Abstract
The genus Andropogon sensu lato is known to be polyphyletic. Accordingly, we here adjust part of the classification of the genus to reflect its evolutionary history and morphological diversity. A plastome phylogeny including 20 new plastome sequences confirms a well-supported clade of species broadly corresponding to Andropogon section Leptopogon. Morphological diversity was assessed across Andropogon sensu lato using specimens held at the K, MO, and A/GH herbaria, GrassBase, and photographs of spikelet pairs, with an emphasis on identifying members of this clade and their distinguishing features. The genus Anatherum is here reestablished, expanded to incorporate 45 of the 131 of Andropogon sensu lato species worldwide, and described and illustrated. Five species names in Anatherum are reinstated and new combinations are made for 40 species and one subspecies. Anatherum is most common and diverse in the Americas but also commonly found across Africa. Few species occur in Europe or Asia. Anatherum inflorescences generally have 2 branches, linear and slender internodes and pedicels with long trichomes, small elliptic to lanceolate spikelets, and flat to concave 2-keeled lower glumes with no intercarinal veins visible. Generic circumscription in this group is complicated by its polyploid history and limited understanding of the relationship between genomic composition and key morphological characters. Five species of doubtful generic affiliation are listed for future analysis.
Introduction
Species in the large grass genus Andropogon L. are ubiquitous in C4 open canopy ecosystems around the world, are significant drivers of these ecosystem functions and are commonly encountered by pastoralist farmers and range managers, ecologists, and conservation biologists (Clayton & Renvoize, Citation1986; Gibson, Citation2009). Understanding and management of grassy ecosystems depends on accurate classification of the plants on which both ecosystem function and local people’s livelihoods depend. In its current circumscription, Andropogon (tribe Andropogoneae Dumort., subfamily Panicoideae A. Braun, family Poaceae Barnhart) includes 131 species distributed on all continents and is often common where it occurs (Nagahama & Norrmann, Citation2012; Welker et al., Citation2020). Andropogon gerardi Vitman is a major component of the tallgrass prairie of North America, A. amethystinus Steud., A. appendiculatus Nees, and A. gayanus Kunth are key components of African savannas and pastures (Acocks & Zacharias, Citation1990; Boonman, Citation1993), while A. gayanus has invaded and now dominates parts of Australia (Shaik et al., Citation2022).
Most species of Andropogon are classified in the monophyletic subtribe Andropogoninae J. Presl (Welker et al., Citation2020), although at least one species assigned to Andropogon falls well outside the subtribe in molecular phylogenies (“A.” burmanicus Bor sister to Eulalia contorta (Brongn.) Kuntze) and will ultimately need to be moved to another genus. Also in Andropogoninae are the genera Schizachyrium Nees, Hyparrhenia Andersson ex E. Fourn., and Diheteropogon (Hack.) Stapf, a placement that has been supported by all molecular phylogenetic studies to date (Arthan et al., Citation2017; Estep et al., Citation2014; Mathews et al., Citation2002; McAllister et al., Citation2018; Skendzic et al., Citation2007; Welker et al., Citation2020). However, the same studies have shown that within Andropogoninae, Andropogon is clearly polyphyletic, with species falling into multiple unrelated clades. The type species, Andropogon distachyos L., is part of a small clade of predominantly Asian species, so the generic name Andropogon is not applicable to the major New World or African radiations, which all have originated separately from the Andropogon sensu stricto clade (Arthan et al., Citation2017; Welker et al., Citation2020).
Relationships and classification of species within Andropogon have always been challenging. Hackel (Citation1889) described Andropogon as “a vast genus, polymorphic, very varied in habit” and divided it into 13 subgenera, most of which are now recognized as separate genera. Hackel’s Andropogon subg. Arthrolophis (Trin.) Hack. corresponds closely to the group now recognized as Andropogon. Stapf (Citation1919) presented a concept of Andropogon similar to that which is followed today, although he noted that “The genus Andropogon, even in the restricted sense in which it is understood here, is probably more heterogeneous than any other genus of Andropogoneae”. He recognized four sections in the genus–Eu-Andropogon, Leptopogon Stapf, Piestium Stapf, Notosolen Stapf–but noted that the latter three are “only loosely connected with Eu-Andropogon and may even be entitled to rank as distinct genera”. Despite this sweeping classification, Stapf (Citation1919) included only 43 species, all from Africa, and did not designate types, thus leaving species from other parts of the world unassigned to section. Nonetheless, his sectional classification has been widely adopted, for example by Campbell (Citation2003) for North American species and Zanin and Longhi-Wagner (Citation2006) for Brazilian species. In the most comprehensive treatment available, Nagahama & Norrmann (Citation2012) extrapolated Stapf’s groups to encompass 39 species from Africa and 60 from the Americas as well as varieties within some of the species. They focused particularly on chromosome numbers and, in a few instances, genomic origins of polyploids, although several dozen species were still unaccounted for.
Poaceae tribe Andropogoneae and subtribe Andropogoninae are distinguished and classified primarily on the basis of their spikelet pairs and synflorescence structures, the rapid evolution of which is responsible for disagreement between the traditional morphology-based classification and monophyletic clades inferred from DNA sequence data (Clayton, Citation1969; Clayton & Renvoize, Citation1986; Kellogg, Citation2015; McAllister et al., Citation2018). Ecological success of these grasses is likely linked to their morphological complexity and variability, enabling reproductive flexibility as the synflorescence structure can initially be challenging to interpret with up to two functionally male, female, bisexual, or sterile flowers/florets in each spikelet, with glumes, lemmas, paleas, and awns which can be reduced or absent and therefore difficult to recognize. In Andropogon fresh synflorescences (called inflorescences in the taxonomic literature) are digitate cylindrical structures which dry out and open as the caryopses mature, often revealing awns and multiple trichomes giving the plant a white fluffy appearance (e.g., Mashau et al., Citation2022). Each sessile spikelet is situated on a branch point of the inflorescence structure, subtended by an internode which gives rise to one internode and one pedicel at its base (e.g., Fish et al., Citation2015, pp. 68–69).
The largest single section of Andropogon sensu lato (s.l.) includes species that are commonly classified in sect. Leptopogon, which looks clearly distinct from Andropogon sensu stricto (s.s.). Stapf (Citation1919) distinguished sections Eu-Andropogon and Leptopogon in tropical Africa from the other two sections by the pedicels and rachis internodes, which he described as linear, although “sometimes slightly widened upwards in sect. Eu-Andropogon”, as well as the minute or absent palea of the seed-bearing floret. In contrast, he described the pedicels and rachis internodes as “distinctly swollen upwards, clavate or cuneate” in sections Piestium and Notosolen, and the palea well developed. Most Andropogon species of the Americas are in sect. Leptopogon with only a few from Africa (Nagahama & Norrmann, Citation2012). The majority are diploid or hexaploid. The same concept of Andropogon sect. Leptopogon was adopted for the genus worldwide by Clayton & Renvoize (Citation1986), describing it as the largest section of Andropogon of 55 species with “lower glumes membranous, without intercarinal nerves, its keels lateral to dorsal and wingless; racemes in a compound panicle, delicate and often plumose; sometimes the sessile spikelet awnless or the pedicelled spikelet suppressed”. In spite of the steady accumulation of phylogenetic data and a growing understanding of the evolution of Andropogon sensu lato (McAllister et al. Citation2018; Welker et al. Citation2020) no global overview has been attempted since, and the American taxa are currently better understood than the African taxa (Nagahama & Norrmann, Citation2012; Norrmann et al., Citation2004).
The polyphyly and morphological diversity in Andropogon s.l. make the current classification misleading, with traits ascribed to any given species often inapplicable to other species that are presumed to be related. Here we propose recognizing Andropogon sect. Leptopogon as a genus distinct from Andropogon s.s. based on morphological and phylogenetic data. Even with the exclusion of Andropogon sect. Leptopogon, the remainder of Andropogon remains polyphyletic; to denote this component of “Andropogon” as a traditional taxonomic concept not representing a monophyletic group, we place the generic name in quotes. This paper reestablishes the genus Anatherum P. Beauv. as the oldest available generic name for the section, and transfers the accepted species and infraspecific taxa summarized by POWO (POWO, Citation2021) from “Andropogon” to Anatherum.
Materials and methods
Phylogenetic reconstruction
We added 20 plastome sequences of species putatively assigned to “Andropogon” sect. Leptopogon to those published by McAllister et al. (Citation2018) (). DNA was extracted using a DNeasy® Plant Kit (Qiagen Inc., Germantown, MD), Illumina TruSeq PCR-Free or TruSeq DNA nano libraries were constructed, and samples were sequenced to a depth of approximately 1X–5X on an Illumina sequencer with paired end reads of 150 bp. Quality control of raw reads was performed by analysing overlap of paired end reads to assess DNA degradation, checking non-Poaceae contamination with Kraken (Wood & Salzberg, Citation2014). Placement of sequences in Andropogoneae was also validated by comparison to a custom database of select gene sequences from previously published plastomes. Plastome sequences were extracted and assembled with Fast-plast (McKain et al., unpublished) and assemblies checked manually for errors. Annotation used GE-seq (Tillich et al., Citation2017). New sequences were added to all putative “Andropogon” sect. Leptopogon sequences available from GenBank, aligned with MAFFT (Katoh & Standley, Citation2014), and a phylogeny constructed with RAxML (Stamatakis, Citation2014) with support assessed by 500 fast bootstrap replicates.
Table 1. Specimens included in phylogenetic analysis and associated GenBank accession numbers for the plastome sequences.
Morphology and taxonomy
To determine whether the “Andropogon” sect. Leptopogon clade is morphologically distinctive, we developed a list of all species of Andropogon s.l. in the RBG Kew (K) herbarium. Co-authors MSV and EAK then independently examined specimens of all species of Andropogon s.l. available at K, MO, and A/GH, which together encompassed the taxonomic diversity of the genus. We then met together to focus on the handful of species whose placement seemed unclear. In addition, we checked and compared spikelet pair length measurements reported by McAllister et al. (Citation2018). Finally, data were retrieved from Clayton et al. (Citation2016) for all species of “Andropogon” sect. Leptopogon to compare with other species of Andropogon s.l. and to compile the generic description. Photographs of spikelets for most species treated here can be retrieved from www.tropicos.org by searching on the species name.
Nomenclature
The generic name Anatherum, based on A. bicorne (L.) P. Beauv., is the oldest available name in “Andropogon” sect. Leptopogon. Accordingly, we provide formal nomenclatural transfers of members of the former “Andropogon” sect. Leptopogon to Anatherum. We inspected the types available in K, MO, and A/GH and verified that all were morphologically consistent with the generic realignment proposed. We have not attempted to revise species concepts or provide typification of names.
Results
Clade structure and synapomorphies
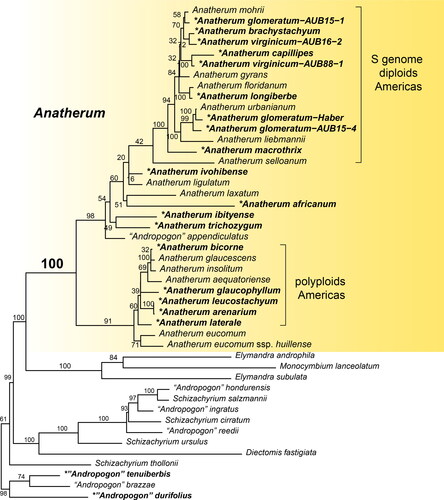
Monophyly of “Andropogon” sect. Leptopogon has been confirmed by adding 20 new plastome sequences to the 14 “A.” sect. Leptopogon sequences presented by McAllister et al. (Citation2018) (). As shown in all previous studies to date, the clade of “Andropogon” sect. Leptopogon is strongly supported as monophyletic and sister to the clade of Elymandra Stapf plus Monocymbium Stapf. These clades together are sister to a clade that includes a mix of species currently classified in “Schizachyrium” and “Andropogon” but not including the type of either genus (S. brevifolium (Sw.) Nees ex Buse and A. distachyos L., respectively). Sister to these is a clade including “A.” tenuiberbis Hack., “A.” brazzae Franch., and “A.” durifolius Renvoize.
Figure 1. Plastome phylogeny of Anatherum and its immediate sister clades. Species with new plastomes are in bold and indicated by asterisk (*); all others were presented by McAllister et al. (Citation2018) except for A. eucomum which was published by Welker et al. (Citation2020). See also . Numbers above branches are fast bootstrap values calculated by RAxML. Sequences from A. mohrii and A. laxatum were published by McAllister et al. (Citation2018) under those names. POWO (Citation2021) considers them to be synonyms of A. liebmannii and A. eucomum ssp. huillense, respectively, but they are phylogenetically distant from their putative conspecifics and are recognized as distinct here pending further investigation.
Most species of “Andropogon” sect. Leptopogon share a common set of characters although it is unclear if any are synapomorphic. The spikelet pairs commonly bear long trichomes on the callus, and in two lines along the internodes and pedicels, giving the inflorescences a characteristic fluffy appearance that can often be spotted from a distance (). Most species have inflorescences with two branches (paired racemes), although a few have inflorescences with three or four branches and occasionally the branches are themselves branched. Internodes and pedicels are generally straight and often slightly flattened, a trait noted by Stapf (Citation1919) and corroborated here. The lower glume of the sessile spikelet has two prominent veins between which the glume is hyaline and often slightly concave. The glume itself is glabrous.
Figure 2. Spikelet morphology of selected Anatherum species and outgroups. Each species is represented by a single photo that includes two or three spikelet pairs from one herbarium specimen, as generated by McAllister et al. (Citation2018), except for Elymandra subulata, which shows a single spikelet pair. Cladogram to the left of the images shows relationships based on the plastome tree in . All images to the same scale. Scale bar, 1 cm.
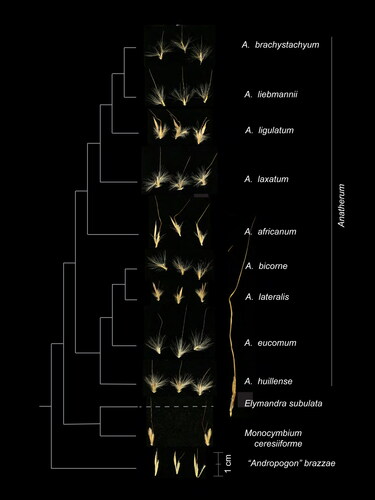
Figure 3. Inflorescences. Thicker lines indicate spikelets, internodes, pedicels, and awns; thinner lines indicate trichomes. (a) Anatherum eucomum, (b) Anatherum ivohibense, (c) Anatherum cordatum, (d) Anatherum africanum, (e) Anatherum glomeratum, (f) Anatherum lindmannii. Scale bar, 4 mm. Drawn by Christabel King.
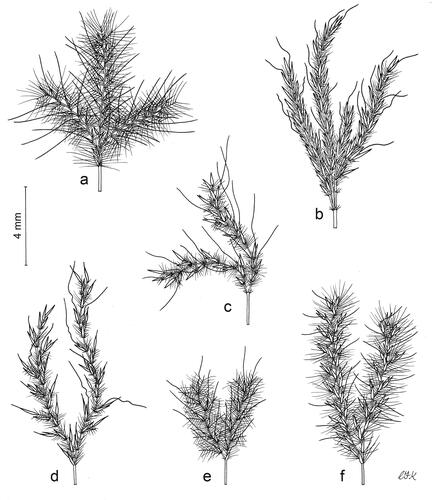
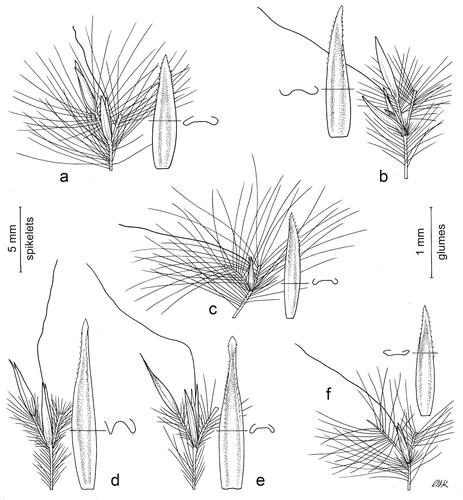
Figure 4. Spikelet pairs. Sessile spikelet on the lower internode, with the pedicel and the pedicelled spikelet where present on the left, and the upper internode on the right. Lower glume of the sessile spikelet is enlarged and the shape of its cross section is shown. Thicker lines indicate spikelets, internodes, pedicels, and awns; thinner lines indicate trichomes. (a) Anatherum lindmannii, (b) Anatherum cordatum, (c) Anatherum eucomum, (d) Anatherum ivohibense, (e) Anatherum africanum, (f) Anatherum glomeratum. Left scale bar, spikelets, 5 mm; right scale bar, glumes, 1 mm. Drawn by Christabel King.
Comparison within “Andropogon” sensu lato
Morphological data collected by McAllister et al. (Citation2018) and the morphological characters scored and compiled in GrassBase (Clayton et al., Citation2016) were used to explore the following characters for their utility in diagnosing “Andropogon” sect. Leptopogon:
Number of branches in the inflorescence.
Shape of the internodes and pedicels.
Length of the sessile spikelet.
Number of veins on the lower glume of the sessile spikelet.
Concavity of the lower glume of the sessile spikelet.
Presence or absence of trichomes on the lower glume of the sessile spikelet.
Distribution of trichomes on the internodes and pedicels.
With a few exceptions, spikelets in most members of “Andropogon” sect. Leptopogon are smaller than those of other species classified in other clades of “Andropogon”. We compared sizes of sessile spikelets using morphometric data from McAllister et al. (Citation2018). Mean sessile spikelet length in “Andropogon” sect. Leptopogon was 4.39 ± 0.99 mm (range 2.6–7.02), whereas for all other species of “Andropogon” together it was 6.15 ± 1.63 mm (range 2.74–13.11). (These measurements were taken from three representative spikelets on each of three herbarium sheets per species, so the extreme values may have been missed.) In most cases, the combination of small spikelets and presence of long trichomes provide a provisional identification for “A.” sect. Leptopogon.
“Andropogon” sect. Leptopogon is distinguished from “Andropogon” s.l. and Andropogon s.s. by its sessile spikelets with lower glumes elliptic to lanceolate with two prominent veins, the glumes flat to concave, most commonly <5 mm long, although up to 6.5 mm in a few species. Other species of Andropogon have sessile spikelets with glumes with more than two veins, or veins indistinct, the glumes nearly flat to deeply grooved and folded into an M-shape, most commonly >5 mm long but sometimes as short as 3 mm. Inflorescence internodes and pedicels are slender and linear in “A.” sect. Leptopogon, and only slightly flared at the apex if at all. In species of “Andropogon” s.l. and Andropogon s.s., inflorescence internodes are broadly clavate to goblet-shaped, flaring distally to a deep cup-shaped joint. “A.” sect. Leptopogon inflorescences generally have two branches, rarely 3–5 or occasionally nine, whereas for “Andropogon” s.l. and Andropogon s.s. species inflorescences generally have three or more branches.
Comparison with related genera
The closely related genus Elymandra has pairs of homogamous spikelets at the base of the racemes, whereas homogamous pairs are lacking in “A.” sect. Leptopogon; in addition, Elymandra spikelets are generally larger with longer and more robust awns (). “Andropogon” sect. Leptopogon is distinguished from Monocymbium by its glabrous glumes, whereas in Monocymbium the glumes are densely pubescent. Racemes of Monocymbium are entirely encased in reddish-brown spatheoles that are generally borne horizontally on the plant. In contrast, spatheoles in “A.” sect. Leptopogon, if present, are upright (Clayton & Renvoize Citation1986; Clayton et al. Citation2016).
Discussion
Many members of Andropogoneae were initially assigned to the Linnaean genus Andropogon, but over time the most distinctive groups have been segregated as separate genera, leaving “Andropogon” sensu lato as the polyphyletic residue. As phylogenetic sampling has improved over the last couple of decades, species nominally in “Andropogon” have been shown clearly to belong to disparate clades. This paper represents a step toward adjusting the generic classification to align with emerging knowledge of phylogenetic relationships.
Our criteria for recognizing a clade at the generic rank include: (1) the group must be monophyletic, with as much evidence as possible from DNA sequence data and (2) the group should be morphologically homogeneous, with one or more traits shared by most members. It is ideal if the shared traits can be shown to be derived (synapomorphic) and shared by all members of the clade. However, one reason that the species of “Andropogon” have not already been placed in separate genera is their lack of distinctive macro-morphological characters. In addition, no formal analysis has been undertaken for the evolution of many morphological traits across the tribe, making the distinction between synapomorphy and symplesiomorphy a plausible hypothesis rather than a firm conclusion. Finally, we adopt a cautious approach with respect to ambiguous data. Species that are placed in the clade by plastome sequences but are morphologically different, and species that are morphologically similar but placed elsewhere by plastome data are left as “Andropogon” incertae sedis, as noted below.
The clade corresponding to “Andropogon” sect. Leptopogon is monophyletic and not directly related to Andropogon s.s. Several authors in the 19th and early 20th centuries, including Stapf (Citation1919), observed that species belonging to this section were morphologically distinct from other Andropogon and assigned them to new genera, although Stapf failed to designate a type. The earliest available generic name was assigned by Palisot de Beauvois (Citation1812) who established the genus Anatherum to accommodate Andropogon bicornis L., which then became Anatherum bicorne (L.) P. Beauv. Other relevant generic names are Euklastaxon Steud., [1855]1854, based on E. tenuifolius Steud. = Andropogon selloanus (Hack.) Hack., Eriopodium Hochst., 1846, in synonymy under Andropogon eucomus Nees, and Dimeiostemon Raf., 1825, based on D. vaginatus Raf. ex B.D. Jacks., an invalid synonym of A. virginicus L.
The name Leptopogon is not available as a generic name. The sectional name was raised to the rank of genus by Roberty (Citation1960), as Leptopogon Roberty. Like Stapf, Roberty also failed to designate a type. Leptopogon Roberty is an illegitimate later homonym of Leptopogon Borzí (Borzí, Citation1907), a genus of cyanobacteria, which are covered by the same nomenclatural code as plants (Turland et al., Citation2018). Hereafter, we refer to “Andropogon” sect. Leptopogon as Anatherum.
Anatherum includes two major subclades, each of which comprise a New World clade derived from a paraphyletic group of African taxa. The diploid species with the S genome (where “S” stands for “selloanus”) characterized by Norrmann et al. (Citation2004) and Nagahama & Norrmann (Citation2012) all form a monophyletic group (). Fluorescent in situ hybridization (FISH) verified that the genomes of A. gyrans Ashe, A. selloanus (Hack.) Hack., and A. macrothrix Trin. were indeed similar, and Norrmann et al. (Citation2004) hypothesized that the other “A.” sect. Leptopogon diploids shared the same basic genome, a hypothesis supported by our phylogenetic data. The hexaploids A. lateralis Nees and A. bicornis bear an S genome but the other genomes are from different unknown sources (Nagahama & Norrmann, Citation2012).
The morphological characters compiled in GrassBase (Clayton et al. Citation2016) identify multiple morphological traits that are shared by all species of Anatherum and distinguish them from other members of Andropogoninae. These data corroborate and extend the de novo herbarium observations made by MSV and EAK. Nagahama and Norrmann (Citation2012) suggested that the two-veined, slightly concave glume might be diagnostic, but the trait can be hard to distinguish from the concave to deeply grooved glumes of other “Andropogon” outside Anatherum, as in “A.” burmanicus Bor and “A.” perligulatus Stapf.
Several species with ambiguous morphology are not transferred here, because molecular data strongly support their placement outside Anatherum (). The species “A.” tenuiberbis, “A.” brazzae, and “A.” durifolius are large plants native to Africa, with highly branched inflorescences of small spikelets. Despite their apparent lack of relationship to Anatherum, they share some morphological traits with species of Anatherum. Andropogon mannii Hook. f. is phylogenetically placed in Andropogon s.s. (and hence not designated with quotes) but some specimens appear morphologically similar to some Anatherum. Andropogon mannii occurs in much of east Africa, from Sudan to the northern provinces of South Africa, but also has disjunct populations in equatorial west Africa. Morphologically, it varies extensively throughout its range and requires closer examination before attempting any re-classification. Our specimen of “Andropogon” appendiculatus is the same one sampled by McAllister et al. (Citation2018) and is firmly placed within the Anatherum clade in our analyses and theirs. However, it has large spikelets and more racemes, which make it morphologically dissimilar to other members of the clade. The species includes complex morphological variation as well as a range of ploidy levels, which suggests that additional samples are warranted. Accordingly, we have deferred transferring it until further work can be undertaken.
“Andropogon” gerardi raises a particular problem in classification in that it is an allohexaploid of hybrid origin (Estep et al., Citation2014). Cytogenetic data show clearly that one of the ancestral genomes came from a species similar and possibly closely related to Anatherum gyrans (Nagahama & Norrmann, Citation2012). Using a different representative of Anatherum (A. virginicum), sequence data later confirmed this result (Estep et al., Citation2014). The other parent is unknown but likely to be a species similar to Schizachyrium. Any generic placement of an intergeneric hybrid will necessarily be arbitrary and placing the species in a new genus may be appropriate. For now, we have chosen to leave “A.” gerardi in “Andropogon” pending further investigation of its ancestry.
Taxonomic treatment
Anatherum P. Beauv., emend.
Type species
Anatherum bicorne (L.) P. Beauv.
Plants perennial, caespitose, occasionally rhizomatous, from 20 cm to 3 m tall. Leaves flat to filiform, 0.5–8(–22) mm wide. Ligules membranous to scarious, usually truncate, with or without apical trichomes. Synflorescence simple or complex, bracteate or not, with each axis ending in an inflorescence of 2 (3–9) racemes, sometimes branched, plumose when dry. Each raceme (1–)2–7(–11) cm long, internodes and pedicels slender, linear, flexuous, 2–6(–10) mm long, usually with long trichomes borne in two vertical lines, the trichomes (1–)2–10(–16) mm long, usually increasing in length distally. Sessile spikelet lanceolate (rarely linear or oblong), (2–)3–6.5(–12) mm long; the callus obtuse, pilose to bearded (rarely glabrous), inserted into a cup-shaped joint; lower glume lanceolate, membranous to coriaceous, glabrous (rarely pubescent), with two prominent veins (2-keeled) and no intercarinal veins, concave (rarely strongly folded or grooved); upper glume membranous to coriaceous, linear, muticous (rarely awned); lower lemma oblong to lanceolate, usually 2-veined; upper lemma lanceolate (rarely oblong), hyaline, generally bifid for a quarter to half of its length, generally with an awn from the sinus (sometimes the lemma entire with an apical awn); the awn straight or geniculate, (2–)5–20(–25) mm long, usually with a twisted column; palea reduced; anthers 1 or 3. Pedicellate spikelet present or absent, if absent then the pedicel ending blindly; if present then represented by one or both glumes or fully developed and staminate, linear to lanceolate, 0–6(–8) mm long; glumes thinly coriaceous to chartaceous, glabrous, apically acute to acuminate, usually mucronate or sometimes awned; awn, if present, slender, straight; stamens, if present, 3; gynoecium absent.
Distribution
North, Central, and South America to Caribbean and Africa, with limited occurrence across Asia.
Species composition
The following 45 species and one subspecies are placed in Anatherum, with species concepts based on the taxonomic compilation by POWO (2013), except for A. laxatum and A. mohrii, q.v. Four species names in Anatherum are reinstated. Thirty-nine species and one subspecies are transferred here to Anatherum. All the previously accepted names are in the genus Andropogon and these are indicated as homotypic synonyms where they differ from the basionym. For full synonymy, distributions, and bibliography see POWO (Citation2021). Names marked with an asterisk are included in the molecular phylogeny; see also . Unmarked names are placed here based on morphology.
* Anatherum aequatoriensis (Hitchc.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon aequatoriensis Hitchc. (Hitchcock, Citation1927, p. 499).
* Anatherum africanum (Franch.) Roberty, (Roberty, Citation1960, p. 207).
Basionym: Andropogon africanus Franch., (Franchet, Citation1895, p. 325).
Anatherum arctatum (Chapm.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon arctatus Chapm. (Chapman, Citation1878, p. 20).
* Anatherum arenarium (Hack.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon arenarius Hack. (Hackel, Citation1885, p. 134).
Anatherum barretoi (Norrmann & Quarín) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon barretoi Norrmann & Quarín (Norrmann & Quarín, Citation2001, p. 171).
* Anatherum bicorne (L.) P. Beauv., (Palisot de Beauvois, Citation1812, p. 128, 150, atlas t. 22, f. 11)
Basionym: Andropogon bicornis L., (Linnaeus, Citation1753 2, p. 1046), nom et type cons., against Andropogon glomeratus (Walter) Britton and Schizachyrium scoparium (Michx.) Nash. (Davidse & Turland, Citation1999). The International Plant Names Index (IPNI, Citation2023) does not list the Linnaean name as the basionym for Anatherum bicorne, referring simply to Anatherum bicorne P. Beauv. However, Palisot de Beauvois’ protologue for Anatherum lists Andropogon bicornis L. as being a species of Anatherum, indicating that the IPNI listing is simply incomplete.
Anatherum bourgaei (Hack.) Roberty, (Roberty, Citation1960, p. 211).
Basionym: Andropogon bourgaei Hack., (Hackel, Citation1885, p. 134).
* Anatherum brachystachyum (Chapm.) Roberty, (Roberty, Citation1960, p. 211).
Basionym: Andropogon brachystachyus Chapm., (Chapman, Citation1883, p. 668).
Anatherum brasiliense (A. Zanin & Longhi-Wagner) Voronts. & E.A.Kellogg, comb. nov., non Anatherum brasiliense Spreng. ex Steud., nom. inval., pro syn.
Basionym: Andropogon brasiliensis A. Zanin & Longhi-Wagner (Zanin & Longhi-Wagner, Citation2003, p. 368).
Anatherum brasiliense Spreng. ex Steud. was published as a synonym when Steudel listed Sprengel’s previously unpublished name, but Steudel did not accept it. The name is therefore invalid.
Anatherum cabanisii (Hack.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon cabanisii Hack. (Hackel, Citation1885, p. 133).
* Anatherum capillipes (Nash) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon capillipes Nash (Nash, Citation1900, p. 431).
Anatherum cordatum (Swallen) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon cordatus Swallen (Swallen, Citation1949, p. 274).
Anatherum cumulicolum (E.L. Bridges & Orzell) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon cumulicola E.L. Bridges & Orzell (Bridges & Orzell, Citation2018, p. 4).
Anatherum curvifolium (Clayton) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon curvifolius Clayton (Clayton, Citation1964, p. 465).
* Anatherum eucomum (Nees) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon eucomus Nees (Nees von Esenbeck, Citation1841, p. 104).
* Anatherum eucomum (Nees) Voronts. & E.A.Kellogg subsp. huillense (Rendle) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon huillensis Rendle (Rendle, Citation1899, p. 146).
Homotypic synonym: Andropogon eucomus subsp. huillensis (Rendle) Sales Citation2002, p. 10).
* Anatherum floridanum (Scribn.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon floridanus Scribn. (Lamson-Scribner, Citation1896, p. 145).
* Anatherum glaucescens (Kunth) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon glaucescens Kunth (von Humboldt et al., Citation1816, p. 186).
* Anatherum glaucophyllum (Roseng., B.R.Arrill. & Izag.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon glaucophyllus Roseng., B.R.Arrill. & Izag. (Rosengurtt, Arrillaga de Maffei, & Izaguirre de Artucio, Citation1970, p. 165).
Anatherum glaziovii (Hack.) Voronts. & E.A.Kellogg comb. nov.
Basionym: Andropogon glaziovii Hack. (von Martius, Citation1883, p. 286).
* Anatherum glomeratum (Walter) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Cinna glomerata Walter (Walter, Citation1788, p. 59).
Homotypic synonym: Andropogon glomeratus (Walter) Britton, Sterns & Poggenb. (Britton et al., Citation1888, p. 67).
* Anatherum gyrans (Ashe) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon gyrans Ashe (Ashe, Citation1898, p. 113).
* Anatherum ibityense (A.Camus) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon ibityensis A.Camus (Camus, Citation1952, p. 213).
Anatherum imerinense (Bosser) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon imerinensis Bosser (Bosser, Citation1968, p. 521).
* Anatherum insolitum (Sohns) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon insolitus Sohns (Maguire & Wurdack, Citation1957, p. 271).
* Anatherum ivohibense (A.Camus) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon ivohibensis A.Camus (Camus, 1924 (publ. 1925), p. 922).
* Anatherum laterale (Nees) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon lateralis Nees (Nees von Esenbeck, Citation1829, p. 329).
* Anatherum laxatum (Stapf) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon laxatus Stapf (Stapf, Citation1919, p. 237).
POWO (Citation2021) considers this a synonym of A. eucomum ssp. huillense but our representatives of the two taxa are unrelated in the plastome tree ().
Anatherum lehmannii (Pilg.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon lehmannii Pilg. (Pilger, Citation1899, p. 24).
* Anatherum leucostachyum (Kunth) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon leucostachyus Kunth (von Humboldt et al., Citation1816, p. 187).
* Anatherum liebmannii (Hack.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon liebmannii Hack. (Hackel, Citation1885, p. 132).
* Anatherum ligulatum (Stapf) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon laxatus var. ligulatus Stapf (Stapf, Citation1919, p. 238).
Homotypic synonym: Andropogon ligulatus (Stapf) Clayton, (Clayton, Citation1977, p. 2).
Anatherum lindmannii (Hack.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon lindmannii Hack. (Lindman, Citation1900, p. 6).
* Anatherum longiberbe (Hack.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon longiberbis Hack. (Hackel, Citation1885, p. 131).
* Anatherum macrothrix (Trin.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon macrothrix Trin. (Trinius, Citation1833, p. 270).
Anatherum miamiensis (E.L.Bridges & Orzell) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon miamiensis E.L.Bridges & Orzell (Bridges & Orzell, Citation2018, p. 13).
* Anatherum mohrii (Hack.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon liebmannii subvar. mohrii Hack. (Hackel, Citation1889, p. 413).
POWO (Citation2021) considers this a synonym of A. liebmannii but our representatives of the two species are unrelated in the plastome tree ().
Anatherum multiflorum (Renvoize) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon multiflorus Renvoize (Renvoize, Citation1998, p. 596).
Anatherum perangustatum (Nash) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon perangustatus Nash (Small, Citation1903, p. 62).
Anatherum pringlei (Scribn. & Merr.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon pringlei Scribn. & Merr. (Lamson-Scribner & Merrill, 1900 (publ. 1901), p. 7).
* Anatherum selloanum (Hack.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon leucostachyus subsp. selloanus Hack. (Hackel, Citation1889, p. 420).
Homotypic synonym: Andropogon selloanus (Hack.) Hack. (Hackel, Citation1904, p. 266).
Anatherum ternarium (Michx.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon ternarius Michx. (Michaux, Citation1803, p. 57).
Anatherum tracyi (Nash) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon tracyi Nash (Nash, Citation1900, p. 433).
* Anatherum trichozygum (Baker) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon trichozygus Baker (Baker, Citation1883, p. 300).
* Anatherum urbanianum (Hitchc.) Voronts. & E.A.Kellogg, comb. nov.
Basionym: Andropogon urbanianus Hitchc. (Hitchcock, Citation1912, p. 424).
* Anatherum virginicum (L.) Spreng., Pl. Min. Cogn. Pug. 2: 16. 1815
Basionym: Andropogon virginicus L., (Linnaeus, Citation1753, p. 1046).
Incertae sedis
These species share some morphological similarity with Anatherum, or in the case of A. appendiculatus are morphologically distinct but phylogenetically placed within it. They are not transferred at this time pending more critical investigation, as noted above. However, they are distinct from Andropogon s.s., so the generic name is placed in quotes.
* “Andropogon” appendiculatus Nees, (Nees von Esenbeck, Citation1841, p. 105).
* “Andropogon” brazzae Franch., (Franchet, Citation1895, p. 326).
“Andropogon” dewetii Mashau & Fish, (Mashau et al., Citation2022, p. 3, 4, f. 1, 2).
* “Andropogon” durifolius Renvoize, (Renvoize, Citation1984, p. 181).
* “Andropogon” mannii Hook.f., (Hooker, Citation1864, p. 232).
Associate Editor: Dr Mark Carine
Acknowledgements
We thank the herbarium management and staff of the Missouri Botanical Garden and Royal Botanic Garden, Kew, for full access to specimens. Thank you to Christabel King for drawing the beautiful plates. Thanks also to editors Mark Carine and Peter Olson and reviewer Jeff Saarela for comments that greatly strengthened the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Full plastome sequences used to construct the tree have been deposited at GenBank.
Additional information
Funding
References
- Acocks, J. P. H., & Zacharias, P. J. K. (1990). Acock’s notes: Key grasses of South Africa. Grassland Society of Southern Africa.
- Arthan, W., McKain, M. R., Traiperm, P., Welker, C. A. D., Teisher, J. K., & Kellogg, E. A. (2017). Phylogenomics of Andropogoneae (Panicoideae: Poaceae) of mainland Southeast Asia. Systematic Botany, 42, 418–431. https://doi.org/10.1600/036364417X696023
- Ashe, W. W. (1898). Notes on grasses. Journal of the Elisha Mitchell Scientific Society, 15, 112–114.
- Baker, J. G. (1883). Contributions to the flora of Madagascar, part 1: Polypetalae. Journal of the Linnean Society of London, Botany, 20, 87–158. https://doi.org/10.1111/j.1095-8339.1883.tb00195.x
- Boonman, G. (1993). East Africa’s grasses and fodders: Their ecology and husbandry. Springer Dordrecht.
- Borzí, A. (1907). Atti Congr. Nat. Ital. Milano, 1906, 372.
- Bosser, J. M. (1968). Note sur les graminées de Madagascar – VII. Adansonia n.s, 8, 513–522.
- Bridges, E. L., & Orzell, S. L. (2018). Revision of the Andropogon ternarius complex of sect. Leptopogon (Poaceae) with two new species from peninsular Florida. Phytoneuron, 2018-80, 1–25.
- Britton, N. L., Sterns, E. E., & Poggenburg, J. F. (1888). Preliminary catalogue of Anthophyta and Pteridophyta reported as growing spontaneously within one hundred miles of New York. Torrey Botanical Club.
- Campbell, C. S. (2003). Andropogon. In M. E. Barkworth, K. M. Capels, S. Long, & M. B. Piep (Eds.), Flora of North America North of Mexico. Magnoliophyta: Commelinidae (in part): Poaceae, part 2 (Vol. 25). Oxford University Press.
- Camus, A. (1924 (publ. 1925). Andropogonées nouvelles de Madagascar. Bulletin de la Société Botanique de France, 71, 921–924. https://doi.org/10.1080/00378941.1924.10836999
- Camus, A. (1952). Andropogon et Nastus nouveaux de Madagascar. Notul. Syst. (Paris), 14, 213–214.
- Chapman, A. W. (1878). An enumeration of some plants – chiefly from the semi-tropical regions of Florida – which are either new, or which have not hitherto been recorded as belonging to the flora of the Southern states (concluded). Botanical Gazette, 3, 17–21. https://doi.org/10.1086/325120
- Chapman, A. W (1883). Flora of the Southern United States (2nd ed.). Ivison, Blakeman, Taylor & Co.
- Clayton, W. D. (1964). Studies in the Gramineae: V. Kew Bulletin. 17, 465–470. https://doi.org/10.2307/4113818
- Clayton, W. D. (1969). A revision of the genus Hyparrhenia. Her Majesty’s Stationery Office.
- Clayton, W. D. (1977). New grasses from Eastern Africa. Studies in the Gramineae: XLII. Kew Bulletin, 32, 1–4.
- Clayton, W. D., & Renvoize, S. A. (1986). Genera graminum: Grasses of the world. Her Majesty’s Stationery Office.
- Clayton, W. D., Vorontsova, M. S., Harman, K. T., & Williamson, H. (2016). GrassBase – The online world grass flora. http://www.kew.org/data/grassbase/index.html
- Davidse, G., & Turland, N. J. (1999). Proposal to conserve the name Andropogon bicornis (Gramineae) with a conserved type. Taxon, 48, 573–574. https://doi.org/10.2307/1224569
- Estep, M. C., McKain, M. R., Vela Diaz, D., Zhong, J., Hodge, J. G., Hodkinson, T. R., Layton, D. J., Malcomber, S. T., Pasquet, R., & Kellogg, E. A. (2014). Allopolyploidy, diversification, and the Miocene grassland expansion. Proceedings of the National Academy of Sciences of the United States of America, 111, 15149–15154. https://doi.org/10.1073/pnas.1404177111
- Fish, L., Mashau, A. C., Moeaha, M. J., & Nembudani, M. T. (2015). Identification guide to southern African grasses. An identification manual with keys, descriptions and distributions (Vol. 36). South African National Biodiversity Institute.
- Franchet, A. R. (1895). Contributions a la Flore du Congo Français. Bulletin de la Société d'Histoire Naturelle d'Autun, 8, 309–391.
- Gibson, D. J. (2009). Grasses and grassland ecology. Oxford University Press.
- Hackel, E. (1885). Andropogoneae novae. Flora, 68, 131–143.
- Hackel, E. (1889). Andropogoneae. In A. DeCandolle & C. DeCandolle (Eds.), Monographiae phanerogamarum (Vol. 6). G. Masson.
- Hackel, E. (1904). Graminee. Bulletin de l'Herbier Boissier, 2, 265–378.
- Hitchcock, A. S. (1912). A new species of Andropogon. Botanical Gazette, 54, 424–424. https://doi.org/10.1086/330934
- Hitchcock, A. S. (1927). The grasses of Ecuador, Peru, and Bolivia. Plant studies, chiefly tropical American. Contr. U.S. Natl. Herb, 24, 291–556.
- Hooker, J. D. (1864). On the plants of the temperate regions of the Cameroons Mountains and islands in the Bight of Benin. Journal of the Proceedings of the Linnean Society of London. Botany, 7, 171–240. https://doi.org/10.1111/j.1095-8312.1864.tb01067c.x
- IPNI (2023). International Plant Names Index. Published on the Internet http://www.ipni.org. The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Herbarium.
- Katoh, K., & Standley, D. M. (2014). MAFFT: Iterative refinement and additional methods. Methods in Molecular Biology (Clifton, N.J.), 1079, 131–146. https://doi.org/10.1007/978-1-62703-646-7_8
- Kellogg, E. A. (2015). Poaceae (K. Kubitzki Ed.). Springer.
- Lamson-Scribner, F. (1896). Grass notes. Bulletin of the Torrey Botanical Club, 23, 141–147. https://doi.org/10.2307/2478154
- Lamson-Scribner, F., & Merrill, E. D. (1900/1901). Some recent collections of Mexican grasses. Bulletin, Division of Agrostology United States Department of Agriculture, 24, 5–30.
- Lindman, C. A. M. (1900). Beiträge zur Gramineenflora südamerikas. Kongliga Svenska Vetenskaps-Akademiens Handlingar, 34, 1–52.
- Linnaeus, C. (1753). Species plantarum (Vol. 2). Laurentius Salvius.
- Maguire, B., & Wurdack, J. J. (1957). The Botany of the Guayana Highland—part II. Memoirs of the New York Botanical Garden, 9, 235–392.
- Mashau, A. C., Fish, L., & de Wet, F. (2022). Andropogon dewetii (Poaceae: Panicoideae: Andropogoneae), a new species from South Africa. Kew Bulletin, 77, 293–299. https://doi.org/10.1007/s12225-022-10012-9
- Mathews, S., Spangler, R. E., Mason-Gamer, R. J., & Kellogg, E. A. (2002). Phylogeny of Andropogoneae inferred from phytochrome B, GBSSI, and ndhF. International Journal of Plant Sciences, 163, 441–450. https://doi.org/10.1086/339155
- McAllister, C. A., McKain, M. R., Li, M., Bookout, B., & Kellogg, E. A. (2018). Specimen-based analysis of morphology and the environment in ecologically dominant grasses: The power of the herbarium. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 374, 201740403. https://doi.org/10.1098/rstb.2017.0403
- McKain, M. R., Wilson, M. C., & Kellogg, E. A. (unpublished). Fast-Plast: A rapid de novo assembly pipeline for whole chloroplast genomes. Git-hub. https://doi.org/10.5281/zenodo.973887
- Michaux, A. (1803). Flora Boreali-Americana (Vol. 1). Levrault.
- Nagahama, N., & Norrmann, G. A. (2012). Review of the genus Andropogon (Poaceae: Andropogoneae) in America based on cytogenetic studies. Journal of Botany, 2012, 1–9. https://doi.org/10.1155/2012/632547
- Nash, G. V. (1900). Some new grasses from the Southern States. Bulletin of the New York Botanical Garden, 1, 429–436.
- Nees von Esenbeck, C. G. D. (1829). Gramineae. In Flora Brasiliensis seu Enumeratio Plantarum (Vol. 2(1)). Stuttgart and Tübingen.
- Nees von Esenbeck, C. G. D. (1841). Florae Africae Australioris Illustrationes Monographicae. Gramineae Palala Press.
- Norrmann, G., Hanson, L., Renvoize, S., & Leitch, I. J. (2004). Genomic relationships among diploid and hexaploid species of Andropogon (Poaceae). Genome, 47, 1220–1224. https://doi.org/10.1139/g04-068
- Norrmann, G. A., & Quarín, C. L. (2001). Andropogon barretoi, una nueva especie de Poaceae del sur de Brasil. Darwiniana, 39, 171–174.
- Palisot de Beauvois, A. M. F. J. (1812). Essai d‘une Nouvelle Agrostographie. Imprimerie de Fain.
- Pilger, R. K. F. (1899). Gramineae Lehmannianae et Stübelianae austro-americanae additis quibusdam ab aliis collectoribus ibi collectis deteminatae et descriptae. Botanische Jahrbücher fur Systematik, 27, 17–36.
- POWO (2021). Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Retrieved November 29, 2021, from http://www.plantsoftheworldonline.org/
- Rendle, A. B. (1899). Catalogue of the African Plants collected by Dr. F. Welwitsch in 1853-61. 2(1): 1–260. British Museum (Natural History).
- Renvoize, S. A. (1984). New grasses from Bahia. Kew Bulletin, 39, 179–183. https://doi.org/10.2307/4107868
- Renvoize, S. A. (1998). Gramineas de Bolivia. Royal Botanic Gardens.
- Roberty, G. (1960). Monographie systématique des Andropogonées du globe. Boissiera, 9, 1–455.
- Rosengurtt, B., Arrillaga de Maffei, B. R., & Izaguirre de Artucio, P. (1970). Gramineas Uruguayas. Universidad de la Republica, Departamento de Publicaciones.
- Sales, F. (2002). Flora Zambesiaca: Mozambique, Federation of Rhodesia and Nyasaland, Bechuanaland Protectorate (p. 10). Kew London.
- Shaik, R. S., Gurusinghe, S., Weston, L. A., & Downey, P. O. (2022). A historical perspective on plant invasion in Australia. In D. R. Clements, M. K. Upadhyaya, S. Joshi, & A. Shrestha (Eds.), Global plant invasions (pp. 129–149). Springer Nature.
- Skendzic, E. M., Columbus, J. T., & Cerros-Tlatilpa, R. (2007). Phylogenetics of Andropogoneae (Poaceae: Panicoideae) based on nuclear ribosomal internal transcribed spacer and chloroplast trnL-F sequences. Aliso, 23, 530–544. https://doi.org/10.5642/aliso.20072301.40
- Small, J. K. (1903). Flora of the Southeastern United States. Small, J. K.
- Stamatakis, A. (2014). RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics (Oxford, England), 30, 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
- Stapf, O. (1919). Order CLVII. Gramineae. Flora of Tropical Africa, 9, 208–265.
- Swallen, J. R. (1949). New grasses from Honduras, Colombia, Venezuela, Ecuador, Bolivia, and Brazil. U. S. Natl. Herb, 29, 251–276.
- Thiers, B. (updated continuously). Index Herbariorum. https://sweetgum.nybg.org/science/ih/.
- Tillich, M., Lehwark, P., Pellizzer, T., Ulbricht-Jones, E. S., Fischer, A., Bock, R., & Greiner, S. (2017). GeSeq – versatile and accurate annotation of organelle genomes. Nucleic Acids Research, 45, W6–W11. https://doi.org/10.1093/nar/gkx391
- Trinius, C. B. (1833). Andropogineorum genera speciesque complures definitionibus novis illustravit. Mémoires de l'Académie impériale des sciences de St.-Pétersbourg, 2, 239–337.
- Turland, N. J., Wiersema, J. H., Barrie, F. R., Greuter, W., Hawksworth, D. L., Herendeen, P. S., Knapp, S., & Kusber, W. H. (Eds.) (2018). International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books.
- von Humboldt, F. W. H., Bonpland, A. J. A., & Kunth, C. S. (1816). Nova genera et species plantarum, 1. Belgium.
- von Martius, C. F. P. (1883). Flora Brasiliensis. 2(3). Frid. Fleischer.
- Walter, T. (1788). Flora Caroliniana. J. Fraser.
- Welker, C. A. D., McKain, M. R., Estep, M. C., Pasquet, R., Chipabika, G., Pallangyo, B., & Kellogg, E. A. (2020). Phylogenomics enables biogeographic analysis and a new subtribal classification of Andropogoneae (Poaceae–Panicoideae). Journal of Systematics and Evolution, 58, 1003–1030. https://doi.org/10.1111/jse.12691
- Wood, D. E., & Salzberg, S. L. (2014). Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biology, 15, R46. https://doi.org/10.1186/gb-2014-15-3-r46
- Zanin, A., & Longhi-Wagner, H. (2003). Taxonomic novelties in Andropogon (Poaceae-Andropogoneae) for Brazil. Novon, 13, 368–375. https://doi.org/10.2307/3393274
- Zanin, A., & Longhi-Wagner, H. (2006). Sinopse do gênero Andropogon L. (Poaceae-Andropogoneae) no Brasil. Revista Brasileira de Botânica, 29, 289–299. https://doi.org/10.1590/S0100-84042006000200010
