Abstract
Micromorphic acrotretide brachiopods first appeared during Cambrian Epoch 2 and subsequently experienced a rapid diversification. However, our knowledge of acrotretide origins and early evolution is hampered by our poor understanding of their earliest representatives. Here, we describe one of the oldest known acrotretides from the Cambrian Series 2 Shuijingtuo Formation of southern Shaanxi and western Hubei, South China. The new genus Palaeotreta gen. nov. can be distinguished from all other genera, mainly by its unusual ontogeny and morphology; in Palaeotreta the pedicle foramen is located mostly outside of the metamorphic shell, the ventral intertrough is very short and the inclination of the ventral pseudointerarea changes from catacline to apsacline. Two ontogenetic stages (T1–T2) of post-metamorphic shell growth can be distinguished in the low cap-like Palaeotreta shannanensis gen. et sp. nov.; this type of development with a relatively long pedicle foramen forming stage (T1), a simple pedicle opening, weakly developed ventral apical process and dorsal median septum, can be considered to represent a plesiomorphy for the acrotretides. By contrast, the more derived Palaeotreta zhujiahensis (Li & Holmer, 2004) is characterized by paedomorphosis, expressed in an early enclosure of pedicle foramen and greatly decreased growth of the ventral intertrough during the intertrough-increasing stage (T3), producing a cap-like shape and retaining catacline inclination of the ventral pseudointerarea. Thus, heterochrony may have played an important role in the morphological divergence of acrotretides during the Cambrian evolutionary radiation, and promoted the subsequent radiation of acrotretides. The ontogenetic study of Palaeotreta gen. nov. also supports the notion that the ventral intertrough of acrotretide brachiopods may have formed by the ‘rolling up’ of the lingulide propareas.
http://zoobank.org/urn:lsid:zoobank.org:pub:2C95FB13-7B15-43A2-B37C-AAA1B74A2D1C
Introduction
Linguliform brachiopods first appeared at the beginning of the Cambrian, and they represent one of the key components of benthic communities during the Cambrian evolutionary radiation (Bassett et al. Citation1999; Z.-F. Zhang et al. Citation2008, Citation2016; Budd & Jackson Citation2016; Z.-L. Zhang et al. Citation2020). The linguliform order Acrotretida, which is uniquely characterized by a diminutive shell size and coniform ventral valve, had a relatively late origination, but subsequently diversified rapidly and became extremely abundant with a cosmopolitan distribution during the Cambrian and Ordovician (Williams & Holmer Citation1992; Holmer & Popov Citation2000; Williams et al. Citation2000; Ushatinskaya Citation2010; Topper et al. Citation2013; Z.-L. Zhang et al. Citation2018a, Citationb; Ushatinskaya & Korovnikov Citation2019). However, many questions concerning their early origin, divergence and phylogenetic relationships with other brachiopod groups remain uncertain.
To date, only six acrotretide genera, including Eohadrotreta, Linnarssonia, Prototreta, Vandalotreta, Kuangshanotreta and Hadrotreta, are known from Cambrian Series 2 (Holmer & Popov Citation2000; Li & Holmer Citation2004; Ushatinskaya Citation2010). Recently published works show that Eohadrotreta rapidly achieved a wide distribution over key palaeocontinents (South China, East Gondwana, West Gondwana, North China), soon after their first appearance in Cambrian Epoch 2 (Wang et al. Citation2012; Popov et al. Citation2015; Z.-L. Zhang et al. Citation2016, Citation2017, Citation2018a; Pan et al. Citation2019; Claybourn et al. Citation2020). Two species of Eohadrotreta (E. zhenbaensis and E.? zhujiahensis) were originally described from South China by Li & Holmer (Citation2004) but the diagnostic features to discriminate these taxa were not clearly defined, making it difficult to evaluate the biostratigraphy and phylogeny of this important genus (Li & Holmer Citation2004; Z.-L. Zhang et al. Citation2017, Citation2018a, Citationb; Z.-L. Zhang Citation2018). A subsequent detailed quantitative analysis of brachiopod ontogeny demonstrated that the developmental pattern of the metamorphic shell was quite different in these two species (Z.-L. Zhang et al. Citation2018a), revealing heterochrony in the ontogeny of the earliest acrotretide representatives during the Cambrian radiation. Numerous morphological differences between E.? zhujiahensis and E. zhenbaensis indicate that they may belong to separate genera (Z.-L. Zhang Citation2018; Z.-L. Zhang et al. Citation2018a).
This paper aims to describe the new acrotretide genus Palaeotreta gen. nov. from the Cambrian Series 2 Shuijingtuo Formation from southern Shaanxi and western Hubei provinces of South China. The ontogeny of Palaeotreta shannanensis gen. et sp. nov. includes the formation of an enclosed pedicle foramen that is mostly located outside of the metamorphic shell, as well as an apsacline ventral pseudointerarea during later ontogenetic stages, which are unlike any other acrotretides from Cambrian Epoch 2. The problematic E.? zhujiahensis is also referred to the new genus Palaeotreta. The new information on the development of the pedicle foramen, pseudointerarea, apical process and median septum is also shown to be important for deciphering phylogeny. Three ontogenetic stages (e.g. pedicle foramen-forming stage [T1], pedicle foramen-enclosing stage [T2] and intertrough-increasing stage [T3]) were identified by Z.-L. Zhang et al. (Citation2018a) for E. zhenbaensis and can also be identified in Palaeotreta.
Geological setting
Neoproterozoic to Palaeozoic sedimentary successions are well developed and distributed on the northern margin of the Yangtze Platform, South China. The acrotretide brachiopods described here come from the lower part of the Shuijingtuo Formation of South China. In southern Shaanxi, Precambrian to Cambrian strata are well exposed at the Xiaoyangba section, approximately 106 km south-east of Hanzhong City (), including (in ascending order) the Ediacaran Dengying Formation, the Cambrian Xihaoping Member of Dengying Formation, the Shuijingtuo Formation and the Shipai Formation (Z.-L. Zhang et al. Citation2018c, Citation2020). The Shuijingtuo Formation (unconformably overlying the Xihaoping Member of the Dengying Formation) is characterized by organic-rich and nodular limestones at the lower boundary. The 31 m thick lower Shuijingtuo Formation is comprised of dark, thin silty limestones, while the 85 m thick upper part consists of siltstones interbedded with thin limestones. Large quantities of phosphatized shelly fossils have been reported from the lower part of the Shuijingtuo Formation at the Xiaoyangba section, which are dominated by the eodiscoid trilobite Hupeidiscus orientalis, the hyolith Paramicrocornus zhenbaensis and bradoriids (Li et al. Citation2012; Z.-L. Zhang Citation2018; Z.-L. Zhang et al. Citation2018c). Associated fossils include the brachiopods Eohadrotreta, Palaeobolus, Botsfordia and Lingulellotreta, the tommotiid Kelenella, hyoliths, molluscs along with chancelloriids, echinoderm plates and sponge spicules (Li & Holmer Citation2004; Yang et al. Citation2015; Z.-F. Zhang et al. Citation2016). The new taxon Palaeotreta shannanensis gen. et sp. nov. is described from a single layer (S4-3) at the base of the Shuijingtuo Formation.
Figure 1. Palaeogeographic map and fossil localities in southern Shaanxi and western Hubei, South China (modified from Z.-F. Zhang et al. Citation2016), noting the Xiaoyangba and Aijiahe sections.
In the Three Gorges area of western Hubei, the Aijiahe section, approximately 25 km north-west of Yichang City (), has been well studied (e.g. Ishikawa et al. Citation2014; Z.-F. Zhang et al. Citation2016; Z.-L. Zhang et al. Citation2016, Citation2018a). The 68 m thick Shuijingtuo Formation (unconformably overlying the Yanjiahe Formation) is composed of three parts. The 10 m thick basal part consists of condensed black shale intercalated with black limestone concretions, yielding the oldest known trilobite, Tsunyidiscus actus, from this region (Z.-F. Zhang et al. Citation2016). The 40 m thick middle part of the succession is dominated by alternating black shale and thin limestones, while the 18 m thick upper part consists of greyish-black argillaceous limestones. Abundant phosphatized shelly fossils have been described from the middle and upper parts of the Shuijingtuo Formation at the Aijiahe section with the acrotretide brachiopod Eohadrotreta zhenbaensis, the eodiscoid trilobite Tsunyidiscus actus and hyoliths the most dominant groups (Z.-F. Zhang et al. Citation2016; Z.-L. Zhang et al. Citation2018c). Other associated fossils include the brachiopods Palaeobolus, Spinobolus and Lingulellotreta, archaeocyaths, molluscs, bradoriids, chancelloriid sclerites and sponge spicules (Steiner et al. Citation2007; Z.-F. Zhang et al. Citation2016; Z.-L. Zhang et al. Citation2016, Citation2018a; Z.-L. Zhang Citation2018). Palaeotreta zhujiahensis (Li & Holmer, Citation2004) is found in the middle and upper parts of the Shuijingtuo Formation in this section.
Material and methods
All specimens described and illustrated are from the Shuijingtuo Formation (Cambrian Series 2) of South China (). To date, 22 ventral and 10 dorsal valves of Palaeotreta shannanensis gen. et sp. nov. and 23 complete conjoined valves, 124 ventral and 115 dorsal valves of Palaeotreta zhujiahensis have been collected from the Xiaoyangba and Aijiahe sections, respectively. The specimens were obtained through acetic acid (∼10%) maceration of samples from thin-bedded limestones following standard techniques (Jeppsson et al. Citation1985). Specimens were manually picked from the acid-resistant residues and selected specimens were imaged using SEM facilities including a Zeiss Supra 35 VP field emission at Uppsala University and a JEOL JSM 7100F-FESEM at Macquarie University. Measurements of length and width of P. shannanensis gen. et sp. nov. were made on the best-preserved specimens, while the measurement data for P. zhujiahensis was illustrated in Z.-L. Zhang et al. Citation2018a (appendix S3–S4). Scatter plots of different specimens showing morphological variation were constructed using PAST v. 3.14 (Hammer et al. Citation2001).
Systematic palaeontology
Phylum Brachiopoda Duméril, Citation1806
Subphylum Linguliformea Williams, Carlsson, Bruton, Holmer & Popov, Citation1996
Class Lingulata Gorjansky & Popov, Citation1985
Order Acrotretida Kuhn, Citation1949
Superfamily Acrotretoidea Schuchert, Citation1893
Family Acrotretidae Schuchert, Citation1893
Genus Palaeotreta gen. nov.
Type species. Palaeotreta shannanensis gen. et sp. nov.; Cambrian Series 2 of southern Shaanxi, South China.
Derivation of name. Palaeo, ‘old’, from the Greek palaiós, describing an ancestral representative of acrotretide brachiopods, with the ending –treta, ‘perforated’ from the Greek trētos.
Description. Shell ventribiconvex, sub-circular to transversely oval, ornamented with evenly distributed pits, about 1 µm in diameter covering the metamorphic shell, and with finely concentric growth lines on the post-metamorphic shell. Ventral valve cap-like to low conical, pseudointerarea vestigial, from catacline to apsacline, with very short intertrough. Incipient pedicle notch is open during the long pedicle foramen-developing stage. Adult pedicle foramen located outside of the metamorphic shell. Growth lines developed on a separate area between the metamorphic shell and pedicle foramen. Apical process vestigial, very close to the pedicle foramen. Cardinal muscle scars and vascula lateralia weakly impressed. Dorsal valve slightly convex. Pseudointerarea orthocline, short with narrow sub-triangular median groove. Median buttress generally well developed, while median septum vestigial. Cardinal muscle scars weakly impressed. Secondary shell layer columns relatively short.
Remarks. The new genus is established for acrotretide species that are similar to Eohadrotreta and Linnarssonia. However, the new genus differs from all other (especially from Cambrian Epoch 2) acrotretides in having the following characters: (1) a catacline to apsacline inclination of ventral pseudointerarea with short intertrough; (2) a pedicle foramen that is located mostly outside the metamorphic shell; (3) a vestigial apical process and median septum; and (4) weakly impressed cardinal muscle scars. Eohadrotreta, Prototreta, Vandalotreta, Hadrotreta and Kotylotreta mainly have procline pseudointerarea with a relatively long intertrough. Although the apsacline ventral pseudointerarea and internal structures (apical process, median septum) of Palaeotreta can be compared with Cambrian Epoch 3 taxa, such as Aphelotreta and the Furongian Linnarssonella, the positions of pedicle foramen and ventral pseudointerarea are different. The ventral apical process and dorsal septum of Palaeotreta are very weakly defined, while Linnarssonia develops a high, boss-like apical process and has a high median septum. Eohadrotreta has a relatively more well-developed apical process and median septum. Vandalotreta has an apical process that forms a boss-like thickening anterior to the internal foramen and Hadrotreta develops apical pits that are situated close to the apical process (Holmer & Popov Citation2000). Kuangshanotreta is difficult to compare with the new genus in the absence of data on shell morphology and ornamentation (Wang et al. Citation2012).
Palaeotreta is the third genus of the Acrotretida that has been described from the Cambrian Series 2 of South China, and this region has a higher diversity of coeval acrotretides as compared to South Australia, Laurentia, Antarctica and Siberia. The most unique character of Palaeotreta is the position of the pedicle foramen, which is located mostly outside of the metamorphic shell from the T2 ontogenetic stage. In this feature it has similarities to some Cambrian Ceratretidae and Ordovician Scaphelasmatidae species (Holmer Citation1989; Holmer & Popov Citation2000); but to date, this character is not known from early Cambrian Acrotretidae.
Palaeotreta shannanensis gen. et sp. nov.
(; )
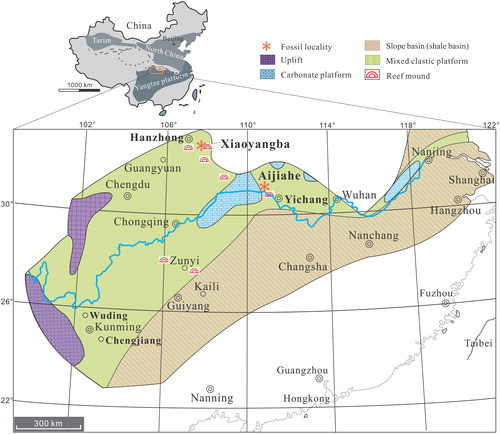
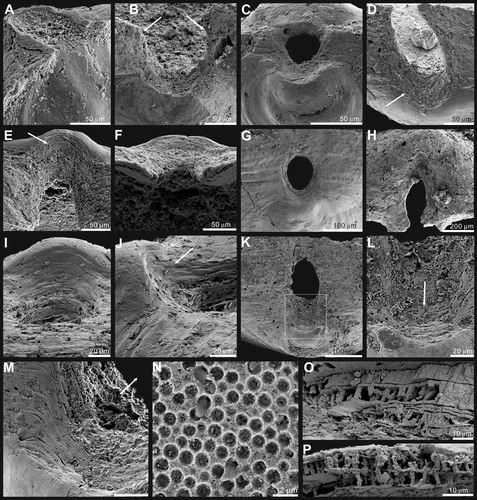
Figure 2. Ontogenetic development of ventral valve of Palaeotreta shannanensis gen. et sp. nov. from the Shuijingtuo Formation of southern Shaanxi. A–H, ventral valves demonstrating pedicle foramen forming stage (T1); A–D, juvenile with unrestricted pedicle notch, ELI-XYB S4-3 AU-06; E–H, small valve with pedicle opening, ELI-XYB S4s-3 AV-04; I–P, ventral valves demonstrating pedicle foramen-enclosing stage (T2); I–L, adult with enclosed pedicle foramen, ELI-XYB S4-3 AU-01; L, interior view, noting vascula lateralia (tailed arrow); M–P, larger valve with very short intertrough, ELI-XYB S4-3 AU-08.
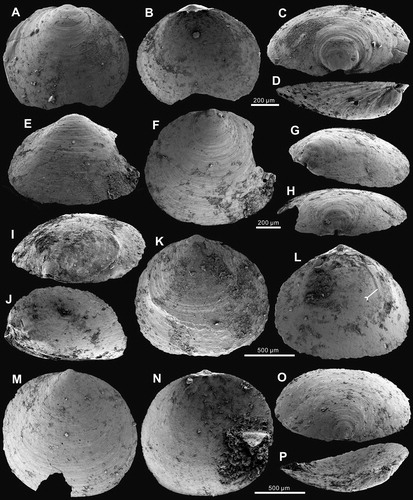
Figure 3. Ontogenetic development of dorsal valve of Palaeotreta shannanensis gen. et sp. nov. from the Shuijingtuo Formation of southern Shaanxi. A–D, juvenile with rudiment median buttress, ELI-XYB S4-3 AV-19; E–G, larger valve with developed median buttress, ELI-XYB S4-3 AU-12; G, oblique lateral view showing weakly developed median septum (arrow); H–K, adult valve with weakly developed median septum, ELI-XYB S4-3 AV-18.
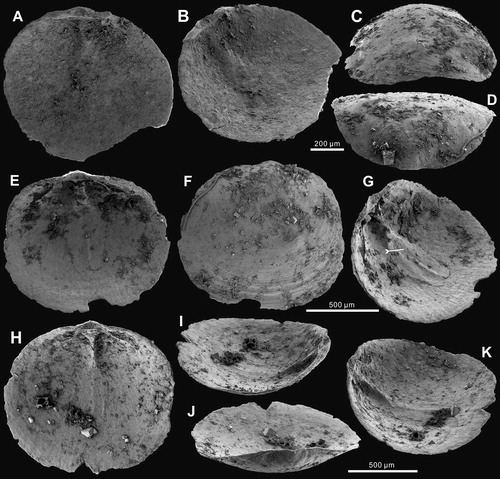
Figure 4. Ornamentation and ultrastructure of the earliest ontogeny of Palaeotreta shannanensis gen. et sp. nov. from the Shuijingtuo Formation of southern Shaanxi. A, enlarged ventral apex, note pronounced halo (arrow) and drape structures (tailed arrow), ELI-XYB S4-3 AU-08; B, oblique lateral view of A, showing the pedicle foramen (arrow) and protegulum (tailed arrow); C, posterior view of ventral apex, show enclosed pedicle foramen outside of metamorphic shell, ELI-XYB S4-3 AU-01; D, enlarged dorsal apex, note pronounced halo (arrow) and drape structures (tailed arrow), ELI-XYB S4-3 AV-15 E, obliquely lateral view of D, showing the protegulum (tailed arrow) and two pairs of larval setal sacs (arrows); F, evenly distributed pitting structures on metamorphic shell, ELI-XYB S4-3 AU-13; G, enlargement of F; H, growth lines and drape structures on post-metamorphic shell, ELI-XYB S4-3 AU-13.
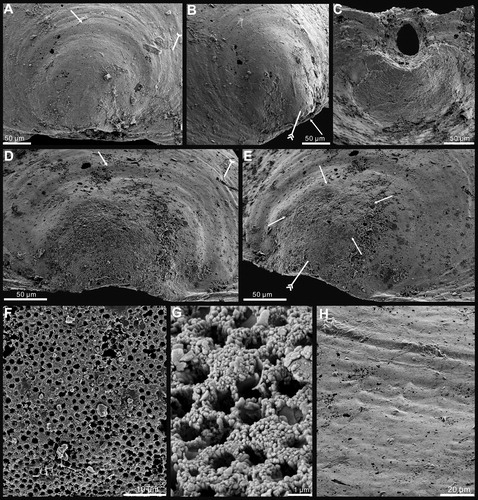
Figure 5. Internal morphology and ultrastructure of Palaeotreta shannanensis gen. et sp. nov. from the Shuijingtuo Formation of southern Shaanxi. A, enlarged ventral posterior end, showing cardinal muscle scars (arrows) and paired vascula lateralia (tailed arrows), ELI-XYB S4-3 AU-07; B, oblique lateral view of A; C, enlarged ventral posterior end, ELI-XYB S4-3 AV-07; D, lateral view of dorsal posterior end, noting cardinal muscle scars by arrows, XYB S4-3 AV-18; E, enlarged dorsal pseudointerarea and median buttress, ELI-XYB S4-3 AU-12; F, enlargement of the terminal of median buttress of E, note weakly developed median septum; G, fine pores on the exterior of columnar lamella by exfoliation of the primary layer, ELI-XYB S4-3 AU-09; H, I, one layer of the secondary columnar structures on valve margin, ELI-XYB S4-3 AV-09, XYB S4-3 AU-07; J, K, remaining base of columns after exfoliation of the covering lamella of H; L, one lamella of very short secondary columns, ELI-XYB S4-3 AU-08; M, re-crystallization of the columns, note hollow in the centre, ELI-XYB S4-3 AV-13.
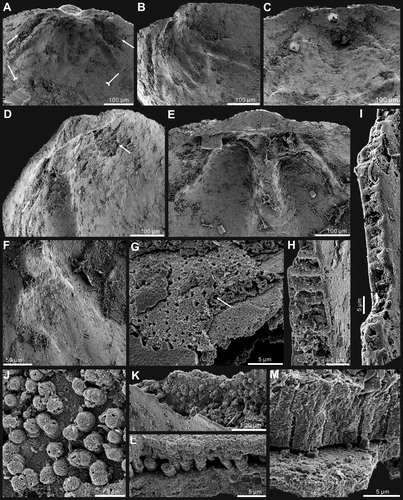
Figure 6. Ontogenetic development of pedicle foramen of Palaeotreta shannanensis gen. et sp. nov. from the Shuijingtuo Formation of southern Shaanxi. A, B, juvenile with unrestricted pedicle notch, note raised propareas (arrows), ELI-XYB S4-3 AV-09; C, D, juvenile with unrestricted pedicle notch, ELI-XYB S4-3 AV-17; E–G, semicircular pedicle foramen soon to be enclosed, ELI-XYB S4-3 AV-05; H, raised propareas (arrows), ELI-XYB S4-3 AV-11; I–L, pedicle foramen, just enclosed with very short intertrough, XYB S4-3 AU-07; M, N, adult with enclosed pedicle foramen, showing the successive growth of propareas at the posterior margin of the metamorphic shell (arrow), ELI-XYB S4-3 AU-01; O, enclosed pedicle foramen is mostly outside the metamorphic shell, ELI-XYB S4-3 AV-07.
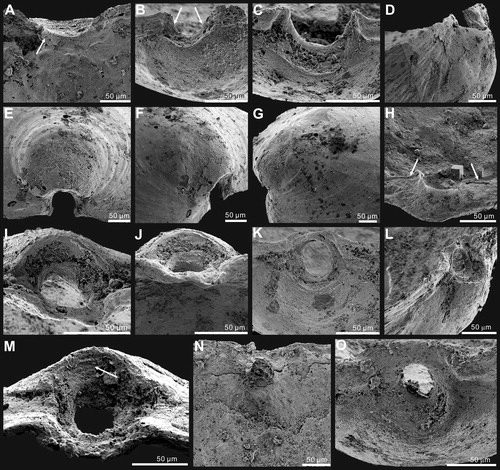
Figure 7. Bivariate plots of ventral valves of Palaeotreta shannanensis gen. et sp. nov. from the Shuijingtuo Formation showing ontogenetic stage T1 (red) and stage T2 (blue). A, plots of valve width – length ratio (W/L), length at maximum width – valve length ratio (Lm/L), and valve height – length ratio (H/L). B, plots of apical process length – valve length ratio (La/L), pedicle foramen length – valve length ratio (Lf/L).
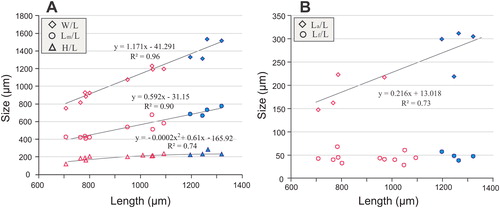
Table 1. Average dimensions and ratios of ventral and dorsal valves of Palaeotreta shannanensis gen. et sp. nov. from the Cambrian Series 2 Shuijingtuo Formation.
Derivation of name. After the occurrence in Shannan, southern Shaanxi Province.
Holotype. ELI-XYB S4-3 AU-01, ventral valve (, length [L] = 1196 µm, width [W] = 1331 µm).
Paratype. ELI-XYB S4-3 AV-18, dorsal valve (, L = 1008 µm, W = 1226 µm).
Type locality. Shuijingtuo Formation (layer S4-3, 0.7 m above the base) at Xiaoyangba section (32° 29′ 29″ N, 107° 57′ 2″ E), Zhenba County, south-eastern Shaanxi, South China. Cambrian Series 2.
Other material. A total of 21 ventral valves and nine dorsal valves were recovered from the same layer (S4-3) in the Shuijingtuo Formation.
Description. Shell ventribiconvex, sub-circular in outline with round posterior margin (). 1.1 µm hemispherical pits evenly distributed on the whole metamorphic shell surface without overlapping (), while the post-metamorphic shell is covered by finely circular growth lines and drape structures (). Shell structure consists of thin-lamella (2 µm) primary layer and thin-lamina (5 µm) secondary columnar layers ().
Ventral valve sub-circular, on average 89% as long as wide with maximum width at mid-length (). The valve is convex, with a cap-like shape (), on average 21% as deep as long, with maximum height almost at mid-valve. Metamorphic shell pronounced at the apex (), occupying 13% of valve length. Pseudointerarea weakly developed, catacline to apsacline. Intertrough poorly defined, very short, occupying on average about 2% of the length and 6% of the width of the valve (). Apical process vestigial, only observed in adult specimens, close to pedicle foramen, occupying 20% of valve length (). Enclosed pedicle foramen almost circular, about 50 µm in diameter, located mostly directly outside of the metamorphic shell, until valve reaches about 1100 µm in length. Growth lines developed at the posterior margin of the metamorphic shell and the lateral side of the pedicle foramen (). Cardinal muscle scars and vascula lateralia weakly impressed.
Dorsal valve sub-circular, on average 87% as long as wide, with maximum width almost at mid-valve (). It is slightly convex (), on average about 19% as deep as long. Pseudointerarea small, orthocline, occupying 6% of the valve length and 36% of valve width (). Median groove sub-triangular, short, about 31% of the pseudointerarea width (). Median buttress generally developed, fading anteriorly (). Median septum vestigial, only developed in adult valve, extending anteriorly for 59% of valve length (). Cardinal muscle scars weakly impressed, occupying 17% of the length and 45% of the width of the valve ().
Remarks. Palaeotreta shannanensis gen. et sp. nov. shares a similar morphology in outline with Eohadrotreta zhenbaensis (from the same locality). However, the new species has a low cap-like shape, small pseudointerarea with very short intertrough, apsacline pseudointerarea in the adult valve, prolonged pedicle foramen-forming stage and a pedicle foramen that is located outside of the metamorphic shell (). Furthermore, the new species has a less developed intertrough as compared with E. zhenbaensis (Z.-L. Zhang et al. Citation2018a); in the latter species the intertrough is becoming remarkably prominent in stage T3, which does not happen in the new species. The shell structure of P. shannanensis is similar to that of E. zhenbaensis. However, the former species has a very thin primary layer about 2 µm thick, and very thin secondary columnar layers. The thickness of columns in P. shannanensis is variable in different parts of the shell, ranging from 4 µm to 8 µm, and are quite short compared to the columns of E. zhenbaensis. The secondary layer insignificantly increases the overall thickness of the ventral valve (Z.-L. Zhang et al. Citation2016), being composed of one to two columnar laminae, resulting in a low cap-like shape in P. shannanensis.
Palaeotreta shannanensis differs from Vandalotreta djagoran (Kruse, Citation1990) and Linnarssonia rowelli (Pelman, Citation1973) in having a lower ventral valve, weakly developed apical process and median septum, apsacline ventral pseudointerarea, as well as a pedicle foramen that is mostly located outside the metamorphic shell (Holmer et al. Citation1996, pl. 13; Ushatinskaya & Korovnikov Citation2019, pl. 4). P. shannanensis has a similar inclination of the ventral pseudointerarea as Aphelotreta khemangarensis (Popov et al. Citation2015), while the ornamentation of the metamorphic shell, pedicle foramen size and the median groove allow the distinction of the two species (Popov et al. Citation2015, fig. 21).
At the Xiaoyangba section, P. shannanensis occurs at the base of the Shuijingtuo Formation, while E. zhenbaensis first occurs 60 cm higher. Therefore, P. shannanensis represents the oldest known acrotretide brachiopod in southern Shaanxi. The diachronous nature of the stratigraphic hiatus at the base of Cambrian Series 2 across southern Shaanxi and western Hubei makes the biostratigraphic correlation between these regions imprecise, and more index fossils from new sections are required to better constrain the time gap and better resolve correlation.
Palaeotreta zhujiahensis (Li & Holmer, 2004)
()
Figure 8. Ontogenetic development of ventral valve of Palaeotreta zhujiahensis from the Shuijingtuo Formation of western Hubei. A–F, ventral valves demonstrating pedicle foramen forming stage (T1); A, oblique dorsal view of a very small conjoined specimen showing unrestricted pedicle notch, ELI-AJH 8-1-2-B AF12; B, lateral view of a small conjoined specimen, ELI-AJH 8-2-3 AD2-07; C, posterior view, box indicates the area shown in , ELI-AJH S05 AG07; D, interior view of C; E, oblique view of a larger juvenile, ELI-AJH 8-2-3 AC-27; F, posterior view of E, note ‘U’-shaped pedicle notch, box indicates the area shown in ; G, lateral view, indicating pedicle foramen-enclosing stage (T2), showing enclosed pedicle foramen outside of the metamorphic shell, ELI-AJH 8-2-D AD2-12; H–N, intertrough-increasing stage (T3); H, posterior view, ELI-AJH S05 AF-16; I–N, adult with short intertrough, ELI-AJH 8-2-1 CE-03; I, exterior view; J, interior view; K, oblique lateral view; L, lateral view; M, posterior view, note the posterior migration of enclosed pedicle foramen, outside the metamorphic shell; N, oblique anterior view.
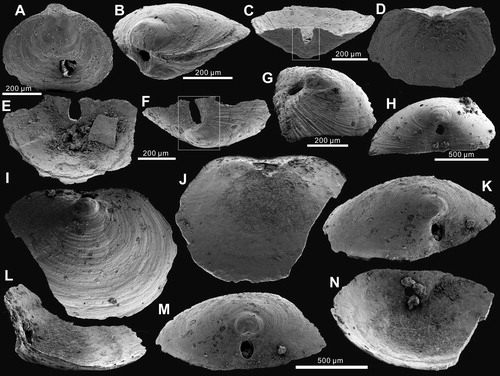
Figure 9. Ontogenetic development of pedicle foramen of Palaeotreta zhujiahensis from the Shuijingtuo Formation of western Hubei. A, enlarged pedicle notch of ; B, juvenile with unrestricted pedicle notch, showing raised propareas (arrows), ELI-AJH 8-2-3 AC-11; C, posterior view of ; D, ‘U’-shaped pedicle notch, note the growth of propareas at the posterior margin of metamorphic shell (arrow); E, F, ‘U’-shaped pedicle foramen is soon to be enclosed, note the growth of propareas (arrow); G, enclosed pedicle foramen with short intertrough, ELI-AJH 8-2-D AD2-12; H, larger adult showing pedicle foramen outside the metamorphic shell, ELI-AJH 8-2-3 CD2-02; I, enlargement of propareas growing at the posterior margin of metamorphic shell and lateral sides of pedicle foramen of G; J, lateral view of I, note propareas growth (arrow); K, posterior view, showing pedicle foramen mostly located outside the metamorphic shell, box indicates the area shown in L, ELI-AJH 8-2-3 AC-22; L, enlarged view showing propareas growth (arrow); M, enlargement of propareas at the posterior margin of metamorphic shell, note pedicle foramen (arrow), ELI-AJH 8-2-1 AE-09; N, pitting structures on metamorphic shell, ELI-AJH 8-2-1 AE-09; O, P, enlarged secondary columnar layer, ELI-AJH 8-2-1 AE-09, ELI-AJH 8-2-1 CE-03.
Citation2004 Eohadrotreta zhujiahensis Li & Holmer: 208, figs 14,15.
Citation2016 Eohadrotreta zhujiahensis Z.-F. Zhang et al.: 342, fig. 6.
Citation2018a Eohadrotreta? zhujiahensis Zhang et al.: 187–197, figs 7–10.
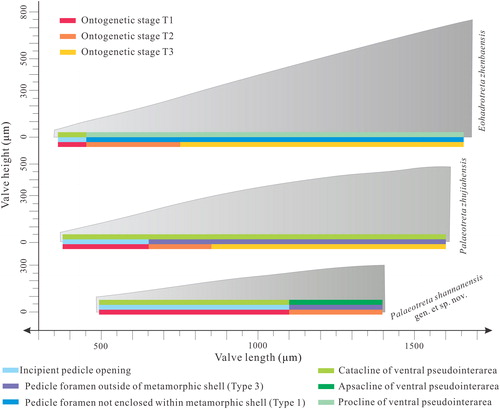
Figure 10. Ontogenetic scheme of Palaeotreta and Eohadrotreta from Cambrian Series 2 of South China, demonstrating transitions of important characters during different ontogenetic stages (modified from Z.-L. Zhang et al. Citation2018a, Claybourn et al. Citation2020).
Citation2018b Eohadrotreta? zhujiahensis Zhang et al.: 4–8, figs 2a, 4.
Holotype. Li & Holmer (Citation2004), NIGP1351 77, conjoined shell (W = 1167 µm).
Type locality. Guojiaba Formation (uppermost) at the Fucheng section of Zhenba County, south-eastern Shaanxi, South China. Cambrian Series 2.
Paratype. ELI-AJH 8-2-1 CE-03, ventral valve (, L = 975 µm, W = 1220 µm) from the upper Shuijingtuo Formation at the Aijiahe section of Yichang, South China.
Other material. A total of 23 conjoined valves, 124 ventral and 115 dorsal valves from the middle and upper parts of the Shuijingtuo Formation at the Aijiahe section (30° 44′ 55.2″ N, 111° 03′ 58.5″ E) of Yichang, western Hubei, South China.
Description. Shell ventribiconvex, transverse oval in outline with slightly straightened posterior margin (). 1.3 µm hemispherical pits evenly distributed on the whole metamorphic shell surface without overlapping (), while post-metamorphic shell covered by finely circular growth lines and drape structures (). Shell structure consists of thin-lamella (2 µm) primary layer and thin-lamina (5–10 µm) secondary columnar layers ().
Ventral valve sub-circular, on average 83% as long as wide with maximum width at the posterior half of valve. It is convex, with a low conical shape (), on average 28% as deep as long, with a maximum height almost at mid-valve. Metamorphic shell pronounced at the apex (), occupying 31% of the valve length. Pseudointerarea weakly developed, almost catacline, divided by a very short intertrough, which is on average about 5% of the length and 11% of the width of the valve (). Apical process weakly developed, occupying on average 30% of valve length, close to pedicle foramen. Pedicle foramen is relatively large, about 90 µm in diameter, enclosed and located directly outside the metamorphic shell until valve reaches about 650 µm in length. Growth lines distinctively developed at the posterior margin of the metamorphic shell (). Cardinal muscle scars and vascula lateralia weakly impressed.
Dorsal valve transversely oval, on average 82% as long as wide, with maximum width almost at mid-valve (see details in Z.-L. Zhang et al. Citation2018a) (). Slightly convex (), on average 17% as deep as long. Pseudointerarea small, orthocline, occupying about 7% of valve length and 38% of valve width. Median groove subtriangular, short, on average 44% of pseudointerarea width. Median buttress moderately developed, fading anteriorly. Median septum vestigial, only developed in adult valve, extending anteriorly at mid-valve. Cardinal muscle scars gently impressed, occupying 22% of the length and 51% of the width of the valve.
Remarks. Based on their similar morphology, Palaeotreta zhujiahensis was originally considered to represent a second species of Eohadrotreta by Li & Holmer (Citation2004). However, Z.-L. Zhang et al. (Citation2018a) demonstrated significant differences in ontogenetic growth between these two species. New material collected from western Hubei demonstrates that P. zhujiahensis has a lower ventral valve, straightened posterior margin, relatively larger pedicle foramen, late enclosure of the pedicle foramen, smaller ventral pseudointerarea, much shorter intertrough, weakly developed growth lines, apical process and median septum, thinner secondary layers and more weakly impressed cardinal muscle scars than those of E. zhenbaensis. Furthermore, the most characteristic feature of P. zhujiahensis is that the pedicle foramen is located directly outside of the metamorphic shell, which fits closely with the diagnosis of the new genus Palaeotreta. The valve shape and ontogenetic development of the pedicle foramen in P. zhujiahensis can be compared with that of P. shannanensis (). However, the former has a catacline inclination of the ventral pseudointerarea and a relatively longer intertrough, which is two times longer than that of P. shannanensis.
The shell structure of P. zhujiahensis is comparable with that of P. shannanensis. Both have a very thin primary layer about 2 µm thick, but P. shannanensis has relatively thinner columnar layers. The thickness of columns in P. zhujiahensis is variable in different shell regions, ranging from 5 µm to 10 µm (), which is quite short compared to the columns in E. zhenbaensis (Zhang et al. Citation2017, fig. 5E, H).
At the Aijiahe section, P. zhujiahensis co-occurs with E. zhenbaensis at the middle part of the Shuijingtuo Formation. Compared to the biostratigraphy with southern Shaanxi (Z.-F. Zhang et al. Citation2016), P. zhujiahensis is slightly younger than P. shannanensis. P. zhujiahensis is the second species discovered in southern Shaanxi and western Hubei (after E. zhenbaensis), but E. zhenbaensis has a much wider palaeogeographical distribution (Z.-L. Zhang et al. Citation2017).
Discussion
Ontogenetic development of Palaeotreta
Recent study of the earliest ontogeny of Cambrian Epoch 2 acrotretide brachiopods has revealed that the primary larval body plan of acrotretides is shared with most early linguliforms, and that the metamorphic shell is formed at the end of the planktotrophic stage (Z.-L. Zhang et al. Citation2018b). The same kind of early ontogenetic characters can also be observed in Palaeotreta shannanensis gen. et sp. nov. The raised lobes at the posterior end of both the ventral and dorsal valves of P. shannanensis are about 50 µm in size (), which is comparable to the protegula of Eohadrotreta zhenbaensis. They are probably secreted initially as a bivalved, organic shell by the larval mantle lobes (Paine Citation1963; Chuang Citation1977; Balinski Citation2001; Z.-L. Zhang et al. Citation2018b). During subsequent peripheral growth of the brephic shell at the planktotrophic stage, two pairs of lobes are preserved as imprints of larval setal sacs on the dorsal side of the larval body (). Pits with an average diameter of 1 µm evenly cover the whole surface of the metamorphic shell (), which may represent imprints of mineralized tablets that potentially protected the larvae against ultraviolet radiation penetrating surface waters (Lüter Citation2004; Williams & Cusack Citation2007; Z.-L. Zhang et al. Citation2018b). The metamorphic shell is separated from the post-metamorphic shell by a pronounced halo when the shell reached about 200 µm in width (; ), which likely represents the completion of larval metamorphosis. A narrow belt about 20 μm wide, outside the metamorphic shell, lacking drape structures indicates the secretion of the neanic shell (). Subsequently, the mature shell is developed by accretionary growth outside the neanic shell, exhibiting all major characters of the adult shell including drape structures () and a three-layer shell (). The growth of the mature shell indicates the development of marginal mantle after completion of metamorphosis (Z.-L. Zhang et al. Citation2018b). Detailed investigation of the earliest ontogeny of P. zhujiahensis has previously been documented by Z.-L. Zhang et al. (Citation2018b). The closely similar features of metamorphosis between Eohadrotreta and Palaeotreta imply that Palaeotreta probably had a life cycle similar to the early Cambrian acrotretide Eohadrotreta and lingulide Eoobolus, which are interpreted to have had a relatively prolonged free-swimming stage (Popov et al. Citation2007; Balthasar Citation2009; Ushatinskaya Citation2016; Z.-L. Zhang et al. Citation2017, Citation2018b).
For the later post-metamorphic ontogeny, Z.-L. Zhang et al. (Citation2018a) demonstrated a strong allometric growth pattern in Eohadrotreta zhenbaensis by quantifying the ontogenetic variations in size and shape in successive ontogenetic stages. This also revealed that Palaeotreta zhujiahensis experienced three stages during the entire ontogenetic sequence, which can be well compared with the almost coeval E. zhenbaensis (; Z.-L. Zhang et al. Citation2018a; Claybourn et al. Citation2020). When compared to the ontogeny of E. zhenbaensis, however, only two ontogenetic stages (T1–T2) can be distinguished during the growth of P. shannanensis (, , ). During the T1 pedicle foramen-forming stage (pedicle opening is not enclosed as a foramen and the ventral intertrough is not developed; Z.-L. Zhang et al. Citation2018a, pp. 187–191), the shape of the ventral valve remains low and cap-like and the cardinal muscle scars, apical process and median septum are vestigial (, ), while the incipient pedicle notch changes from semicircular to circular (). In the subsequent T2 pedicle foramen-enclosing stage (enclosure of pedicle notch to form a foramen and development of an incipient apical process and median septum; Z.-L. Zhang et al. Citation2018a, pp. 191–197), the marginal accretionary growth increases the length and width of both valves, while the ventral valve maintains a low cap-like form (). At the same time, cardinal muscle scars are weakly impressed, while the apical process and median septum are weakly developed (). The inclination of the ventral pseudointerarea changes from catacline to apsacline (), and the accelerated shell growth along the posterior margin of the metamorphic shell produces a posterior migration of the enclosed pedicle foramen (). It is important to note that the stage T2 of P. shannanensis is much delayed until valves reach about 1100 µm in length, which is twice the size compared to E. zhenbaensis. Furthermore, the boundary delineating the pedicle foramen from the metamorphic shell is developed early in stage T1 (), and successive growth lines of the propareas near the posterior margin of the metamorphic shell and lateral sides of pedicle foramen are well developed in stage T2 (). These remarkable ontogenetic variations are also well demonstrated in the entire three ontogenetic stages of P. zhujiahensis ().
The morphology of juvenile shells is quite similar between Palaeotreta and Eohadrotreta, which makes it difficult to distinguish them when a complete ontogeny is lacking (Li & Holmer Citation2004; Z.-F. Zhang et al. Citation2016; Z.-L. Zhang Citation2018). However, the heterochronic process probably occurred in later ontogenetic stages, resulting in quite different morphologies in adults of Palaeotreta and Eohadrotreta (; Klingenberg, Citation1998, Citation2016; Webster & Zelditch Citation2005). A strong allometric growth pattern was verified in E. zhenbaensis especially during the T3 intertrough-increasing stage (complete development of ventral intertrough, apical process, median septum and imprint of vascula lateralia; Z.-L. Zhang et al. Citation2018a, p. 197) by the rapid increase in the ventral intertrough length (Z.-L. Zhang et al. Citation2018a). Nonetheless, this ontogenetic variation in stage T3 for P. shannanensis is completely lacking, resulting in a very small ventral pseudointerarea and short intertrough (). As the growth of the intertrough is an important event in acrotretide brachiopods, the relatively slow development of the intertrough (c. 1.6% length of ventral valve) in P. shannanensis results in a change in inclination of the ventral pseudointerarea from catacline to apsacline, producing a low, cap-like valve shape (; ). In contrast, the ventral valve of E. zhenbaensis becomes more conical with a very long intertrough (c. 24.5% length of ventral valve) and the inclination of the ventral pseudointerarea changes from catacline to procline during stage T3 (Z.-L. Zhang et al. Citation2018a). Compared with the slightly older P. shannanensis, P. zhujiahensis demonstrates a relatively rapid growth during ontogenetic stage T1, as the pedicle foramen is enclosed at an earlier time when the ventral valve reaches 650 µm in length, contrasting with 1100 µm in P. shannanensis. Thus, there is an earlier termination of shape change in the younger P. zhujiahensis during ontogenetic stage T1 (Webster & Zelditch Citation2005). Moreover, the growth of the ventral pseudointerarea greatly decreases during ontogenetic stage T3 (increasing 0.8% of the length and 3.5% of the width of the ventral valve; Z.-L. Zhang et al. Citation2018a, appendix S3), producing a cap-like shape, catacline ventral pseudointerarea and posterior migration of the pedicle foramen outside of the metamorphic shell as well (). The morphological similarity between the adult valves (650–1600 µm) of P. zhujiahensis and juvenile valves (< 1110 µm) of P. shannanensis indicates paedomorphosis (progenesis) of P. zhujiahensis (; Z.-L. Zhang et al. Citation2018a). Thus, heterochrony plays an important role in the differentiation of species in the new genus Palaeotreta.
Changes in shape through ontogeny and the different growth patterns in the two species of Palaeotreta may correspond to subtle modification in life mode during the gradual cumulative increase in size (Gould Citation1966; Raup & Stanley Citation1971; Tomašových et al. Citation2008; Baker & Logan Citation2011; Z.-L. Zhang et al. Citation2018a). However, the life habit of extinct acrotretides is still not well understood (Popov & Holmer Citation1994; Williams et al. Citation2000). Rare but exquisitely preserved fossils may suggest that acrotretides attached to shell fragments or even to other benthic animals like sponges to achieve secondarily tiered niches (Mergl Citation2002; Holmer et al. Citation2005; Henderson & Dann Citation2010; Wang et al. Citation2012; Topper et al. Citation2015a, Citationb). The low cap-like shape and apsacline ventral pseudointerarea indicate that P. shannanensis is likely to have had a mode of life similar to Treptotreta with a thin pedicle anchorage at the apex of the ventral valve (Henderson & Dann Citation2010). The development of a slightly longer intertrough whilst maintaining a catacline pseudointerarea in P. zhujiahensis may have aided stable attachment to the substrate.
Phylogenetic implications
The phylogenetic relationships of the Order Acrotretida to other groups within the Class Lingulata are still poorly resolved, though acrotretides possess many important unique characters, such as diminutive body size, conical shape of the ventral valve with enclosed pedicle foramen, simplified muscular system, developed apical process and often a raised median septum in the dorsal valve (Williams & Holmer Citation1992; Holmer & Popov Citation1996, Citation2000; Williams et al Citation2000; Holmer et al. Citation2008). Recent studies have revealed that bearing an incipient pedicle notch in the juvenile, pitted metamorphic shell and the presence of columnar shell structures in acrotretides demonstrates a close relationship with the oldest known lingulide brachiopods (Holmer & Popov Citation1996; Ushatinskaya & Korovnikov Citation2014; Z.-L. Zhang Citation2018; Z.-L. Zhang et al. Citation2018a, Citation2020). As the position of the developing pedicle foramen in relation to the metamorphic shell is one of the most taxonomically important characters in acrotretides (Rowell Citation1977; Holmer & Popov Citation2000; Topper et al. Citation2013; Z.-L. Zhang et al. Citation2018a), investigation of the ontogeny of the pedicle foramen provides a new way of considering acrotretide phylogeny. The incipient pedicle notch in juvenile specimens of Palaeotreta during ontogenetic stage T1 is interpreted to represent a similar ancestral type of pedicle opening between the ventral and dorsal valves that occurs in linguliform brachiopods (, 8A, B; see also Beecher Citation1891; Rowell Citation1977; Popov Citation1992). The weakly developed ventral pseudointerarea is comparable with the raised pseudointerarea above the valve floor in older lingulides such as Eoobolus (Ushatinskaya & Holmer Citation2001; Ushatinskaya & Korovnikov Citation2014; Z.-L. Zhang et al. Citation2020). In forming a high conical ventral valve, the cardinal muscles move posteriorly to the raised pseudointerarea in acrotretides (). During later ontogeny, the slow growth of the intertrough results in a catacline pseudointerarea in P. zhujiahensis, whilst the lack of major growth of the intertrough changes the pseudointerarea from catacline to apsacline in P. shannanensis (). In contrast, the rapid growth of the intertrough in E. zhenbaensis results in a significant gradual transition of the ventral valve pseudointerarea from catacline to procline (). The new evidence presented here supports the hypothesis that the ventral intertrough in acrotretide brachiopods evolved by the gradual and variable ‘rolling up’ of the lingulide propareas along the posterior margin of the ventral valve in association with changes in the inclination of the ventral intertrough (Popov Citation1992; Holmer & Popov Citation2000; Li & Holmer Citation2004; Z.-L. Zhang et al. Citation2018a). A distinct evolutionary lineage from basal botsfordiids that developed an open delthyrium and vestigial ventral pseudointerarea to more derived acrothelids which have an enclosed pedicle foramen and raised ventral pseudointerarea probably indicates that this monophyletic clade is derived from the lingulides (Popov et al. Citation2015; Smith et al. Citation2016; Claybourn et al. Citation2020; Zhang et al. Citation2020).
There are three main positions of the pedicle foramen in the fossil record of acrotretides concerning its position related to the metamorphic shell. Type 1 is not enclosed in the metamorphic shell, Type 2 is completely enclosed in the metamorphic shell and Type 3 is mostly located outside of the metamorphic shell. The first type, where the pedicle foramen is partly enclosed in the metamorphic shell, occurred widely across genera beginning in Cambrian Epoch 2, such as Linnarssonia, Prototreta (or Homotreta), Eohadrotreta, Hadrotreta and Vandalotreta (Holmer & Popov Citation2000; Ushatinskaya Citation2010). The second and third types (Type 2–3), where accelerated shell growth along the posterior margin of metamorphic shell resulted in anterior or posterior migration of the pedicle foramen respectively, become common later in Cambrian Epoch 3 and the Ordovician (e.g. Apsotreta and Scaphelasma; see also Holmer Citation1989; Holmer & Popov Citation2000; Topper et al. Citation2013; Popov et al. Citation2015). However, this is the first discovery of the Type 3 during Cambrian Epoch 2 in Palaeotreta from South China ().
The morphological diversity amongst acrotretide brachiopods in Cambrian Stage 2 of South China includes great variation in the position of the pedicle foramen, shell growth patterns and inclination of the ventral pseudointerarea (). The low cap-like shape, vestigial apical process and median septum, very short intertrough and longer pedicle foramen-forming stage (T1) probably imply that P. shannanensis is one of the ancestral stocks of acrotretides. This is also supported by its early occurrence in the Shuijingtuo Formation of southern Shaanxi. Compared with the other two species P. zhujiahensis and E. zhenbaensis (Z.-L. Zhang et al. Citation2018a), P. shannanensis has a more restricted distribution and lower abundance, which may indicate this ancestral acrotretide was not suited to life in distal mixed clastic platforms. By contrast, E. zhenbaensis has more derived characters exemplified by faster growth rates during accretionary shell growth, especially during stage T3, enabling a wider geographical distribution and increased abundance in shallow carbonate environments (Li & Holmer Citation2004; Betts et al. Citation2017; Z.-L. Zhang et al. Citation2018a; Claybourn et al. Citation2020). The evidence presented here suggests evolutionary modification to ontogenetic changes resulted in the early divergence of Palaeotreta and Eohadrotreta, and played an important role in the early evolution of acrotretides. The early Cambrian widespread acrotretides Hadrotreta and Prototreta, developing a long ventral intertrough and dorsal median septum, may have a similar heterochronic process to E. zhenbaensis during the T3 intertrough-increasing stage. Heterochrony also probably drove rapid morphological variation in acrotretides, explaining their subsequent radiation and optimal adaptation across a wide range of shallow marine depositional environments (Klingenberg Citation1998; Bassett et al. Citation1999, Citation2002; Tomašových et al. Citation2008; Baker & Carlson Citation2010; Baker & Logan Citation2011). These adaptations contributed to the success of younger acrotretide brachiopods, especially during the transition from Cambrian Epoch 2 to Epoch 3 and their subsequent radiation during the Great Ordovician Biodiversification Event.
Associate Editor: Rebecca Freeman
Acknowledgements
The authors would like to thank Zhifei Zhang from Northwest University for his original contribution to this project and valuable discussion. We are also grateful to Qingchun Feng for sample preparation in Xi’an, and Michael Streng at Uppsala University, Sue Lindsay and Chao Shen of the Microscopy Unit at Macquarie University for assistance with SEM imaging. Financial support for this project was provided by Macquarie University Research Fellowships (2019 MQRF), National Natural Science Foundation of China (41425008, 41720104002, 41621003, 41890844), Strategic Priority Research Program of Chinese Academy of Sciences (XDB26000000), Ministry of Science and 111 project of Ministry of Education of China (D17013), Zhongjian Yang Scholarship, Swedish Research Council (VR 2018-03390), Opening Foundation of Shaanxi Key Laboratory of Early Life and Environments and 1000 Talent Shaanxi Province Fellowship. Financial support from the China Scholarship Council (CSC 201806970026) for F.-Y. Chen to have a 14-month study at Macquarie University is acknowledged. We are also very grateful to Associate Editor Rebecca L. Freeman and one anonymous reviewer whose constructive comments greatly improved the manuscript.
References
- Baker, P. G. & Carlson, S. J. 2010. The early ontogeny of Jurassic thecideoid brachiopods and its contribution to the understanding of thecideoid ancestry. Palaeontology, 53(3), 645–667. doi:10.1111/j.1475-4983.2010.00941.x
- Baker, P. G. & Logan, A. 2011. Support from early juvenile Jurassic, Cretaceous and Holocene thecideoid species for a postulated common early ontogenetic development pattern in thecideoid brachiopods. Palaeontology, 54(1), 111–131. doi:10.1111/j.1475-4983.2010.01023.x
- Balinski, A. 2001. Embryonic shells of Devonian linguloid brachiopods. Systematics Association Special Volume, 63, 91–101.
- Balthasar, U. 2009. The brachiopod Eoobolus from the Early Cambrian Mural Formation (Canadian Rocky Mountains). Paläontologische Zeitschrift, 83(3), 407–418. doi:10.1007/s12542-009-0026-4
- Bassett, M. G., Popov, L. E. & Holmer, L. E. 1999. Organophosphatic brachiopods: patterns of biodiversification and extinction in the early Palaeozoic. Geobios, 32(2), 145–163. doi:10.1016/S0016-6995(99)80026-6
- Bassett, M. G., Popov, L. E. & Holmer, L. E. 2002. Brachiopods: Cambrian-Tremadoc precursors to Ordovician radiation events. Geological Society of London, Special Publications, 194(1), 13–23. doi:10.1144/GSL.SP.2002.194.01.02
- Beecher, C. E. 1981. Development of the Brachiopoda. American Journal of Science, 41, 343–357. doi:10.2475/ajs.s3-41.244.343
- Betts, M. J., Paterson, J. R., Jago, J. B., Jacquet, S. M., Skovsted, C. B., Topper, T. P. & Brock, G. A. 2017. Global correlation of the early Cambrian of South Australia: shelly fauna of the Dailyatia odyssei Zone. Gondwana Research, 46, 240–279. doi:10.1016/j.gr.2017.02.007
- Budd, G. E. & Jackson, I. S. 2016. Ecological innovations in the Cambrian and the origins of the crown group phyla. Philosophical Transactions of the Royal Society B, 371(1685), 20150287. doi:10.1098/rstb.2015.0287
- Chuang, S. H. 1977. Larval development in Discinisca (Inarticulata, Brachiopoda). American Zoologist, 17, 39–52. doi:10.1093/icb/17.1.39
- Claybourn, T. M., Skovsted, C. B., Holmer, L. E., Pan, B., Myrow, P. M., Topper, T. P. & Brock, G. A. 2020. Brachiopods from the Byrd Group (Cambrian Series 2, Stage 4) Central Transantarctic Mountains, East Antarctica: biostratigraphy, phylogeny and systematics. Papers in Palaeontology, 6(3), 349–383. doi:10.1002/spp2.1295
- Duméril, A. M. C. 1806. Zoologie analytique ou méthode naturelle de classification des animaux. XXIV Allais, Paris, 344 pp.
- Gorjansky, V. I & Popov, L. E. 1985. The morphology, systematic position, and origin of inarticulate brachiopods with carbonate shells. Paleontologicheskii Zhurnal, 3, 3–13. [in Russian]
- Gould, S. J. 1966. Allometry and size in ontogeny and phylogeny. Biological Reviews, 41(4), 587–638. doi:10.1111/j.1469-185X.1966.tb01624.x
- Hammer, Ø., Harper, D. A. & Ryan, P. D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm
- Henderson, R. A. & Dann, A. L. 2010. Substrate control of benthos in a Middle Cambrian near-shore, epeiric palaeoenvironmental setting. Palaeogeography, Palaeoclimatology, Palaeoecology, 292(3–4), 474–487. doi:10.1016/j.palaeo.2010.04.016
- Holmer, L. E. 1989. Middle Ordovician phosphatic inarticulate brachiopods from Västergötland and Dalarna, Sweden. Fossils and Strata, 26, 1–172.
- Holmer, L. E. & Popov, L. E. 1996. Early Paleozoic radiation and classification of organo-phosphatic brachiopods. Pp. 1–121 in P. Copper & J. Jin (eds) Brachiopods. Proceedings of the Third International Brachiopod Congress. Sudbury, Ontario, Canada. 2 September 1995.
- Holmer, L. E. & Popov, L. E. 2000. Order Acrotretida. Pp. 321–440 in R. L. Kaesler (ed.) Treatise on invertebrate paleontology, part H, Brachiopoda (revised) volume 2. Geological Society of America, Boulder and University of Kansas, Lawrence.
- Holmer, L. E., Popov, L. E. & Wrona, R. D. 1996. Early Cambrian lingulate brachiopods from glacial erratics of King George Island (South Shetland Islands), Antarctica. Palaeontologia Polonica, 55, 37–50.
- Holmer, L. E., Popov, L. E., Streng, M. & Miller, J. F. 2005. Lower Ordovician (Tremadocian) lingulate brachiopods from the House and Fillmore formations, Ibex area, western Utah, USA. Journal of Paleontology, 79(5), 884–906. doi:10.1666/0022-3360(2005)079[0884:LOTLBF]2.0.CO;2
- Holmer, L. E., Popov, L. E. & Streng, M. 2008. Organophosphatic stem group brachiopods: implications for the phylogeny of the subphylum Linguliformea. Fossils and Strata, 54, 3–11.
- Ishikawa, T., Ueno, Y., Shu, D., Li, Y., Han, J., Guo, J. & Komiya, T. 2014. The δ13C excursions spanning the Cambrian explosion to the Canglangpuian mass extinction in the Three Gorges area, South China. Gondwana Research, 25(3), 1045–1056. doi:10.1016/j.gr.2013.03.010
- Jeppsson, L., Fredholm, D. & Mattiasson, B. O. 1985. Acetic acid and phosphatic fossils: a warning. Journal of Paleontology, 59, 952–956. doi:10.1017/S0022336000040798
- Klingenberg, C. P. 1998. Heterochrony and allometry: the analysis of evolutionary change in ontogeny. Biological Reviews, 73(1), 79–123. doi:10.1111/j.1469-185X.1997.tb00026.x
- Klingenberg, C. P. 2016. Size, shape, and form: concepts of allometry in geometric morphometrics. Development Genes and Evolution, 226(3), 113–137. doi:10.1007/s00427-016-0539-2
- Kruse, P. D. 1990. Cambrian palaeontology of the Daly Basin. Northern Territory Geological Survey, Report, 7, 1–58.
- Kuhn, O. 1949. Lehrbuch der paläozoologie. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart, 326 pp.
- Li, G. X. & Holmer, L. E. 2004. Early Cambrian lingulate brachiopods from the Shaanxi Province, China. GFF, 126(2), 193–211. doi:10.1080/11035890401262193
- Li, G., Steiner, M., Zhu, M. & Zhao, X. 2012. Early Cambrian eodiscoid trilobite Hupeidiscus orientalis from South China: ontogeny and implications for affinities of Mongolitubulus-like sclerites. Bulletin of Geosciences 87, 159–169. doi:10.3140/bull.geosci.1224
- Lüter, C. 2004. How brachiopods get covered with nanometric silicon chips. Proceedings of the Royal Society of London. Series B: Biological Sciences, 271(6), S465–S467. doi:10.1098/rsbl.2004.0226
- Mergl, M. 2002. Linguliformean and craniiformean brachiopods of the Ordovician (Třenice to Dobrotivá Formations) of the Barrandian, Bohemia. Acta Musei Nationalis Pragae, Series B, Natural History, 58, 1–82.
- Paine, R. T. 1963. Ecology of the brachiopod Glottidia pyramidata. Ecological Monographs, 33, 187–213. doi:10.2307/1942626
- Pan, B., Skovsted, C. B., Brock, G. A., Topper, T. P., Holmer, L. E., Li, L. Y. & Li, G. X. 2019. Early Cambrian organophosphatic brachiopods from the Xinji Formation, at Shuiyu section, Shanxi Province, North China. Palaeoworld. doi:10.1016/j.palwor.2019.07.001
- Pelman, Yu. L. 1973. Some inarticulate brachiopods from the Lower and Middle Cambrian of the Olenek River basin (lower reaches). Tr. Inst. Geol. Geofiz. Sib. Otd. Akad. Nauk SSSR, 49, 69–80.
- Popov, L. E. 1992. The Cambrian radiation of brachiopods. Pp. 399–423 in J. H. Lipps & P. W. Signor (eds). Origin and early evolution of the Metazoa. Plenum Press, New York.
- Popov, L. E. & Holmer, L. E. 1994. Cambrian-Ordovician lingulate brachiopods from Scandinavia, Kazakhstan, and South Ural Mountains. Fossils and Strata, 35, 1–156.
- Popov, L. E., Egerquist, E. & Holmer, L. E. 2007. Earliest ontogeny of Middle Ordovician rhynchonelliform brachiopods (Clitambonitoidea and Polytoechioidea) implications for brachiopod phylogeny. Lethaia, 40, 85–96. doi:10.1111/j.1502-3931.2006.00008.x
- Popov, L. E., Holmer, L. E., Hughes, N. C., Ghobadi Pour, M. & Myrow, P. M. 2015. Himalayan Cambrian brachiopods. Papers in Palaeontology, 1(4), 345–399. doi:10.1002/spp2.1017
- Raup, D. M. & Stanley, S. M. 1971. Principles of paleontology. W. H. Freeman and Company, San Francisco, 388 pp.
- Rowell, A. J. 1977. Valve orientation and functional morphology of the foramen of some siphonotretacean and acrotretacean brachiopods. Lethaia, 10(1), 43–50. doi:10.1111/j.1502-3931.1977.tb00590.x
- Schuchert, C. 1893. A classification of the Brachiopoda. American Geologist, 11, 141–167.
- Smith, P. M., Brock, G. A. & Paterson, J. R. 2016. Linguliformean brachiopods from the early Templetonian (Cambrian series 3, stage 5) Giles Creek Dolostone, Amadeus Basin, Northern Territory. Australasian Palaeontological Memoirs, 49, 125–143.
- Steiner, M., Li, G., Qian, Y., Zhu, M. & Erdtmann, B. D. 2007. Neoproterozoic to early Cambrian small shelly fossil assemblages and a revised biostratigraphic correlation of the Yangtze Platform (China). Palaeogeography, Palaeoclimatology, Palaeoecology, 254(1–2), 67–99. doi:10.1016/j.palaeo.2007.03.046
- Tomašových, A., Carlson, S. J. & Labarbera, M. 2008. Ontogenetic niche shift in the brachiopod Terebratalia transversa: relationship between the loss of rotation ability and allometric growth. Palaeontology, 51(6), 1471–1496. doi:10.1111/j.1475-4983.2008.00809.x
- Topper, T. P., Harper, D. A. & Ahlberg, P. 2013. Reappraisal of the brachiopod Acrotreta socialis von Seebach, 1865: clarifying 150 years of confusion. GFF, 135(2), 191–203. doi:10.1080/11035897.2013.811440
- Topper, T. P., Strotz, L. C., Holmer, L. E. & Caron, J. B. 2015a. Survival on a soft seafloor: life strategies of brachiopods from the Cambrian Burgess Shale. Earth-Science Reviews, 151, 266–287. doi:10.1016/j.earscirev.2015.10.015
- Topper, T. P., Strotz, L. C., Holmer, L. E., Zhang, Z., Tait, N. N. & Caron, J. B. 2015b. Competition and mimicry: the curious case of chaetae in brachiopods from the middle Cambrian Burgess Shale. BMC Evolutionary Biology, 15(1), 42. doi:10.1186/s12862-015-0314-4
- Ushatinskaya, G. T. 2010. Stratigraphic and geographic distribution of acrotretids (Brachiopoda, Lingulata) in the Middle and Late Cambrian. Paleontological Journal, 44(9), 1164–1175. doi:10.1134/S0031030110090029
- Ushatinskaya, G. T. 2016. Protegulum and brephic shell of the earliest organophosphatic brachiopods. Paleontological Journal, 50(2), 141–152. doi:10.1134/S0031030116020088
- Ushatinskaya, G. T. & Holmer, L. E. 2001. Brachiopoda. Pp. 120–132 in Alexander, E. M., J. B. Jago, A. Y. Rozanov & A. Y. Zhuravlev (eds) The Cambrian biostratigraphy of the Stansbury Basin, South Australia. Transactions, Palaeontological Institute, Moscow.
- Ushatinskaya, G. T. & Korovnikov, I. V. 2014. Revision of the early-middle Cambrian Lingulida (Brachiopoda) from the Siberian Platform. Paleontological Journal, 48(1), 26–40. doi:10.1134/S0031030114010158
- Ushatinskaya, G. T. & Korovnikov, I. V. 2019. Revision of the early and middle Cambrian Acrotretids (Brachiopoda, Linguliformea) from the Siberian Platform. Paleontological Journal, 53(7), 689–714.
- Wang, H., Zhang, Z., Holmer, L. E., Hu, S., Wang, X. & Li, G. X. 2012. Peduncular attached secondary tiering acrotretoid brachiopods from the Chengjiang fauna: Implications for the ecological expansion of brachiopods during the Cambrian explosion. Palaeogeography, Palaeoclimatology, Palaeoecology, 323, 60–67. doi:10.1016/j.palaeo.2012.01.027
- Webster, M. & Zelditch, M. L. 2005. Evolutionary modifications of ontogeny: heterochrony and beyond. Paleobiology, 31(3), 354–372. doi:10.1666/0094-8373(2005)031[0354:EMOOHA]2.0.CO;2
- Williams, A. & Cusack, M. 2007. Chemicostructural diversity of the brachiopod shell. Pp. 2396–2521 in P. A. Selden (ed.) Treatise on invertebrate paleontology, Part H, Brachiopoda (revised) volume 6. Geological Society of America, Boulder and University of Kansas, Lawrence.
- Williams, A. & Holmer, L. E. 1992. Ornamentation and shell structure of acrotretoid brachiopods. Palaeontology, 35(3), 657–692. doi:10.1111/j.0031-0239.2004.00404.x
- Williams, A., Carlson, S. J., Brunton, C. H. C., Holmer, L. E. & Popov, L. E. 1996. A supra‐ordinal classification of the Brachiopoda. Philosophical Transactions of the Royal Society B, 351, 1171–1193. doi:10.1098/rstb.1996.0101
- Williams, A. Brunton, C. H. C. & Mackinnon, D. I. 2000. Morphology. Pp. 321–440 in R. L. Kaesler (ed) Treatise on invertebrate paleontology, part H, Brachiopoda (revised) volume 2. Geological Society of America, Boulder and University of Kansas, Lawrence.
- Yang, B., Steiner, M. & Keupp, H. 2015. Early Cambrian palaeobiogeography of the Zhenba–Fangxian Block (South China): Independent terrane or part of the Yangtze Platform? Gondwana Research, 28(4), 1543–1565. doi:10.1016/j.gr.2014.09.020
- Zhang, Z.-F., Robson, S. P., Emig, C. & Shu, D. G. 2008. Early Cambrian radiation of brachiopods: a perspective from South China. Gondwana Research, 14, 241–254. doi:10.1016/j.gr.2007.08.001
- Zhang, Z.-F., Zhang, Z. L., Li, G. X. & Holmer, L. E. 2016. The Cambrian brachiopod fauna from the first-trilobite age Shuijingtuo Formation in the Three Gorges area of China. Palaeoworld, 25(3), 333–355. doi:10.1016/j.palwor.2015.10.001
- Zhang, Z.-L. 2018. Early Cambrian phosphatic-shelled brachiopods from South China. PhD dissertation, Northwest University. [in Chinese with English abstract].
- Zhang, Z.-L., Zhang, Z. F. & Wang, H. Z. 2016. Epithelial cell moulds preserved in the earliest acrotretid brachiopods from the Cambrian (Series 2) of the Three Gorges area, China. GFF, 138(4), 455–466. doi:10.1080/11035897.2016.1143528
- Zhang, Z.-L., Zhang, Z. F. & Holmer, L. E. 2017. Studies on the shell ultrastructure and ontogeny of the oldest acrotretid brachiopods from South China. Acta Palaeontologica Sinica, 56(4), 483–503. [in Chinese with English abstract])
- Zhang, Z.-L., Zhang, Z. F., Holmer, L. E. & Chen, F. Y. 2018a. Post‐metamorphic allometry in the earliest acrotretoid brachiopods from the lower Cambrian (Series 2) of South China, and its implications. Palaeontology, 61(2), 183–207. doi:10.1111/pala.12333
- Zhang, Z.-L., Popov, L. E., Holmer, L. E. & Zhang, Z. F. 2018b. Earliest ontogeny of early Cambrian acrotretoid brachiopods- first evidence for metamorphosis and its implications. BMC Evolutionary Biology, 18(1), 42. doi:10.1186/s12862-018-1165-6
- Zhang, Z.-L., Skovsted, C. B. & Zhang, Z. F. 2018c. A hyolithid without helens preserving the oldest hyolith muscle scars; palaeobiology of Paramicrocornus from the Shujingtuo Formation (Cambrian Series 2) of South China. Palaeogeography, Palaeoclimatology, Palaeoecology, 489, 1–14. doi:10.1016/j.palaeo.2017.07.021
- Zhang, Z.-L., Chen, F. Y. & Zhang, Z. F. 2020. Earliest phosphatic-shelled brachiopods from the carbonates of South China—their diversification, ontogeny, and distribution. Earth Science Frontiers, 27(6), 127–151. doi:10.13745/j.esf.sf.2020.6.4
