publication No. 1, Fauna IIICc3, 33-112. UNESCO Singapore.
- Serène, R. (1971). Observations préliminaires sur des Brachyoures nouveaux ou mal connus du Sud-Est Asiatique (Crustacea Decapoda). Bulletin du Muséum national d’Histoire naturelle, (2)42(5), 903–918.
- Serène, R. (1984). Crustacés Décapodes Brachyoures de l‘océan Indien occidental et de la mer Rouge, Xanthoidea: Xanthidae et Trapeziidae. Avec un addendum par Crosnier A.: Carpiliidae et Menippidae. Faune tropicale, 24, 1–243.
- Serène, R., & Nguyen, V. L. (1958). Chlorodopsis (Brachyure) du Viêtnam. Annales de la Faculté des Sciences. Saigon, 36, 87–147. pls. 1–4.
- Serène, R., & Nguyen, V. L. (1959). Note additionnelle sur les espèces de Chlorodopsis (Brachyures). Annales de la Faculté des Sciences, Saigon, 42, 301–340.
- Stamatakis, A. (2014). RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
- Targioni Tozzetti, A. (1877). Zoología del viaggio intorno al globo della R. Pirocorvetta Magenta durante gli anni 1865–68. Crostacei Brachiuri e Anomuri. Pubblicazioni del Reale Istituto di Studi Superiori Pratici e di Perfezionamento in Firenze, Sezione di Scienze Fisiche e Naturali. Vol. 1(24) Reale Istituti di Studi Superiori, Sezione di Scienza Fisiche e Naturali. 257pp, pls. 1–13.
- Titus, B. M., Daly, M., Hamilton, N., Berumen, M. L., & Baeza, J. A. (2018). Global species delimitation and phylogeography of the circumtropical ‘sexy shrimp’ Thor amboinensis reveals a cryptic species complex and secondary contact in the Indo‐West Pacific. Journal of Biogeography, 45, 1275–1287. https://doi.org/10.1111/jbi.13231
- Ward, M. (1935). Notes on a collection of crabs from Christmas Island, Indian Ocean. Bulletin of the Raffles Museum, 9, 5–28. pls. I–III.
- Ward, M. (1942). Notes on the Crustacea of the Desjardins Museum, Mauritius Institute, with descriptions of new genera and species. The Mauritius Institute Bulletin, 2, 49–108. pls. 5–6.
- Waterman, T. H. (1953). Xiphosura from Xuong-Ha. American Scientist, 41, 292–302.
- Wickham, H., Chang, W., & Wickham, M. H. (2016). Package ‘ggplot2’. Create elegant data visualizations using the grammar of graphics. Version, 2, 1–189.