Abstract
The phylogenetic relationships of the major lineages within Dinosauria have come under intense scrutiny in recent years. In 2017, a radical new hypothesis of early dinosaur evolution, the ‘Ornithoscelida hypothesis’, was proposed, prompting a flurry of work in this area. However, instead of untangling the phylogenetic tree of dinosaurs as hoped, this further research unravelled the scientific consensus on dinosaur origins and their early relationships. Multiple hypotheses have now been proposed, including several that position the ‘traditionally’ non-dinosaurian silesaurids within Dinosauria. There is no sign of an emerging consensus, with all possible combinations of the three major dinosaur clades (Ornithischia, Theropoda and Sauropodomorpha) having been recovered by recent phylogenetic analyses. The existence of several ‘wildcard taxa’ and clades with uncertain affinities around the base of Dinosauria complicates efforts to differentiate these hypotheses. Recent studies have suggested that the data sets used to investigate the phylogenetic relationships of Dinosauria might be fatally flawed. The construction of new data sets with a stronger focus on the logical underpinning of characters, in addition to the inclusion of newly described or redescribed taxa, will likely hold the key to resolving this debate.
Introduction
Dinosaurs (including their living representatives, birds) are one of the most familiar and charismatic groups of animals, and their fossil record has been the subject of intense scrutiny (e.g. Agnolin et al., Citation2019; Barrett et al., Citation2009; Butler et al., Citation2010; Cashmore & Butler, Citation2019; Mannion & Upchurch, Citation2010; Upchurch et al., Citation2002, Citation2011). Despite this, many aspects of the clade’s early evolutionary history remain shrouded in mystery, due to a paucity of Middle and Late Triassic fossil sites, the rarity of early dinosaur fossils, and disagreements over the relationships of many early-occurring taxa (e.g. Brusatte et al., Citation2010d; Langer et al., Citation2010, Citation2013; Nesbitt, Citation2011; Norman et al., Citation2022). Although there has been general agreement regarding dinosaur monophyly and the interrelationships of the major dinosaur lineages since the dawn of the cladistic era in the 1980s (Bakker & Galton, Citation1974; Gauthier, Citation1986; Novas, Citation1996), recent years have witnessed a proliferation of new phylogenetic tree topologies for the clade that have challenged the ‘traditional’ consensus (Baron et al., Citation2017a; Cabreira et al., Citation2016; Černý & Simonoff, Citation2023; Müller & Garcia, Citation2020; Parry et al., Citation2017). Establishing the interrelationships of the major dinosaur lineages is central to understanding the clade’s early evolutionary history because a robust and stable phylogenetic tree provides a framework for macroevolutionary analyses, including those on evolutionary rates (Brusatte et al., Citation2010b, Citation2014), diversity and disparity (e.g. Barrett et al., Citation2009; Brusatte et al., Citation2008; Butler et al., Citation2010; Upchurch et al., Citation2011), body size (e.g. Benson et al., Citation2014, Citation2018) and biogeography (e.g. Dunne et al., Citation2023; Lee et al., Citation2019; Marsola et al., Citation2019), among many others, in addition to forming the basis for understanding character evolution in the clade (Nesbitt et al., Citation2009). The aim of the current paper is not just to summarize the current state of the debate but also to synthesize existing critiques of previous studies on dinosaur interrelationships. Our goal is to evaluate the key barriers preventing a consensus on early dinosaur phylogeny, and to identify the lines of research that are likely to overcome these barriers.
Institutional abbreviations
CPBA, Cátedra de Paleontologıá de la Facultad de Ciencias Exactas de la Universidad de Buenos Aires, Argentina; NHMUK, Natural History Museum, London, United Kingdom; PVL, Colección de Paleontologia de Vertebrados de la Fundación Instituto Miguel Lillo, Tucumán, Argentina; SAM-PK, Iziko South African Museum, Cape Town, South Africa; UFSM, Laboratório de Estratigrafia e Paleobiologia, Universidade Federal de Santa Maria, Brazil.
A brief history of dinosaur classification
Dinosauria was erected in 1842 for an advanced suborder of reptiles, containing the genera Megalosaurus, Iguanodon and Hylaeosaurus (Owen, Citation1842). Owen’s proposal echoed that of von Meyer (Citation1832), who had previously grouped Megalosaurus and Iguanodon into his ‘Pachypoda’ on the basis of similarities in their limb anatomy, although von Meyer did not elaborate further. Owen (Citation1842, p. 103) mentioned von Meyer’s proposal in a footnote, noted the absence of detail in the latter’s publication, and went on to list numerous characters that he regarded as unique to dinosaurs, which he considered to be a ‘… distinct tribe, or suborder …’ (Owen, Citation1842, p. 103), based on their large body size and features of the sacrum, ribs and hind limb. Hence, Owen (Citation1842) is almost universally credited with recognising this group. However, it is unclear whether Owen considered the founding members of Dinosauria to be particularly closely related. Although he included them within the same order, which might imply that he regarded them as a ‘natural’ group, Owen noted that Megalosaurus was more similar to crocodiles in some features and that Hylaeosaurus and Iguanodon were more like lizards in others (Owen, Citation1842, pp. 111, 120).
Subsequently, the recognition of many more dinosaur taxa during the mid-nineteenth century led to discussions over their classification, with the proposal of numerous subgroups or orders (e.g. Cope, Citation1866, Citation1867, Citation1869, Citation1883; Huxley, Citation1870; Marsh, Citation1882). Cope (Citation1869) divided dinosaurs into three major groups, which were distinguished primarily by features of the tibia and tarsus, though other characters of the dentition and ilium were also considered: Orthopoda (consisting of Hadrosauridae, Iguanodontidae and Scelidosauridae); Goniopoda (containing Megalosaurus, Poikilopleuron, ‘Laelaps’ and ‘Coelosaurus’); and Symphypoda (Compsognathus and ‘Ornithotarsus’ only). Huxley (Citation1870) dismissed Cope’s classification, citing conflicting patterns of tarsal fusion, and proposed a different model, resulting in another tripartite subdivision. In Huxley’s (Citation1870) scheme, Dinosauria was divided into Megalosauridae (equivalent to Goniopoda), Scelidosauridae and Iguanodontidae (formerly united in Orthopoda). Compsognathus longipes was considered closely related to dinosaurs, but not referable to any of its three component groups, and he proposed the name Ornithoscelida for Dinosauria plus Coelophysis (Huxley, Citation1870). Interestingly, Euskelosaurus, Thecodontosaurus and Cetiosaurus, which would later be placed in Sauropodomorpha (von Huene, Citation1932), were initially distributed among these three groups, being placed in Megalosauridae, Scelidosauridae and Iguanodontiae, respectively (Huxley, Citation1870). Adding further to the debate, Marsh (Citation1877, Citation1878, Citation1881, Citation1882) erected a plethora of dinosaurian orders and families based on the new taxa being described from the western USA, thereby providing many of the names in current use. He elevated Dinosauria to a subclass containing five orders (Marsh, Citation1882): Sauropoda, Stegosauria, Ornithopoda, Theropoda and Hallopoda (for Hallopus, now regarded as a crocodylomorph: Walker, Citation1970). Most of these orders were similar in taxonomic content to those proposed by Cope and Huxley, with the notable exception of Sauropoda, which was recognised as a formal group for the first time (Marsh, Citation1878). Following this work, Cope (Citation1883) emended his original classification to four subdivisions: Orthopoda (Ornithopoda plus Stegosauria), Goniopoda (= Theropoda), Opisthocoelia (= Sauropoda) and Hallopoda.
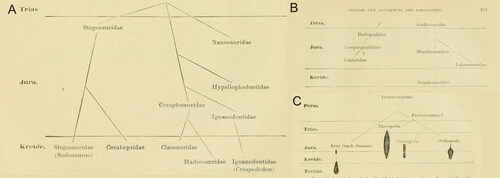
Entering this debate, Seeley (Citation1888a, Citation1888b) proposed instead that dinosaurs should be classified into two distinct orders: the ‘lizard-hipped’ Saurischia and the ’bird-hipped’ Ornithischia (see for a summary of the nomenclature prior to 1888). In addition to the most frequently cited eponymous differences in pelvic morphology, Seeley distinguished ornithischians from saurischians on the basis of several other features: the presence of vertebral pneumaticity in saurischians; differences in basicranial morphology; and the frequent possession of osteoderms in ornithischians (although noting that they may be ‘so reduced as to be unrecognisable’ in the latter: Seeley, Citation1888a, p. 171). Theropoda and Sauropoda were included within Saurischia; Ornithopoda and Stegosauria were included in Ornithischia. This bipartite division was adopted by other dinosaur workers and quickly became the consensus view of higher-level dinosaur classification for much of the twentieth century (e.g. Charig, Citation1982; von Huene, Citation1914, Citation1932; Romer, Citation1945; Steel, Citation1969, Citation1970; Weishampel et al., Citation1990, Citation2004), although Nopcsa (Citation1901) expressed a minority opinion that sauropods and ornithischians formed a group separate from theropods (and also, incidentally, produced the first branching diagrams of dinosaur relationships, see ). Palaeontologists continued to discuss the exact composition of the various dinosaur families throughout the twentieth century, with Triassic taxa the subject of particularly intensive debate, but the basic saurischian/ornithischian dichotomy was retained, with the only substantial modification occurring when sauropodomorphs (sauropods plus ‘prosauropods’) were recognised as a distinct group that was part of Saurischia (Charig et al., Citation1965; von Huene, Citation1932). For most of the twentieth century, it was thought that the two dinosaur orders were descended from different ‘thecodont’ ancestors (= polyphyletic, in cladistic parlance) (e.g. Charig, Citation1976; von Huene, Citation1914; Romer, Citation1945; Seeley, Citation1888a; Thulborn, Citation1975). However, the advent of cladistic methodology in the 1960s–1970s led some palaeontologists to propose new classifications based on patterns of common ancestry rather than phenetic resemblance. Bakker and Galton (Citation1974) were the first to propose that dinosaurs were monophyletic, itself a revolutionary idea (see Charig, Citation1976; Thulborn, Citation1975), but this study also identified various features (related primarily to herbivory) that seemed to undermine the ‘traditional’ dichotomy, leading to the suggestion that sauropodomorphs were allied with ornithischians, rather than theropods, breaking Saurischia apart (see also Bonaparte, Citation1976; Nopcsa, Citation1901). This hypothesis, with its ornithischian/sauropodomorph clade that was later dubbed Phytodinosauria (Bakker, Citation1986), was developed further by Paul (Citation1984) who suggested that ‘segnosaurs’ (= therizinosaurs) bridged the morphological gap between sauropodomorphs and ornithischians. Cooper (Citation1985) also proposed a sister-group relationship between sauropodomorphs (termed Pachypodosauria) and ornithischians, which he united in Ornithischiformes. However, the ‘phytodinosaur’ hypothesis was rejected by most of the phylogenetic analyses carried out in the 1980s–2010s, not least as the discovery of new, well-preserved therizinosaur material showed that these animals were deeply nested within Theropoda (e.g. Clark et al., Citation1994; Xu et al., Citation1999). The application of formal cladistic analyses to palaeontology during the 1980s–1990s confirmed dinosaur monophyly, and the fundamental basal divergence into monophyletic ornithischian/saurischian lineages remained the accepted view of dinosaur relationships (e.g. Gauthier, Citation1986; Novas, Citation1996). Some studies even tested the internal relationships of the two clades separately, based on the assumption of monophyly (e.g. Gauthier, Citation1986; Maryanska & Osmólska, Citation1985; Norman, Citation1984; Sereno, Citation1984, Citation1986).
Figure 1. The earliest attempts at visualizing dinosaur relationships using a branching tree from Nopcsa (Citation1901). A, Nopcsa’s reconstruction of the internal relationships of Ornithischia; B, Nopcsa’s reconstruction of the internal relationships of Theropoda; and C, Nopsca’s reconstruction of the phylogenetic relationships of dinosaurs and birds.
Subsequent phylogenetic studies of early dinosaurs, and archosaurs more broadly, continued to support (or assume) the Ornithischia/Saurischia basal dichotomy (e.g. Langer & Benton, Citation2006; Langer et al., Citation2010; Nesbitt et al., Citation2009; Sues et al., Citation2011; Yates, Citation2003). However, these studies often used only a single outgroup and rarely evaluated the statistical support for the relationships recovered (Langer & Benton, Citation2006; Nesbitt et al., Citation2009). The taxonomic sampling of these data sets was also problematic, being heavily dominated by saurischians, with ornithischians represented by only one or two early-branching taxa (usually Lesothosaurus and/or Pisanosaurus) (Nesbitt et al., Citation2009; Sues et al., Citation2011) or a supraspecific ‘Ornithischia’ operational unit (Langer & Benton, Citation2006; Yates, Citation2003). Many of the ornithischian characters coded into these analyses were either dinosaur symplesiomorphies or putative synapomorphies supporting ornithischian monophyly (Baron et al., Citation2017a; Langer et al., Citation2010; Nesbitt et al., Citation2009; Padian, Citation2012). Using a supraspecific ‘Ornithischia’ operational taxonomic unit (OTU) is problematic for other reasons also, as it relies on assumptions about ornithischian monophyly and character polarity that may be incorrect (see Prendini, Citation2001 for a discussion of these issues). As a result, these studies made several untested assumptions about character state evolution in early dinosaurs and ignored the possibility that characters shared between ornithischians and theropods might be homologous, such as the theropod-like manus (hand) morphology of the ornithischian Heterodontosaurus (Baron et al., Citation2017a; Galton, Citation2014; Norman et al., Citation2011). These issues heavily biased these studies towards recovering the Saurischia-Ornithischia status quo.
The modern Ornithoscelida hypothesis and its aftermath
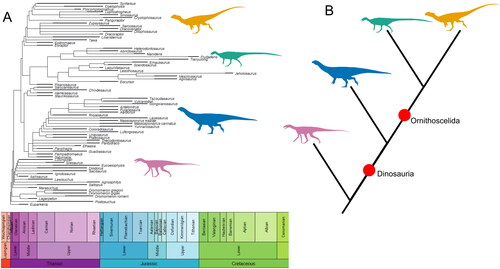
In 2017, a new hypothesis of dinosaur evolution proposed a tree topology that conflicted with the long-established saurischian/ornithischian dichotomy (Baron et al., Citation2017a). Theropoda was recovered outside Saurischia, so that the latter clade, although monophyletic, was composed solely of Sauropodomorpha + Herrerasauridae. In turn, this more restricted version of Saurischia was proposed as the sister group of a new clade, composed of Theropoda + Ornithischia, for which the name Ornithoscelida Huxley, Citation1870 was resurrected. This new topology (see ), termed the ‘Ornithoscelida hypothesis’ hereafter, was recovered using the largest early dinosaur data set then available, which contained 457 characters and 75 taxa, including 14 ornithischians, providing the first large sample of that clade in such a data set, and a range of outgroups (Baron et al., Citation2017a). Ornithoscelida was well supported by this analysis with a Bremer support value of 4, as were Ornithischia (Bremer support 4), and Theropoda, Sauropodomorpha, and Herrerasauridae (Bremer supports of 3). More poorly supported clades included Saurischia (Bremer support 2), a Dinosauria + Silesauridae clade (Bremer support 2), and Dinosauria itself (Bremer support 1). However, the low support recovered for Dinosauria and Saurischia was found to be caused by a small number of fragmentary early-branching taxa: when these taxa were removed from the data set, these clades were better supported (Bremer support values of 3 and 4, respectively) (Baron et al., Citation2017a). Nevertheless, several ‘wildcard’ taxa continue to frustrate attempts to build consensus around early dinosaur relationships. A good example is Agnosphitys cromhallensis, from the Late Triassic of the UK, which has been variously identified as a non-dinosaurian dinosauriform (Fraser et al., Citation2002), a theropod (Yates, Citation2007a), the earliest-diverging sauropodomorph (Ezcurra, Citation2010), and most recently a silesaurid (Baron et al., Citation2017a).
Figure 2. The ’Ornithoscelida’ hypothesis represented as both a time-scaled phylogeny based on the tree from Baron et al. (Citation2017a) (A) and as a simplified topology highlighting the relationships between major clades (B). Major clades represented by silhouettes are: Silesauridae (pink), Sauropodomorpha (blue), Ornithischia (green), and Theropoda (orange). Silhouettes sourced from phylopic.org: Asilisaurus kongwe by Scott Hartman attribution 3.0 unported edited (pink), Riojasaurus incertus by Tasman Dixon CC0 1.0 license (blue), Scutellosaurus lawleri by Scott Hartman public domain mark 1.0 (green), and Dilophosaurus wetherilli by Tasman Dixon CC0 1.0 license (orange). Time-scaled phylogeny generated using the R package strap (Bell & Lloyd, Citation2015).
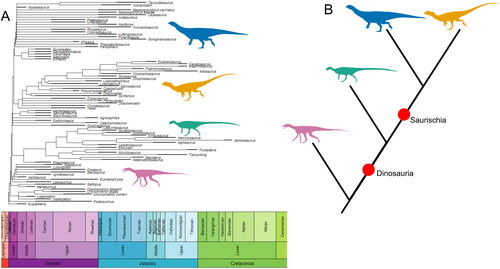
The Ornithoscelida hypothesis kickstarted a fierce scientific debate over the phylogenetic relationships within Dinosauria. Although the Baron et al. (Citation2017a) data set was lauded for the breadth of taxa sampled and the wealth of characters coded, its conclusions were challenged and have not been widely accepted (e.g. Goloboff & Sereno, Citation2021; Langer et al., Citation2017). A derivative study used an expanded data set, with nine additional taxa, and extensive character state re-scorings, with c. 2500 character states (∼10%) of the original data set rescored and 68 out of the 75 taxa at least partially rescored (Langer et al., Citation2017). These extensive re-scorings led this study to recover the ‘traditional’ Ornithischia/Saurischia dichotomy (see ) (Langer et al., Citation2017). The Langer et al. study also proposed that the synapomorphies of Ornithoscelida identified by Baron et al. (Citation2017a) were better optimized as a mixture of dinosaurian symplesiomorphies, character states acquired convergently by ornithischians and theropods, and character states with more complex distributions (Langer et al., Citation2017). In turn, the character re-scorings of Langer et al. were challenged by the original authors of the data set (Baron et al., Citation2017b). Moreover, when Templeton tests were applied to the two competing topologies, it was found that there was no statistical difference between support for the Ornithoscelida and ‘traditional’ hypotheses, with the latter being only two steps shorter than the Ornithoscelida topology (Langer et al., Citation2017). In addition, the Phytodinosauria hypothesis (Bakker, Citation1986; Cooper, Citation1985) was recovered as only four steps less parsimonious than the ‘traditional’ tree (Langer et al., Citation2017). As all possible arrangements of the three major dinosaur clades are almost equally parsimonious this demonstrates that the early evolutionary history of the group remains unsettled and should be regarded as an open question.
Figure 3. The ’traditional’ hypothesis represented as both a time-scaled phylogeny based on the tree from Langer et al. (Citation2017) (A) and as a simplified topology highlighting the relationships between major clades (B). Major clades represented by silhouettes are: Silesauridae (pink), Sauropodomorpha (blue), Ornithischia (green), and Theropoda (orange). Silhouettes sourced from phylopic.org: Asilisaurus kongwe by Scott Hartman attribution 3.0 unported edited (pink), Riojasaurus incertus by Tasman Dixon CC0 1.0 license (blue), Scutellosaurus lawleri by Scott Hartman public domain mark 1.0 (green), and Dilophosaurus wetherilli by Tasman Dixon CC0 1.0 license (orange). Time-scaled phylogeny generated using the R package strap (Bell & Lloyd, Citation2015).
All the aforementioned analyses were conducted using maximum parsimony, but this is not the only method for generating phylogenetic trees from morphological data, and alternative Bayesian analyses were also used to test the Ornithoscelida hypothesis (Parry et al., Citation2017). Bayesian methods consistently recovered a monophyletic Ornithoscelida, even when using the heavily modified Langer et al. (Citation2017) data set that supported the ‘traditional’ hypothesis under maximum parsimony (Parry et al., Citation2017). Once again, a small number of enigmatic ‘wildcard’ taxa were placed in unexpected locations in the trees generated. In particular, the dinosauriform Saltopus elginensis, usually considered to be earlier branching (with respect to dinosaurs) than silesaurids (Benton & Walker, Citation2010; Langer et al., Citation2013; Nesbitt, Citation2011), was recovered as the sister taxon of Dinosauria (Parry et al., Citation2017). Although this unexpected result was poorly supported, it highlights how the uncertainties surrounding early dinosaur relationships extend to other early-branching avemetatarsalians. The Parry et al. (Citation2017) study also tested the effects of partitioning character data by both skeletal region (e.g. cranial, postcranial) and origin (e.g. a novel character vs a character from a previous study). Anatomical partitioning was conducted because it has been shown that craniodental characters can recover tree topologies that differ from those produced by postcranial characters, and that dental characters may be less phylogenetically informative than other osteological characters (Mounce et al., Citation2016; Sansom et al., Citation2017). Moreover, partitioning the data into novel vs legacy characters can demonstrate the impact of the former on the recovered topology (Parry et al., Citation2017). However, no matter which partitions were excluded or included, the fundamental ornithoscelidan topology was recovered in each case, although individual partitions failed to completely resolve the majority of nodes or clades (Parry et al., Citation2017). These results suggest that support for the ‘Ornithoscelida hypothesis' is based on character states that are distributed across the entire skeleton, but that this signal is not strong enough within individual partitions to resolve the phylogeny. Although the novel characters included in the analyses by Baron et al. (Citation2017a) were not needed to recover Ornithoscelida, they were found to increase resolution within Dinosauromorpha (Parry et al., Citation2017). These findings suggest that more extensive character sampling from across the entire skeleton and the development of novel characters will be key tools in the quest to unravel the early evolutionary history of dinosaurs.
The herrerasaurid question
One aspect of early dinosaur evolution that warrants detailed consideration is the position of the clade Herrerasauridae. Gnathovorax cabreirai from the Carnian-aged Santa Maria Formation of Brazil can be considered an archetypal herrerasaurid, a 2–3 m long bipedal carnivore with recurved, serrated teeth and well-developed clawed hands (Pacheco et al., Citation2019). The clade’s eponym, Herrerasaurus ischigualastensis, reached lengths of ∼6 m and was one of the largest, most abundant predators within its ecosystem (Rogers et al., Citation1993; Sereno & Novas, Citation1992). Herrerasaurids represent Dinosauria’s first invasion of the large predator niche, which theropods occupied for the rest of the Mesozoic. Many fragmentary and/or problematic taxa have been assigned to Herrerasauridae and, if taken at face value, these might suggest that the clade had a near-global distribution and a temporal range that extended into the Norian (Baron & Williams, Citation2018; Cau, Citation2018; Griffin et al., Citation2022; Niedźwiedzki et al., Citation2014; Novas et al., Citation2021). The clade Herrerasauridae is defined as ‘Herrerasaurus, Staurikosaurus, their most recent common ancestor plus all of its descendants’ (Langer et al., Citation2010, p. 66); however, it is possible that some of the problematic taxa referred to above actually fall outside Herrerasauridae sensu stricto, in which case it would be more appropriate to refer to the stem-based (sometimes referred to as branch-based) clade Herrerasauria, ‘All dinosaurs that share a more recent common ancestor with Herrerasaurus than with Liliensternus and Plateosaurus’ (Langer et al., Citation2010, p. 66), as proposed by Baron and Williams (Citation2018).
Herrerasaurids are unusual among early dinosaurs in being recovered frequently as a clade rather than as successive outgroups to other dinosaur lineages (e.g. Alcober & Martínez, Citation2010; Baron & Williams, Citation2018; Pacheco et al., Citation2019), although the synapomorphies uniting this clade have varied drastically between analyses (Alcober & Martínez, Citation2010; Garcia et al., Citation2021; Langer & Benton, Citation2006; Novas, Citation1994). The phylogenetic position of herrerasaurids is, therefore, a key part of the puzzle when considering dinosaur intrarelationships (Baron & Williams, Citation2018). Unfortunately, the phylogenetic affinities of herrerasaurids have proved exceptionally problematic, with the clade being recovered as the sister group to Dinosauria (e.g. Baron & Williams, Citation2018; Fraser et al., Citation2002), as early-diverging theropods (e.g. Ezcurra & Novas, Citation2007; Sereno, Citation1999), as the sister group of Theropoda + Sauropodomorpha within Saurischia (e.g. Ezcurra, Citation2006; Langer & Benton, Citation2006), and in an unresolved relationship with both theropods and sauropodomorphs (Langer et al., Citation2017). Under the Ornithoscelida hypothesis, herrerasaurids remain in Saurischia as the sister clade of Sauropodomorpha alone (Baron et al., Citation2017a). The previous recovery of herrerasaurids as within, or closely related to, Theropoda was suggested to result from the convergent acquisition of hypercarnivory in the early evolution of both clades (Baron et al., Citation2017a). A variation on the Ornithoscelida hypothesis has also been proposed in which herrerasaurids are the sister group to Dinosauria, but with the unexpected inclusion of the enigmatic taxon Saltopus elginensis, from the Carnian of Scotland, in Herrerasauridae (Baron & Williams, Citation2018). This topology, if correct, would dramatically increase the potential geographical and size ranges of the clade’s members (Baron & Williams, Citation2018). With the description of the exceptionally complete Gnathovorax cabreirai (Pacheco et al., Citation2019) and the unnamed large but fragmentary UFSM 11330 from the Carnian of Brazil (‘large Saturnalia’: Garcia et al., Citation2021) a wealth of new information on herrerasaurid morphology has become available in recent years. Some of this suggests unusual character distributions across Avemetatarsalia: for example, Gnathovorax possesses a foramen on the ischium that is also found in the lagerpetid Ixalerpeton polesinensis (Garcia et al., Citation2021). Much of this new morphological data has yet to be incorporated into broader-scale studies of dinosaur interrelationships, despite its potential impact on the position of herrerasaurids within Dinosauria and on the position of other avemetatarsalian clades relative to Dinosauria.
Ornithischian silesaurids
One of the biggest mysteries surrounding the early evolution of dinosaurs is the cause of the ‘ornithischian gap’ or ‘ornithischian crisis’. These terms refer to a clear hiatus, in both time and morphology, between the earliest-known dinosaurs and the earliest-known ornithischians (e.g. Baron, Citation2019; Butler et al., Citation2007; Irmis et al., Citation2007b; Padian, Citation2012). A review of Late Triassic ornithischians found that out of 24 proposed occurrences (many of which were isolated teeth), only three could confidently be assigned to Ornithischia (Irmis et al., Citation2007b): Pisanosaurus mertii (PVL 2577); SAM-PK-K8025 (later described as Eocursor parvus: Butler et al., Citation2007); and CPBA-V-14091 an isolated heterodontosaurid maxilla from Argentina. However, the latter two specimens have since been re-dated to Early Jurassic in age, leaving P. mertii as the sole Late Triassic ornithischian (Olsen et al., Citation2010). Proposed explanations for this gap have included: misidentification of early ornithischians as members of different dinosauriform clades (Padian, Citation2012); exceptional rarity of ornithischians until their diversification in the Early Jurassic (Agnolin & Rozadilla, Citation2017; Butler et al., 2017); nesting of Ornithischia within another dinosaurian clade (Baron, Citation2019); or that the early evolution of ornithischians occurred in regions where the Late Triassic fossil record is poorly sampled or absent (Bordy et al., Citation2020). However, as the post-Ornithoscelida debate continued, a new hypothesis of early dinosaur relationships was proposed that would effectively close the ornithischian gap.
The most recent hypothesis of early dinosaur relationships focusses not on the three major dinosaur clades, but on a relatively recently discovered clade of dinosauriformes, the Silesauridae (Nesbitt et al., Citation2010). This clade was initially identified as the sister group to Dinosauria and described on the basis of five taxa: Silesaurus opolensis (Dzik, Citation2003), Asilisaurus kongwe (Nesbitt et al., Citation2010), Eucoelophysis baldwini (Ezcurra, Citation2006), Sacisaurus agudoensis (Ferigolo & Langer, Citation2007), and Lewisuchus admixtus/Pseudolagosuchus major (Agnolín et al., Citation2022; Ezcurra & Martinez, Citation2016; Ezcurra et al., Citation2020b) (NB, Pseudolagosuchus major is widely considered synonymous with Lewisuchus admixtus: e.g. Agnolín et al., Citation2022; Ezcurra et al., Citation2020a). The number of taxa assigned to the clade has increased rapidly in the past decade, based on newly discovered or re-identified specimens including Kwanasaurus williamparkeri and Amanasaurus nesbitti (Martz & Small, Citation2019; Müller & Garcia, Citation2023), resulting in as many as 12 named silesaurid taxa in some recent phylogenetic analyses (Mestriner et al., Citation2023; Müller & Garcia, Citation2020; Norman et al., Citation2022).
Silesaurids possessed a unique bauplan, involving a combination of gracile, elongate limbs, a quadrupedal stance, and ‘beaked’ lower jaws (Langer et al., Citation2013; Nesbitt et al., Citation2010). They appeared in Gondwanan faunas in the Middle Triassic and achieved a near-global distribution in the Late Triassic (Langer et al., Citation2013; Norman et al., Citation2022). The potentially Anisian age of Asilisaurus kongwe and Lutungutali sitwensis would make them (along with the coeval potential dinosaur Nyasasaurus) the earliest known dinosauriforms (Nesbitt et al., Citation2010, Citation2013; Peecook et al., Citation2013), although recent dating studies suggest they might be Carnian in age (Marsicano et al., Citation2016; Peecook et al., Citation2017). Silesaurids are interesting for their ecological variability, possibly having undergone a transition from carnivory to herbivory/omnivory (Barrett et al., Citation2010; Kubo & Kubo, Citation2014; Nesbitt et al., Citation2010), and for their anatomical novelty, with some possessing a hooked mandibular beak that might be homologous with the predentary bone, whose recognition led to the initial suggestion that they might have been ornithischians (Ferigolo & Langer, Citation2007). These dietary and anatomical transitions are similar to those predicted to occur in the earliest ornithischians, and the temporal ranges of most silesaurid taxa (Carnian–Norian) are close to the predicted age of the most recent common ancestor of ornithischians (Boyd, Citation2015; Irmis et al., Citation2007b). Two putative Late Triassic ornithischians, Pisanosaurus mertii and Technosaurus smalli, have recently been re-classified as silesaurids by some authors (Agnolin & Rozadilla, Citation2017; Martz et al., Citation2012), emphasizing the similarities between these clades.
Pisanosaurus mertii has long been considered crucial to understanding early dinosaur evolution (Bonaparte, Citation1976; Irmis et al., Citation2007b; Norman et al., Citation2004), so its proposed referral to Silesauridae is highly significant. Pisanosaurus mertii was initially described as the oldest known ornithischian based on PVL 2577, a single articulated (but incomplete) specimen that was extensively weathered prior to discovery (Bonaparte, Citation1976; Casamiquela, Citation1967). Crucially, the pelvis is preserved largely as natural moulds which are difficult to interpret, leading to much debate, not just over its morphology but also regarding which surface of the pelvis (medial or lateral) is actually preserved (Agnolin & Rozadilla, Citation2017; Bonaparte, Citation1976). Furthermore, the combination of an unusually derived dental morphology and a plesiomorphic ‘non-dinosaurian’ hind limb in P. mertii has led to the suggestion that the material is chimeric (Norman et al., Citation2004, Citation2011; Sereno, Citation1991), although this hypothesis is at odds with the holotype’s articulated state as described and figured in the original description (Casamiquela, Citation1967). As noted above, Pisanosaurus mertii was recently considered not only the world’s oldest ornithischian but also the only Triassic one (Olsen et al., Citation2010). Unexpectedly, P. mertii was recovered as a silesaurid during the debate over early dinosaur relationships (Baron et al., Citation2017b). At approximately the same time, a thorough re-description of the available material was published (Agnolin & Rozadilla, Citation2017) that identified several synapomorphies of Silesauridae, a clade that was unknown at the time of P. mertii’s initial description (Bonaparte, Citation1976; Casamiquela, Citation1967). This new placement of Pisanosaurus was also recovered by a subsequent study that noted that this reinforced the ‘ornithischian gap’: as silesaurids were initially regarded as non-dinosaurs (e.g. Nesbitt, Citation2011), the removal of Pisanosaurus from Ornithischia had the consequence of removing all definitive ornithischians from the record until the Early Jurassic (Baron, Citation2019). Nevertheless, it should be noted that this hypothesis has not been universally accepted, with some authors maintaining that the silesaurid features seen in P. mertii are plesiomorphic for dinosauriformes (Desojo et al., Citation2020). However, subsequent work has suggested that Pisanosaurus, and silesaurids more generally, might reduce or even eliminate the ‘ornithischian gap’.
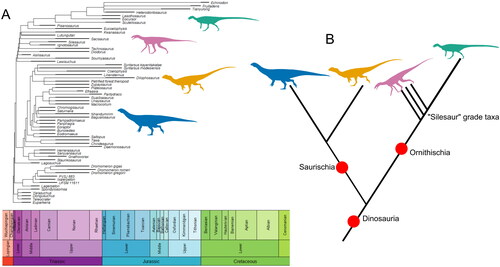
The idea that silesaurids were early ornithischians, rather than non-dinosaur dinosauriforms, was proposed before Silesauridae had been formally defined (Ferigolo & Langer, Citation2007). This ‘ornithischians as silesaurids’ hypothesis was based purely on anatomical similarities rather than a phylogenetic analysis (Ferigolo & Langer, Citation2007). Most of the formative phylogenetic analyses on silesaurid relationships placed them as the sister group to dinosaurs (Baron et al., Citation2017a; Brusatte et al., Citation2010a; Nesbitt, Citation2011; Nesbitt et al., Citation2010). However, other topologies have been recovered in which silesaurids were split, with some taxa forming a small clade at the base of Ornithischia, while other taxa referred to the group were placed among a polytomy of non-dinosaurian dinosauriforms instead (Langer & Ferigolo, Citation2013). Initially, only three silesaurids (Sacisaurus agudoensis, Diodorus scytobrachion and Silesaurus opolensis) were placed within Ornithischia, but later analyses increased the number of taxa within this ‘ornithischian silesaurid’ clade (Cabreira et al., Citation2016; Pacheco et al., Citation2019). When the new interpretations of Pisanosaurus mertii and Technosaurus smalli were included alongside a wider sample of early-branching dinosauromorphs, a new variation of this topology was recovered, with the silesaurid clade collapsing into a paraphyletic grade at the base of Ornithischia (see ) (Müller & Garcia, Citation2020; Norman et al., Citation2022). These new topologies were accompanied by the resurrection of some historical taxonomic names to reflect clades recovered by the ornithischian silesaurid hypothesis – for example, Prionodontia for the clade uniting thyreophorans and neornithischians, which is equivalent to the clade Genasauria within ‘traditional’ Ornithischia (Norman et al., Citation2022).
Figure 4. The ’Ornithischian silesaur’ hypothesis represented as both a time-scaled phylogeny based on the tree from Müller and Garcia (Citation2020) (A) and as a simplified topology highlighting the relationships between major clades (B). Major groups represented by silhouettes are: silesaurids (recovered as a paraphyletic grade in this analysis) (pink), Sauropodomorpha (blue), Ornithischia (green), and Theropoda (orange). Silhouettes sourced from phylopic.org: Asilisaurus kongwe by Scott Hartman attribution 3.0 unported edited (pink), Riojasaurus incertus by Tasman Dixon CC0 1.0 license (blue), Scutellosaurus lawleri by Scott Hartman public domain mark 1.0 (green), and Dilophosaurus wetherilli by Tasman Dixon CC0 1.0 license (orange). Time-scaled phylogeny generated using the R package strap (Bell & Lloyd, Citation2015).
Other developments in Avemetatarsalia
Although the focus of this review is the debate around the internal relationships of Dinosauria, it is necessary to consider developments in our understanding of archosaur phylogeny as a whole. The emergence of the ‘ornithischian silesaurid’ hypothesis demonstrates that the early evolutionary history of Dinosauria cannot be studied without examining the phylogenetic relationships of other avemetatarsalian (‘bird-line’) archosaur clades (Brusatte et al., Citation2010a; Müller & Garcia, Citation2020; Nesbitt, Citation2011; Nesbitt et al., Citation2017a; Norman et al., Citation2022). If one were to examine Middle Triassic faunas, ignoring each clade’s later successes or failures, the earliest dinosaurs would be seen to represent just one lineage among a diverse radiation of avemetatarsalian groups (see ) (Padian, Citation2012). Our understanding of this radiation has expanded exponentially in recent years, with several new clades being discovered (Nesbitt et al., Citation2010, Citation2017a), and a clear division between ‘pterosaur line’ and ‘dinosaur line’ avemetatarsalians is starting to appear (see ) (Ezcurra et al., Citation2020a; Foffa et al., Citation2022). Our rapidly changing understanding of non-dinosaurian avemetarsalians potentially impacts the phylogenetic relationships within Dinosauria. Anatomical characteristics that were thought to be derived dinosaurian traits have now been found to have more complex distributions across Avemetatarsalia, challenging previous ideas of character polarity and of homoplasy vs synapomorphy (Müller et al., Citation2023; Nesbitt et al., Citation2017a).
Figure 5. A phylogenetic tree of Avemetatarsalia with Pseudosuchia as an outgroup. Based on the topology from Nesbitt et al. (Citation2023). Silhouettes sourced from phylopic.org: Asilisaurus kongwe by Scott Hartman attribution 3.0 unported edited (pink), Columba by Ferran Sayol CC0 1.0 (blue), Peteinosaurus zambellii by Tasman Dixon CC0 1.0 (yellow), Ixalerpeton polesinensis by Scott Hartman attribution 3.0 unported edited (orange), Teleocrater rhadinos by Scott Hartman attribution 3.0 unported edited (blue), and Alligator missisipensis by Ferran Sayol CC0 1.0 (green).
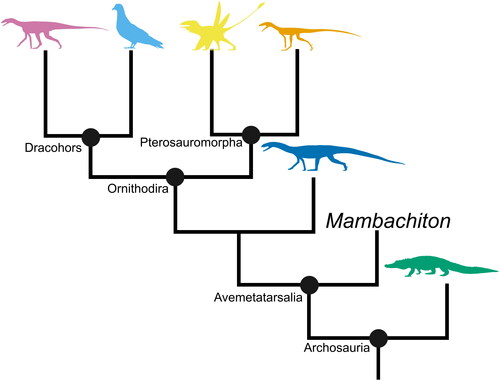
Several new clades of avemetatarsalians have been described in recent years, for example Aphanosauria (Nesbitt et al., Citation2017a). Members of Aphanosauria had been known for decades, but it was not until the formal description of NHMUK PV R6795 as the holotype of Teleocrater rhadinus that they were united in a monophyletic group positioned close to the root of Avemetatarsalia. The clade includes T. rhadinus as well as the fragmentary and problematic taxa Spondylosoma absconditum, Yarasuchus deccanensis and Dongosuchus efremovi (Galton, Citation2000; Nesbitt et al., Citation2017a, Citation2017b; Niedźwiedzki et al., Citation2016; Sen, Citation2005). These taxa were non-cursorial quadrupedal carnivores, a bauplan more typical of early-branching archosaurs or pseudosuchians, and the clade appears to have had a near-global distribution during the Middle Triassic, but to have disappeared by the Late Triassic (Nesbitt et al., Citation2017a; although these dates are debated, see above). The unusual anatomy of aphanosaurs combines a plesiomorphic, pseudosuchian-like body plan with anatomical features (such as a supratemporal fossa on the frontal) that had previously been considered dinosaur synapomorphies (Nesbitt et al., Citation2017a, Citation2017b). The status of aphanosaurs as the earliest-diverging avemetatarsalians was short lived, however, being usurped by Mambachiton fiandohana from Madagascar just five years later (Nesbitt et al., Citation2023). Mambachiton is notable not only for being the earliest-diverging avemetatarsalian (see ) but also for possessing an extensive array of dorsal osteoderms reminiscent of those seen in stem archosaurs and pseudosuchians (Nesbitt et al., Citation2023). The discovery of these early-diverging avemetatarsalians fundamentally changes our understanding of character evolution in bird-line archosaurs, challenging the assumption that avemetatarsalians were ancestrally cursorial and lacked osteoderms: this shifts the acquisition of these elements of the ancestral dinosaurian bauplan tipward in the phylogenetic tree (Nesbitt et al., Citation2017a, Citation2023). In addition to the discovery of new clades and taxa, the shifting phylogenetic positions of long-established clades within Avemetatarsalia can also impact our understanding of early dinosaur evolution. A good example of this is another major group of early avemetatarsalians, the lagerpetids. In recent years, lagerpetids have experienced a substantial increase in both the number of named taxa, e.g. Maehary bonapartei, Kongonaphon kely and Venetoraptor gassenae (Kammerer et al., Citation2020; Kellner et al., Citation2022; Müller et al., Citation2023), and the amount of described material (e.g. the first described braincase from the clade: Bronzati et al., Citation2023). Lagerpetids are small-bodied, cursorial ornithodirans known from Ladinian–Rhaetian-aged deposits from across Gondwana and Laurasia (Ezcurra et al., Citation2020a; Foffa et al., Citation2022; Langer et al., Citation2013). Renewed interest in the clade was sparked by their recovery as the sister group to pterosaurs (in the clade Pterosauromorpha) in some analyses (Müller et al., Citation2018; Ezcurra et al., Citation2020a; Foffa et al., Citation2022). This shifted lagerpetids from their long-established position as ‘dinosaur line’ avemetatarsalians (Baron et al., Citation2017a; Nesbitt, Citation2011; Nesbitt et al., Citation2017a; Padian, Citation2012; and references therein) to ‘pterosaur line’ avemetatarsalians, which has important implications for our understanding of character evolution around the origin of dinosaurs, and may in turn impact our understanding of higher-level dinosaur relationships. The increase in described material and taxa has helped settle several debates regarding lagerpetid anatomy but has also created new ones. For example, the locomotor style of lagerpetids had been reconstructed as bipedal (Irmis et al., Citation2007a; Kammerer et al., Citation2020; Sereno & Arcucci, Citation1994), quadrupedal (Demuth et al., Citation2023), facultatively bipedal (Foffa et al., Citation2022), and indeterminate (Pintore et al., Citation2022). The discovery of the first well-preserved lagerpetid manus, belonging to Venetoraptor gassenae, has confirmed that at least some lagerpetids were bipedal (Müller et al., Citation2023) and had well-developed grasping hands, a feature previously thought to have been acquired by the earliest dinosaurs and possibly giving them an evolutionary advantage over other Triassic archosaur groups (Baron et al., Citation2017a; Müller et al., Citation2023). The presence of grasping hands in this lagerpetid suggests that this feature may have had a deeper evolutionary origin within Avemetatarsalia than previously thought (Müller et al., Citation2023). Whether the grasping hands of lagerpetids are homologous with those of early dinosaurs or a case of convergence has yet to be tested, but it is clear that lagerpetids must be considered during discussions of early dinosaur evolution, even if the clade is the sister group to pterosaurs rather than dinosaurs.
The importance of re-evaluating named taxa
Several early dinosaur taxa that might provide vital phylogenetic information have been described in recent years, including the sauropodomorph Mbiresaurus raathi and the theropod Notatesseraeraptor frickensis (Griffin et al., Citation2022; Zahner & Brinkmann, Citation2019). It is easy to assume that palaeontological debates can only be answered by the discovery of new fossils like these. Increasingly, however, the importance of better character creation, character coding, and re-interpretations of key taxa is being recognised (Baron, Citation2022; Brazeau, Citation2011; Müller & Dias-da-Silva, Citation2019; Sereno, Citation2007). Several taxa that are key to the debates over early dinosaur evolution and avemetatarsalian interrelationships have recently received detailed re-descriptions, including the perennially problematic Scleromochlus taylori (Foffa et al., Citation2022) and the early-branching thyreophorans Scutellosaurus lawleri (Breeden et al., Citation2021) and Scelidosaurus harrisonii (the latter taking an extraordinary 160 years to receive a detailed anatomical description; Norman, Citation2020a, Citation2020b, Citation2020c). Scleromochlus taylori has been re-described twice recently, one proposing a radical re-interpretation of the taxon as a frog-like saltator (Bennett, Citation2020) and the other (based on extensive computed tomographic (CT) scanning) recovering it as the earliest-diverging lagerpetid (Foffa et al., Citation2022, Citation2023). The new interpretation of Scleromochlus taylori as an early lagerpetid provides an abundance of morphological reference data enabling important characters to be compared across Pterosauromorpha, improving the diagnostic power and optimization of these characters (Foffa et al., Citation2022, Citation2023).
A good example of the importance of re-examining and re-interpreting known early dinosaur taxa is Eoraptor lunensis, from the late Carnian–early Norian Ischigualasto Formation of Argentina, which was initially described as the earliest-branching theropod (Sereno et al., Citation1993). Subsequently, two schools of thought developed, with some studies continuing to recover Eoraptor as a theropod (Nesbitt et al., Citation2009; Tykoski, Citation2005), and others finding it to be an early-diverging saurischian (Brusatte et al., Citation2010d; Langer & Benton, Citation2006; Langer et al., Citation2010). The description of another Ischigualasto Formation dinosaur, Eodromaeus murphi, led to a drastic reinterpretation of E. lunensis as an early-branching sauropodomorph (Martinez et al., Citation2011). A detailed osteological description of E. lunensis, based on the now fully prepared holotype, as well as additional referred specimens, revealed key sauropodomorph synapomorphies, including a distinct ‘twisted’ proximal phalanx of manus digit 1 so that the tip of the ungual is turned inwards, cementing its position as an early sauropodomorph (Sereno et al., Citation2012). The changing phylogenetic position of E. lunensis demonstrates how even taxa known from exceptionally well-preserved specimens may need detailed re-interpretation and re-examination. The affinities of several important taxa remain heavily debated, such as Chilesaurus diegosuarezi, which is recovered as either an aberrant tetanuran theropod (Baron, Citation2022; Chimento et al., Citation2017; Novas et al., Citation2015), a very early-diverging ornithischian (Baron & Barrett, Citation2017, Citation2018; Müller & Dias-da-Silva, Citation2019), or a sauropodomorph (Müller et al., Citation2018): detailed re-examination and description of such taxa is an obvious avenue for future work.
An insoluble problem?
The collapse of the consensus around dinosaur origins ‘post-Ornithoscelida’ and the resulting proliferation of alternative hypotheses around broad-scale dinosaur relationships might lead to the suggestion that this is an intractable problem: the question – ‘How are the major clades of dinosaur related?’ – may be one without answer. The earliest divergences within Dinosauria might be a ‘hard polytomy’ of the type proposed (by some authors) to exist at the base of Neoaves (Suh, Citation2016). This might be the case if the diversification of the earliest dinosaurs was extremely rapid, as some have suggested during the radiation of Neoaves after the K–Pg boundary (Suh, Citation2016), making it impossible to tease apart the internal relationships of the clade. Even with extensive molecular data sets, one neoavian taxon in particular, the hoatzin (Opisthocomus hoazin), has proved exceptionally difficult to classify, with its phylogenetic affinities changing dramatically between analyses (Prum et al., Citation2015; Wang et al., Citation2022; Wu et al., Citation2024). If the hoatzin is considered a modern analogue for ‘wildcard’ dinosaur taxa like Chilesaurus diegosaurezi (Baron & Barrett, Citation2017, Citation2018; Novas et al., Citation2015), it could suggest that some taxa have relationships that are intrinsically difficult to resolve regardless of the quality of data (molecular or morphological) which is available. However, the initial radiation of dinosaurs appears to have been relatively slow. Fragmentary body fossils, trace fossils and phylogenetic inference suggest that the earliest dinosaurs had appeared by the Anisian (247.2–242 mya) but diverse dinosaur faunas are currently only seen for the first time in the fossil record in the late Carnian (∼227 mya) (Benton et al., Citation2014; Brusatte et al., Citation2010c; Marsicano et al., Citation2007; Nesbitt et al., Citation2013). Moreover, macroevolutionary studies have found that dinosaur diversity increased steadily throughout their early evolutionary history, and although there was a marked increase in disparity, this did not occur until the Carnian–Norian boundary (Brusatte et al., Citation2008). Current evidence suggests, therefore, that the rapid initial radiation needed to produce a hard polytomy at the base of Dinosauria did not occur. In fact, it may be misleading to think about the dinosaur origin as a radiation in its own right; rather, we should perhaps consider early dinosaurs as an integral part of the wider avemetatarsalian radiation (Padian, Citation2012).
If we reject the hypothesis that the interrelationships of the different dinosaur clades are a hard polytomy, an alternative explanation is needed to account for the continued lack of consensus around their relationships. Missing data is at least partially responsible for this continued instability. Some of these sources of missing data are impossible to overcome; for example, sadly there will never be a molecular tree of early dinosaur relationships, which is regrettable as this data source has proved critical in resolving other areas of vertebrate evolutionary history where competing morphological phylogenies have provided conflicting results – see O’Leary et al. (Citation2013) with respect to mammalian phylogeny and Rio and Mannion (Citation2021) for attempts to reconcile multiple morphological and molecular data sets relating to the relationships of gharial-like taxa within Crocodylia. The incompleteness of the fossil record is another source of missing data, as most early dinosaurs and their closest relatives are known from extremely fragmentary remains: for example, the putative earliest dinosaur Nyasasaurus parringtoni is known from only a partial humerus and a handful of vertebrae (Nesbitt et al., Citation2013), and the silesaurid Amanasaurus is based on the proximal and distal ends of a single femur (Müller & Garcia, Citation2023). We would expect the earliest-diverging members of the major dinosaurian clades to have been less morphologically distinct from each other than their later descendants: if this assumption is correct, this would make the accurate assignment of fragmentary taxa even more difficult. Missing anatomical information also makes it hard to distinguish between homology and homoplasy: for example, it is unclear whether the traits related to hypercarnivory in herrerasaurids are homologous with those in theropods or are analogous traits caused by the convergent adoption of similar diets (Baron et al., Citation2017a). A different form of missing data is caused by the uneven geographical and temporal sampling of relevant taxa, reflecting both the incomplete nature of the fossil record and anthropogenic biases. For example, recent discoveries in Africa highlight the importance of areas outside western Gondwana (South America) to our understanding of dinosaur and avemetatarsalian evolution (Griffin et al., Citation2022; Nesbitt et al., Citation2013; Peecook et al., Citation2013). For example, the archosaur fauna of the Tanzanian Manda Formation contains three key avemetatarsalian taxa: the earliest ?dinosaur Nyasasaurus; the most completely known aphanosaur, Teleocrater; and the silesaurid Asilisaurus (Nesbitt et al., Citation2013, Citation2017a, Citation2020). Another area that may be important to our understanding of early dinosaur evolution is India, with a pair of early-branching sauropodomorphs, Nambalia roychowdhurii and Jaklapallisaurus asymmetrica, and the early saurischian Alwalkeria maleriensis (although chimeric as originally described) already known from the subcontinent (Novas et al., Citation2010; Remes & Rauhut, Citation2005), although its Triassic fauna remains understudied compared to those from southern Africa or South America. It is easy to overemphasize the importance of new fossil discoveries when solving palaeontological problems, although there are many examples where the discovery of new taxa has proved pivotal in changing our understanding of dinosaur evolution (e.g. the dramatic impact of the feathered dinosaurs from the Jehol Biota on our understanding of bird origins: Xu et al., Citation2020). However, the importance of re-interpreting existing taxa has increasingly been recognised, especially using modern techniques such as CT scanning, as recently applied to Scleromochlus taylori (Foffa et al., Citation2022, Citation2023). Moreover, several studies have noted the positive impact of improved sampling, in terms of additional taxa and character codings for existing taxa, on phylogenetic tree accuracy, emphasizing the importance of both new and re-described existing taxa both in general (Koch & Parry, Citation2020; Pollock et al., Citation2002) and with specific reference to the problem of early dinosaur relationships (Baron et al., Citation2017a; Langer et al., Citation2017). However, paradoxically, in the case of dinosaur origins and establishing their early evolutionary relationships, an influx of new data seems to have resulted in the destruction of the existing consensus, and a lack of clarity, rather than enhancing support for the status quo. New discoveries have contributed to the erosion of the former consensus, as shown by the recent proposal of the ‘ornithischian silesaurid’ hypothesis (Müller & Garcia, Citation2020; Norman et al., Citation2022). This is especially puzzling as missing data is considered one of the main causes of the current lack of consensus on dinosaur relationships. Although these new taxa and clades have reduced the once wide morphological gulf between dinosaurs and non-dinosaurian archosaurs, this progress has also reduced the number of anatomical characteristics unique to Dinosauria (Nesbitt et al., Citation2010, Citation2017a; Padian, Citation2012). For example, the description of the aphanosaur Teleocrater rhadinus shifted the former dinosaur synapomorphy of a supratemporal fossa on the frontal bone to the base of Ornithodira (Nesbitt et al., Citation2017a). This shifting pattern of synapomorphies and symplesiomorphies has added confusion to early dinosaur relationships rather than helping alleviate it. While new data are likely to help resolve early dinosaur relationships, new data alone will not be sufficient to resolve this debate. We must then consider whether factors intrinsic to the methods and data sets in use may be contributing to, or even causing, the uncertainty surrounding early dinosaur relationships.
Data sets under scrutiny
The current debate on dinosaur intrarelationships is often simplified to discussion of only two hypotheses, the ‘traditional’ Ornithischia/Saurischia dichotomy (Langer et al., Citation2017) vs Ornithoscelida (Baron et al., Citation2017a). However, this ignores other possibilities, like the Phytodinosauria hypothesis, which have received support in some analyses (Langer et al., Citation2017; Parry et al., Citation2017). An additional layer of complication is the ‘ornithischian silesaurid’ hypothesis (Müller & Garcia, Citation2020; Norman et al., Citation2022): although this has only been proposed as a variation of the Ornithischia/Saurischia topology, it is not mutually exclusive with the other two hypotheses (e.g. a topology with silesaurids as early-branching ornithischians within Phytodinosauria). Some studies have found support for even more radical relationships within Dinosauria, such as ornithischians nested deeply among sauropodomorphs or theropods (Baron, Citation2019). Even if we ignore theoretically possible but unpublished hypotheses (e.g. ornithischian silesaurs within Ornithoscelida), at least six competing topologies (Ornithischia/Saurischia, Ornithoscelida, Phytodinosauria, ornithischian Silesauridae, Ornithischia within Sauropodomorpha, and Ornithischia within Theropoda) have been recovered by analyses of current data sets.
Prompted by this high degree of uncertainty, two recent studies have used statistical techniques to evaluate why current early dinosaur data sets produce such conflicting results (Černý & Simonoff, Citation2023; Goloboff & Sereno, Citation2021). One of these studies applied statistical techniques developed to identify the causes of phylogenetic conflict within phylogenomic data sets, namely a difficulty measure and a character-wise support metric (Černý & Simonoff, Citation2023). The difficulty measure aims to assess the ‘ruggedness’ of tree space under maximum likelihood and whether tree topologies will converge on a single global optimal topology (‘easy’) or several local optimal topologies (‘difficult’) (Černý & Simonoff, Citation2023; Haag et al., Citation2022). Perhaps unsurprisingly, both the Baron et al. and Langer et al. data sets received high difficulty scores, suggesting that resolving the phylogenetic relationships of early dinosaurs using either data set would be difficult, if not impossible (Černý & Simonoff, Citation2023). Character-wise support techniques assess how the support for various hypotheses is distributed across individual characters in the data set (Černý & Simonoff, Citation2023; Shen et al., Citation2017). Application of the latter found that these data sets contained many conflicting phylogenetic signals between characters and included a number of ‘outlier’ characters that strongly favoured particular hypotheses (Černý & Simonoff, Citation2023). Furthermore, the piecemeal re-scoring of individual character states that Langer et al. (Citation2017) applied to the Baron et al. (Citation2017a) data set were found to drastically reduce the stability of the results (Černý & Simonoff, Citation2023). However, only 39% of the character state score differences (across 153 of 457 characters) between the Langer et al. and Baron et al. data sets were found to have demonstrable impacts on the recovered phylogenetic tree (Goloboff & Sereno, Citation2021). The additional taxa included in the Langer et al. (Citation2017) data set were found to have a notable impact on the recovered topologies, although they alone were not enough to recover the ‘traditional’ Ornithischia/Saurischia topology (Goloboff & Sereno, Citation2021). Langer et al. (Citation2017) acknowledged these weaknesses in their data set, noting that an in-depth analysis of character construction was needed, and concluded that ‘a more critical evaluation of characters – how they are defined and scored, whether they are independent from one another, how different authors have used them – is the best tool for untangling the roots of the dinosaur family tree’ (Langer et al., Citation2017, p. E2). Although these results suggest that current phylogenetic data sets are seriously flawed, this can be viewed in a positive light because it suggests that it is human errors during data set construction that are responsible for the current uncertainty around dinosaur relationships (Černý & Simonoff, Citation2023). Problems relating to data set construction and character scoring are likely to be much more tractable than insurmountable issues with the fossil record. The application of novel phylogenetic methodologies could also play a role in building this new consensus, for example using fossilized birth-death (FBD) process techniques that might enable the origins of various clades to be constrained more accurately (Heath et al., Citation2014).
In short, it is likely that the different approaches to character creation and coding taken by researchers when constructing and modifying phylogenetic data sets are contributing to the phylogenetic uncertainty around the base of Dinosauria (Černý & Simonoff, Citation2023; Langer et al., Citation2010). Inevitably, we must consider the possibility that the cause of this debate may not be wholly or even largely the result of a poor-quality fossil record, but rather reflects problems relating to character construction and sampling, the accuracy of state scores, and issues of taxon sampling, which affect all current data sets.
The importance of phylogeny
Our understanding of early dinosaur evolution has changed dramatically in the twenty-first century, with techniques such as ancestral state reconstruction giving us an unprecedented ability to investigate the characteristics of early dinosaurs. Ancestral state reconstruction enables inference of information lost because of the varied biases inherent in the fossil/geological record, allowing research to predict the integument (e.g. Barrett et al., Citation2015; Yang et al., Citation2019), metabolism (Wiemann et al., Citation2022), and even the eggshell structure and colour of the earliest dinosaur (Norell et al., Citation2020; Wiemann et al., Citation2018). The findings of these ancestral state reconstructions are not universally accepted: for example, the soft-shelled eggs predicted by one of these studies has been challenged (Legendre et al., Citation2020) and the integument of early dinosaurs is still a subject of intense debate (Benton et al., Citation2019; Campione et al., Citation2020; Cincotta et al., Citation2022; Godefroit et al., Citation2020; Hendrickx et al., Citation2022; Yang et al., Citation2019). The biggest issue with this type of study is that a robust, well resolved, and widely accepted phylogenetic tree is an essential component of successful ancestral state reconstruction. If the phylogeny of early dinosaurs is unstable and unresolved, as the profusion of hypotheses discussed above illustrates, this threatens the validity of the results of all analytical techniques that need to incorporate information on dinosaur phylogeny (Baron et al., Citation2017a; Černý & Simonoff, Citation2023; Goloboff & Sereno, Citation2021; Langer et al., Citation2017; Müller & Garcia, Citation2020). Some studies have attempted to overcome this problem by running their analyses using both the Ornithoscelida and ‘traditional’ topologies (e.g. Felice et al., Citation2020; Hendrickx et al., Citation2022), but not the other topologies available, thereby underestimating the level of uncertainty surrounding broad-scale dinosaur relationships (Černý & Simonoff, Citation2023). An example of the impact of tree topology on macroevolutionary hypotheses concerning early dinosaurs relates to reconstructing the ancestral diet of Dinosauria. A recent study used biomechanical and morphological analyses of dentition to characterize ecological diversity among early dinosaurs: however, it was unable to conclusively identify the ancestral ecology of the clade due to uncertain tree topology (Ballell et al., Citation2022). Under the Ornithoscelida topology, the ancestral ecology of Dinosauria was found to be carnivorous; by contrast, under the ornithischian/saurischian hypothesis, an ambiguously omnivorous/carnivorous ancestral diet was estimated (Ballell et al., Citation2022; see also Barrett et al., Citation2010). The ornithischian silesaurid hypothesis further complicates matters by adding taxa with carnivorous, omnivorous and possibly insectivorous diets to the base of the otherwise entirely herbivorous group (Ballell et al., Citation2022; see also Barrett et al., Citation2010). While the debate over dinosaur relationships continues, with a range of viable but often radically divergent topologies in the literature, efforts to test hypotheses about the macroevolution of dinosaurs will be severely hampered.
A paradigm shift?
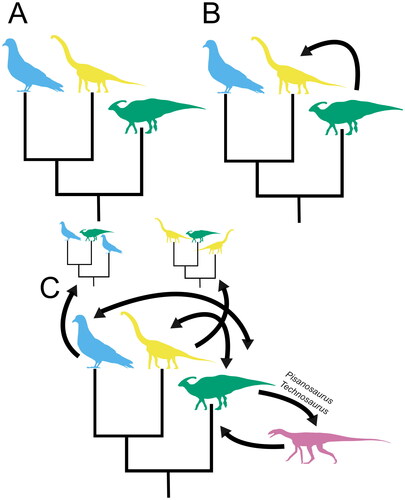
The debate around early dinosaur relationships remains unresolved and we currently lack a robust model for the clade’s early evolution. It may be worth taking a step back and asking ‘Do we have any good models for the current state of this debate?’ (i.e. the collapse of a long-held consensus followed by a proliferation of alternative hypotheses and conflicting results). We propose that our understanding of dinosaur phylogenetic relationships is following a ‘paradigm shift’ model, as put forward by the philosopher Thomas Kuhn, and that we are currently ‘mid-shift’ (see ) (Kuhn, Citation1962). The Ornithischia/Saurischia paradigm that had dominated the study of early dinosaur relationships has been substantially weakened, and may be on the verge of collapse, but despite considerable research effort a new paradigm has yet to emerge (Bakker & Galton, Citation1974; Baron et al., Citation2017a, Citation2017b; Cabreira et al., Citation2016; Langer et al., Citation2017; Müller & Garcia, Citation2020; Norman et al., Citation2022). It should be noted, however, that this does not necessarily imply that the new paradigm will be radically different from the old, as it is still possible that a modified and/or better statistically supported variation of the Ornithischia/Saurischia model will become accepted as the new consensus. By examining past phylogenetic controversies that have gone through a comparable paradigm shift, it may be possible to make predictions about the future of the debate. The phylogeny of Sauropodomorpha is an obvious candidate for such a comparison because, like the debate around early dinosaur relationships, it focussed on an early Mesozoic group with at least some key taxa known primarily from fragmentary material (e.g. Galton & Upchurch, Citation2004; Sereno, Citation1999; Upchurch et al., Citation2007; Yates, Citation2004, Citation2007b). The pre-cladistic view was that sauropods emerged from within ‘prosauropods’, or in cladistic terms the monophyletic sauropod clade emerged from within a paraphyletic prosauropod grade (Bonaparte, Citation1986; Cooper, Citation1984). In the 1980s and 1990s, early cladistic analyses found support for the hypothesis that sauropodomorphs formed two monophyletic sister groups, Prosauropoda and Sauropoda (Galton, Citation1990; Galton & Upchurch, Citation2004; Gauffre, Citation1996; Sereno, Citation1999), and this created a ’sauropod gap’ equivalent to the ’ornithischian gap’, with a temporal and morphological gap between the earliest sauropods in the Jurassic and the last common ancestor of sauropods and prosauropods. There followed a period of instability where both topologies were recovered (Pol & Powell, Citation2007; Yates & Kitching, Citation2003), punctuated by the description of key new taxa like Antetonitrus ingenipes, which combined many plesiomorphic ‘prosauropod’ traits with several derived sauropod traits associated with graviportal locomotion (McPhee et al., Citation2014; Yates & Kitching, Citation2003). A new consensus emerged with Sauropoda deeply nested within Sauropodomorpha, with the former ‘prosauropods’ reduced to a grade of non-sauropod sauropodomorphs (e.g. Apaldetti et al., Citation2011, Citation2013; Upchurch et al., Citation2007; Yates, Citation2004, Citation2007b). Based on this timeline we might expect the field of early dinosaur research to return to a consensus within 10–15 years of the ‘starting gun’ being fired, in this case the proposal of the Ornithoscelida hypothesis in 2017 (Baron et al., Citation2017a). The problem of early dinosaur relationships is much more intensely studied than that of early sauropodomorph phylogeny and this concentrated research effort may allow this timeline to be accelerated. However, this may be balanced or even outweighed by the scale of the problem, not helped by the ever increasing diversity and disparity of relevant taxa documented above, slowing the field’s shift towards a new paradigm. It is extremely difficult to predict exactly when a new paradigm will emerge.
Figure 6. The current state of our understanding of dinosaur relationships visualized. A, the Ornithischia/Saurischia paradigm, the long-standing consensus about dinosaur relationships supported by dinosaur phylogenetic studies until recently. B, the paradigm is challenged, with the Ornithoscelida hypothesis of Baron et al. (Citation2017a) providing the first major challenge to the consensus. C, the paradigm collapses; an intensive period of research focussed on the problem of broad-scale dinosaur relationships creates a proliferation of competing hypotheses. Silhouettes sourced from phylopic.org: Columba by Ferran Sayol CC0 1.0 (blue), Brachiosaurus altithorax by Michael P. Taylor CC0 1.0 (yellow), Parasaurolophus walkeri by Jack Mayer Wood CC0 1.0 (green), and Asilisaurus kongwe by Scott Hartman attribution 3.0 unported edited (pink).
Conclusions
The exact relationships between the major clades of dinosaurs remain an open question, but are critical for understanding their evolutionary history. In recent years, our understanding of early dinosaur evolution appears to have moved away from, rather than towards, a consensus. A century-long consensus on the broad-scale phylogenetic relationships within dinosaurs has collapsed since the Ornithoscelida hypothesis was proposed (Baron et al., Citation2017a), despite a spirited debate in the literature (e.g. Baron et al., Citation2017b; Langer et al., Citation2017; Müller & Garcia, Citation2020; Norman et al., Citation2022; Parry et al., Citation2017), returning us to a period of uncertainty that mirrors the early debates on dinosaur intrarelationships. There appear to be three major competing hypotheses: the ‘traditional’ Ornithischia/Saurischia dichotomy, the Ornithoscelida hypothesis, and the ornithischian silesaurid hypothesis (Baron et al., Citation2017a; Langer et al., Citation2017; Müller & Garcia, Citation2020). However, this masks the true level of uncertainty surrounding early dinosaur relationships, with a wide range of alternative hypotheses receiving at least some statistical support from current data sets (Baron et al., Citation2017b; Černý & Simonoff, Citation2023; Parry et al., Citation2017). There is little sign of a consensus re-emerging: recent developments suggest that although current data sets collate a lot of relevant and useful character data, they are also fundamentally flawed (Černý & Simonoff, Citation2023). Some studies propose that a complete overhaul of these data sets is the only solution to this debate (Černý & Simonoff, Citation2023; Goloboff & Sereno, Citation2021; Langer et al., Citation2017). It is possible, and indeed necessary, that a new consensus on broad-scale dinosaur relationships can be built through improved character construction and coding, a wider sample of taxa with a focus on non-dinosaurian avemetetarsalians, and an in-depth statistical evaluation of the resulting data set.
Associate Editor: Kimberley Chapelle
Acknowledgements
JL was funded by the London Natural Environment Research Council (Grant number NE/S007729/1). We thank the Editors-in-Chief for inviting this review, two anonymous referees for their constructive comments on an earlier draft, and the Associate Editor (Kimberley Chapelle) for their handling of the MS.
Disclosure statement
The authors report there are no competing interests to declare.
Table 1. Table modified from the one provided by Seeley (Citation1888a), in which he collated the different nomenclatures proposed for the internal dinosaur clades up to that time.
References
- Agnolín, F., Brissón Egli, F., Ezcurra, M. D., Langer, M. C., & Novas, F. E. (2022). New specimens provide insights into the anatomy of the dinosauriform Lewisuchus admixtus Romer, 1972 from the upper Triassic levels of the Chañares Formation, NW Argentina. The Anatomical Record, 305, 1119–1146. https://doi.org/10.1002/ar.24731
- Agnolin, F. L., Motta, M. J., Brissón Egli, F., Lo Coco, G., & Novas, F. E. (2019). Paravian phylogeny and the dinosaur-bird transition: An overview. Frontiers in Earth Science, 6, 252. https://doi.org/10.3389/feart.2018.00252
- Agnolin, F., & Rozadilla, S. (2017). Phylogenetic reassessment of Pisanosaurus mertii Casamiquela, 1967, a basal dinosauriform from the Late Triassic of Argentina. Journal of Systematic Palaeontology, 16, 853–878. https://doi.org/10.1080/14772019.2017.1352623
- Alcober, O., & Martínez, R. (2010). A new herrerasaurid (Dinosauria, Saurischia) from the Upper Triassic Ischigualasto Formation of northwestern Argentina. ZooKeys, 63, 55–81. https://doi.org/10.3897/zookeys.63.550
- Apaldetti, C., Martinez, R. N., Alcober, O. A., & Pol, D. (2011). A new basal sauropodomorph (Dinosauria: Saurischia) from Quebrada del Barro Formation (Marayes-El Carrizal Basin), northwestern Argentina. PLoS ONE, 6, e26964. https://doi.org/10.1371/journal.pone.0026964
- Apaldetti, C., Pol, D., & Yates, A. M. (2013). The postcranial anatomy of Coloradisaurus brevis (Dinosauria: Sauropodomorpha) from the Late Triassic of Argentina and its phylogenetic implications. Palaeontology, 56, 277–301. https://doi.org/10.1111/j.1475-4983.2012.01198.x
- Bakker, R. T. (1986). The dinosaur heresies: New theories unlocking the mystery of the dinosaurs and their extinction. Morrow.
- Bakker, R. T., & Galton, P. M. (1974). Dinosaur monophyly and a new class of vertebrates. Nature, 248, 168–172. https://doi.org/10.1038/248168a0
- Ballell, A., Benton, M. J., & Rayfield, E. J. (2022). Dental form and function in the early feeding diversification of dinosaurs. Science Advances, 8, eabq5201. https://doi.org/10.1126/sciadv.abq5201
- Baron, M. G. (2019). Pisanosaurus mertii and the Triassic ornithischian crisis: Could phylogeny offer a solution? Historical Biology, 31, 967–981. https://doi.org/10.1080/08912963.2017.1410705
- Baron, M. G. (2022). The effect of character and outgroup choice on the phylogenetic position of the Jurassic dinosaur Chilesaurus diegosaurezi. Palaeoworld. https://doi.org/10.1016/j.palwor.2022.12.001
- Baron, M. G., & Barrett, P. M. (2017). A dinosaur missing-link? Chilesaurus and the early evolution of ornithischian dinosaurs. Biology Letters, 13, 20170220. https://doi.org/10.1098/rsbl.2017.0220
- Baron, M. G., & Barrett, P. M. (2018). Support for the placement of Chilesaurus within Ornithischia: A reply to Müller et al. Biology Letters, 14, 20180002. https://doi.org/10.1098/rsbl.2018.0002
- Baron, M. G., Norman, D. B., & Barrett, P. M. (2017a). A new hypothesis of dinosaur relationships and early dinosaur evolution. Nature, 543, 501–506. https://doi.org/10.1038/nature21700
- Baron, M. G., Norman, D. B., & Barrett, P. M. (2017b). Baron et al. reply. Nature, 551, E4–E5. https://doi.org/10.1038/nature24012
- Baron, M. G., & Williams, M. (2018). A re-evaluation of the enigmatic dinosauriform Caseosaurus crosbyensis from the Late Triassic of Texas, USA and its implications for early dinosaur evolution. Acta Palaeontologica Polonica, 63, 129–145. https://doi.org/10.4202/app.00372.2017
- Barrett, P. M., Butler, R. J., & Nesbitt, S. J. (2010). The roles of herbivory and omnivory in early dinosaur evolution. Earth and Environmental Science Transactions of The Royal Society of Edinburgh, 101, 383–396. https://doi.org/10.1017/S1755691011020111
- Barrett, P. M., Evans, D. C., & Campione, N. E. (2015). Evolution of dinosaur epidermal structures. Biology Letters, 11, 20150229. https://doi.org/10.1098/rsbl.2015.0229
- Barrett, P. M., McGowan, A. J., & Page, V. (2009). Dinosaur diversity and the rock record. Proceedings of the Royal Society B, 276, 2667–2674. https://doi.org/10.1098/rspb.2009.0352
- Bell, M. A., & Lloyd, G. T. (2015). strap: An R package for plotting phylogenies against stratigraphy and assessing their stratigraphic congruence. Palaeontology, 58(2), 379–389. https://doi.org/10.1111/pala.12142
- Bennett, S. C. (2020). Reassessment of the Triassic archosauriform Scleromochlus taylori: neither runner nor biped, but hopper. PeerJ, 8, e8418. https://doi.org/10.7717/peerj.8418
- Benson, R. B. J., Campione, N. E., Carrano, M. T., Mannion, P. D., Sullivan, C., Upchurch, P., & Evans, D. C. (2014). Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. PLoS Biology, 12, e1001853. https://doi.org/10.1371/journal.pbio.1001853
- Benson, R. B. J., Hunt, G., Carrano, M. T., & Campione, N. E. (2018). Cope’s Rule and the adaptive landscape of dinosaur evolution. Palaeontology, 61, 13–48. https://doi.org/10.1111/pala.12329
- Benton, M. J., Dhouailly, D., Jiang, B., & McNamara, M. (2019). The early origin of feathers. Trends in Ecology & Evolution, 34, 856–869. https://doi.org/10.1016/j.tree.2019.04.018
- Benton, M. J., Forth, J., & Langer, M. C. (2014). Models for the rise of the dinosaurs. Current Biology, 24, R87–R95. https://doi.org/10.1016/j.cub.2013.11.063
- Benton, M. J., & Walker, A. D. (2010). Saltopus, a dinosauriform from the Upper Triassic of Scotland. Earth and Environmental Science Transactions of The Royal Society of Edinburgh, 101, 285–299. https://doi.org/10.1017/S1755691011020081
- Bonaparte, J. F. (1976). Pisanosaurus mertii Casamiquela and the origin of the Ornithischia. Journal of Paleontology, 50, 808–820.
- Bonaparte, J. F. (1986). The early radiation and phylogenetic relationships of the Jurassic sauropod dinosaurs, based on vertebral anatomy. In K. Padian (Ed.), The beginning of the Age of Dinosaurs: Faunal change across the Triassic–Jurassic boundary (pp. 247–258). Cambridge University Press.
- Bordy, E. M., Abrahams, M., Sharman, G. R., Viglietti, P. A., Benson, R. B. J., McPhee, B. W., Barrett, P. M., Sciscio, L., Condon, D., Mundil, R., Rademan, Z., Jinnah, Z., Clark, J. M., Suarez, C. A., Chapelle, K. E. J., & Choiniere, J. N. (2020). A chronostratigraphic framework for the upper Stormberg Group: Implications for the Triassic–Jurassic boundary in southern Africa. Earth-Science Reviews, 203, 103120. https://doi.org/10.1016/j.earscirev.2020.103120
- Boyd, C. A. (2015). The systematic relationships and biogeographic history of ornithischian dinosaurs. PeerJ, 3, e1523. https://doi.org/10.7717/peerj.1523
- Brazeau, M. D. (2011). Problematic character coding methods in morphology and their effects. Biological Journal of the Linnean Society, 104, 489–498. https://doi.org/10.1111/j.1095-8312.2011.01755.x
- Breeden, B. T., Raven, T. J., Butler, R. J., Rowe, T. B., & Maidment, S. C. R. (2021). The anatomy and palaeobiology of the early armoured dinosaur Scutellosaurus lawleri (Ornithischia: Thyreophora) from the Kayenta Formation (Lower Jurassic) of Arizona. Royal Society Open Science, 8(7), 201676. https://doi.org/10.1098/rsos.201676
- Bronzati, M., Langer, M. C., Ezcurra, M. D., Stocker, M. R., & Nesbitt, S. J. (2023). Braincase and neuroanatomy of the lagerpetid Dromomeron gregorii (Archosauria, Pterosauromorpha) with comments on the early evolution of the braincase and associated soft tissues in Avemetatarsalia. Anatomical Record. https://doi.org/10.1002/ar.25334
- Brusatte, S. L., Benton, M. J., Desojo, J. B., & Langer, M. C. (2010a). The higher-level phylogeny of Archosauria (Tetrapoda: Diapsida). Journal of Systematic Palaeontology, 8, 3–47. https://doi.org/10.1080/14772010903537732
- Brusatte, S. L., Benton, M. J., Lloyd, G. T., Ruta, M., & Wang, S. C. (2010b). Macroevolutionary patterns in the evolutionary radiation of archosaurs (Tetrapoda: Diapsida). Earth and Environmental Science Transactions of The Royal Society of Edinburgh, 101, 367–382. https://doi.org/10.1017/S1755691011020056
- Brusatte, S. L., Benton, M. J., Ruta, M., & Lloyd, G. T. (2008). The first 50 Myr of dinosaur evolution: Macroevolutionary pattern and morphological disparity. Biology Letters, 4, 733–736. https://doi.org/10.1098/rsbl.2008.0441
- Brusatte, S. L., Lloyd, G. T., Wang, S. C. & Norell, M. A. (2014). Gradual assembly of avian body plan culminated in rapid rates of evolution across the dinosaur-bird transition. Current Biology, 24, 2386–2392. https://doi.org/10.1016/j.cub.2014.08.034
- Brusatte, S. L., Nesbitt, S. J., Irmis, R. B., Butler, R. J., Benton, M. J., & Norell, M. A. (2010c). The origin and early radiation of dinosaurs. Earth-Science Reviews, 101, 68–100. https://doi.org/10.1016/j.earscirev.2010.04.001
- Brusatte, S. L., Niedźwiedzki, G., & Butler, R. J. (2010d). Footprints pull origin and diversification of dinosaur stem lineage deep into Early Triassic. Proceedings of the Royal Society B, 278, 1107–1113. https://doi.org/10.1098/rspb.2010.1746
- Butler, R. J., Benson, R. B. J., Carrano, M. T., Mannion, P. D., & Upchurch, P. (2010). Sea level, dinosaur diversity and sampling biases: Investigating the ‘common cause’ hypothesis in the terrestrial realm. Proceedings of the Royal Society B, 278, 1165–1170. https://doi.org/10.1098/rspb.2010.1754
- Butler, R. J., Smith, R. M. H., & Norman, D. B. (2007). A primitive ornithischian dinosaur from the Late Triassic of South Africa, and the early evolution and diversification of Ornithischia. Proceedings of the Royal Society B, 274, 2041–2046. https://doi.org/10.1098/rspb.2007.0367
- Cabreira, S. F., Kellner, A. W. A., Dias-da-Silva, S., Roberto da Silva, L., Bronzati, M., Marsola, J. C. de A., Müller, R. T., Bittencourt, J. de S., Batista, B. J., Raugust, T., Carrilho, R., Brodt, A., & Langer, M. C. (2016). A unique Late Triassic dinosauromorph assemblage reveals dinosaur ancestral anatomy and diet. Current Biology, 26, 3090–3095. https://doi.org/10.1016/j.cub.2016.09.040
- Campione, N. E., Barrett, P. M., & Evans, D. C. (2020). On the ancestry of feathers in Mesozoic dinosaurs. In C. Foth & O. W. M. Rauhut (Eds.) The evolution of feathers: From their origin to the present (pp. 213–243). Springer International Publishing. https://doi.org/10.1007/978-3-030-27223-4_12
- Casamiquela, R. M. (1967). Un nuevo dinosaurio ornitisquio triasico (Pisanosaurus mertii; Ornithopoda) de la Formacion Ischigualasto, Argentina. Ameghiniana, 5, 47–64.
- Cashmore, D. D., & Butler, R. J. (2019). Skeletal completeness of the non-avian theropod dinosaur fossil record. Palaeontology, 62, 951–981. https://doi.org/10.1111/pala.12436
- Cau, A. (2018). The assembly of the avian body plan: A 160-million-year long process. Bollettino della Societa Paleontologica Italiana, 57, 1–25. https://doi.org/10.4435/BSPI.2018.01
- Černý, D., & Simonoff, A. L. (2023). Statistical evaluation of character support reveals the instability of higher-level dinosaur phylogeny. Scientific Reports, 13, 9273. https://doi.org/10.1038/s41598-023-35784-3
- Charig, A. J. (1976). ‘Dinosaur monophyly and a new class of vertebrates’: A critical review. In A. d'A. Bellairs & C. B. Cox (Eds.), Morphology and biology of reptiles (pp. 66–104). Academic Press.
- Charig, A. J. (1982). Problems in dinosaur phylogeny: A reasoned approach to their attempted resolution. Geobios, 15, 113–126. https://doi.org/10.1016/S0016-6995(82)80106-X
- Charig, A. J., Attridge, J., & Crompton, A. W. (1965). On the origin of the sauropods and the classification of the Saurischia. Proceedings of the Linnean Society, 176, 197–221. https://doi.org/10.1111/j.1095-8312.1965.tb00944.x
- Chimento, N. R., Agnolin, F. L., Novas, F. E., Ezcurra, M. D., Salgado, L., Isasi, M. P., Suarez, M., Cruz, R. D. L., Rubilar-Rogers, D., & Vargas, A. O. (2017). Forelimb posture in Chilesaurus diegosuarezi (Dinosauria, Theropoda) and its behavioral and phylogenetic implications. Ameghiniana, 54, 567–575. https://doi.org/10.5710/AMGH.11.06.2017.3088
- Cincotta, A., Nicolaï, M., Nascimento Campos, H. B., McNamara, M., D’Alba, L., Shawkey, M. D., Kischlat, E.-E., Yans, J., Carleer, R., Escuillié, F., & Godefroit, P. (2022). Pterosaur melanosomes support signalling functions for early feathers. Nature, 604, 684–688. https://doi.org/10.1038/s41586-022-04622-3
- Clark, J. M., Perle, A., & Norell, M. A. (1994). The skull of Erlicosaurus andrewsi, a Late Cretaceus [sic] ‘segnosaur’ (Theropoda: Therizinosauridae) from Mongolia. American Museum Novitates, 3115, 1–39.
- Cooper, M. R. (1984). A reassessment of Vulcanodon karibaensis Raath (Dinosauria: Saurischia) and the origin of the Sauropoda. Palaeontologica africana, 25, 203–231.
- Cooper, M. R. (1985). A revision of the ornithischian dinosaur Kangnasaurus coetzeei Haughton, with a classification of the Ornithischia. Annals of the South African Museum, 95, 281–317.
- Cope, E. D. (1869). Synopsis of the extinct Batrachia, Reptilia and Aves of North America. Transactions of the American Philosophical Society, 14, 1–252 + viii + pls I–XIV. https://doi.org/10.2307/1005355
- Cope, E. D. (1866). [Untitled]. Proceedings of the Academy of Natural Sciences of Philadelphia, 18, 316–317.
- Cope, E. D. (1867). [Untitled]. Proceedings of the Academy of Natural Sciences of Philadelphia, 19, 234–235.
- Cope, E. D. (1883). On the characters of the skull in the Hadrosauridae. Proceedings of the Academy of Natural Sciences of Philadelphia, 35, 97–107 + pls IV–VII.
- Demuth, O. E., Wiseman, A. L. A., & Hutchinson, J. R. (2023). Quantitative biomechanical assessment of locomotor capabilities of the stem archosaur Euparkeria capensis. Royal Society Open Science, 10, 221195. https://doi.org/10.1098/rsos.221195
- Desojo, J. B., Fiorelli, L. E., Ezcurra, M. D., Martinelli, A. G., Ramezani, J., Da Rosa, Á. A. S., von Baczko, M. B., Trotteyn, M. J., Montefeltro, F. C., Ezpeleta, M. & Langer, M. C. (2020). The Late Triassic Ischigualasto Formation at Cerro Las Lajas (La Rioja, Argentina): Fossil tetrapods, high-resolution chronostratigraphy, and faunal correlations. Scientific Reports, 10, 12782. https://doi.org/10.1038/s41598-020-67854-1
- Dunne, E. M., Farnsworth, A., Benson, R. B. J., Godoy, P. L., Greene, S. E., Valdes, P. J., Lunt, D. J., & Butler, R. J. (2023). Climatic controls on the ecological ascendancy of dinosaurs. Current Biology, 33, 206–214. https://doi.org/10.1016/j.cub.2022.11.064
- Dzik, J. (2003). A beaked herbivorous archosaur with dinosaur affinities from the early Late Triassic of Poland. Journal of Vertebrate Paleontology, 23, 556–574. https://doi.org/10.1671/A1097
- Ezcurra, M. D. (2006). A review of the systematic position of the dinosauriform archosaur Eucoelophysis baldwini Sullivan & Lucas, 1999 from the Upper Triassic of New Mexico, USA. Geodiversitas, 28, 649–684.
- Ezcurra, M. D. (2010). A new early dinosaur (Saurischia: Sauropodomorpha) from the Late Triassic of Argentina: A reassessment of dinosaur origin and phylogeny. Journal of Systematic Palaeontology, 8, 371–425. https://doi.org/10.1080/14772019.2010.484650
- Ezcurra, M. D., & Martínez, R. (2016). Dinosaur precursors and early dinosaurs of Argentina. In F. Agnolíin, G. L. Lio, F. Brissón Egli, N. R. Chimento, & F. Novas (Eds.), Historia evolutiva y paleobiogeografía de los vertebrados de América del Sur (pp. 97–107). MACN.
- Ezcurra, M. D., Nesbitt, S. J., Bronzati, M., Dalla Vecchia, F. M., Agnolin, F. L., Benson, R. B. J., Brissón Egli, F., Cabreira, S. F., Evers, S. W., Gentil, A. R., Irmis, R. B., Martinelli, A. G., Novas, F. E., Roberto da Silva, L., Smith, N. D., Stocker, M. R., Turner, A. H., & Langer, M. C. (2020a). Enigmatic dinosaur precursors bridge the gap to the origin of Pterosauria. Nature, 588, 445–449. https://doi.org/10.1038/s41586-020-3011-4
- Ezcurra, M. D., Nesbitt, S. J., Fiorelli, L. E., & Desojo, J. B. (2020b). New specimen sheds light on the anatomy and taxonomy of the early Late Triassic dinosauriforms from the Chañares Formation, NW Argentina. The Anatomical Record, 303, 1393–1438. https://doi.org/10.1002/ar.24243
- Ezcurra, M. D., & Novas, F. E. (2007). Phylogenetic relationships of the Triassic theropod Zupaysaurus rougieri from NW Argentina. Historical Biology, 19, 35–72. https://doi.org/10.1080/08912960600845791
- Felice, R. N., Watanabe, A., Cuff, A. R., Hanson, M., Bhullar, B.-A. S., Rayfield, E. R., Witmer, L. M., Norell, M. A. & Goswami, A. (2020). Decelerated dinosaur skull evolution with the origin of birds. PLoS Biology, 18, e3000801. https://doi.org/10.1371/journal.pbio.3000801
- Ferigolo, J., & Langer, M. C. (2007). A Late Triassic dinosauriform from south Brazil and the origin of the ornithischian predentary bone. Historical Biology, 19, 23–33. https://doi.org/10.1080/08912960600845767
- Foffa, D., Dunne, E. M., Nesbitt, S. J., Butler, R. J., Fraser, N. C., Brusatte, S. L., Farnsworth, A., Lunt, D. J., Valdes, P. J., Walsh, S., & Barrett, P. M. (2022). Scleromochlus and the early evolution of Pterosauromorpha. Nature, 610, 313–318. https://doi.org/10.1038/s41586-022-05284-x
- Foffa, D., Nesbitt, S. J., Butler, R. J., Brusatte, S. L., Walsh, S., Fraser, N. C., & Barrett, P. M. (2023). The osteology of the Late Triassic reptile Scleromochlus taylori from μCT data. The Anatomical Record. https://doi.org/10.1002/ar.25335
- Fraser, N. C., Padian, K., Walkden, G. M., & Davis, A. L. M. (2002). Basal dinosauriform remains from Britain and the diagnosis of the Dinosauria. Palaeontology, 45, 79–95. https://doi.org/10.1111/1475-4983.00228
- Galton, P. M. (1990). Basal Sauropodomorpha – Prosauropoda. In D. B. Weishampel, P. Dodson, & H. Osmólska (Eds.), The Dinosauria (pp. 320–344). University of California Press.
- Galton, P. M. (2000). Are Spondylosoma and Staurikosaurus (Santa Maria Formation, Middle–Upper Triassic, Brazil) the oldest saurischian dinosaurs? PalZ, 74, 393–423. https://doi.org/10.1007/BF02988109
- Galton, P. M. (2014). Notes on the postcranial anatomy of the heterodontosaurid dinosaur Heterodontosaurus tucki, a basal ornithischian from the Lower Jurassic of South Africa. Revue de Paleobiologie, 33, 97–141.
- Galton, P. M., & Upchurch, P. (2004). Prosauropoda. In D. B. Weishampel, P. Dodson, & H Osmólska (Eds.), The Dinosauria (Second Edition) (pp. 232–258). University of California Press.
- Garcia, M. S., Müller, R. T., Pretto, F. A., Da-Rosa, Á. A. S., & Dias-Da-Silva, S. (2021). Taxonomic and phylogenetic reassessment of a large-bodied dinosaur from the earliest dinosaur-bearing beds (Carnian, Upper Triassic) from southern Brazil. Journal of Systematic Palaeontology, 19, 1–37. https://doi.org/10.1080/14772019.2021.1873433
- Gauffre, F. (1996). Phylogénie des dinosaures prosauropodes et etude d’uprosauropode du Trias Supérieur d’Afrique australe. [Unpublished PhD thesis]. Muséum national d'histoire naturelle, Paris.
- Gauthier, J. (1986). Saurischian monophyly and the origin of birds. Memoirs of the California Academy of Sciences, 8, 1–55.
- Godefroit, P., Sinitsa, S. M., Cincotta, A., McNamara, M. E., Reshetova, S. A., & Dhouailly, D. (2020). Integumentary structures in Kulindadromeus zabaikalicus, a basal neornithischian dinosaur from the Jurassic of Siberia. In C. Foth & O. W. M. Rauhut (Eds.), The evolution of feathers (pp. 47–65). Springer International Publishing. https://doi.org/10.1007/978-3-030-27223-4_4
- Goloboff, P. A., & Sereno, P. C. (2021). Comparative cladistics: Identifying the sources for differing phylogenetic results between competing morphology-based datasets. Journal of Systematic Palaeontology, 19, 761–786. https://doi.org/10.1080/14772019.2021.1970038
- Griffin, C. T., Wynd, B. M., Munyikwa, D., Broderick, T. J., Zondo, M., Tolan, S., Langer, M. C., Nesbitt, S. J., & Taruvinga, H. R. (2022). Africa’s oldest dinosaurs reveal early suppression of dinosaur distribution. Nature, 609, 313–319. https://doi.org/10.1038/s41586-022-05133-x
- Haag, J., Höhler, D., Bettisworth, B., & Stamatakis, A. (2022). From easy to hopeless – predicting the difficulty of phylogenetic analyses. Molecular Biology and Evolution, 39, msac254. https://doi.org/10.1093/molbev/msac254
- Heath, T. A., Huelsenbeck, J. P. & Stadler, T. (2014). The fossilized birth–death process for coherent calibration of divergence-time estimates. Proceedings of the National Academy of Sciences, 111, E2957–E2966. https://doi.org/10.1073/pnas.1319091111
- Hendrickx, C., Bell, P. R., Pittman, M., Milner, A. R. C., Cuesta, E., O’Connor, J., Loewen, M., Currie, P. J., Mateus, O., Kaye, T. G., & Delcourt, R. (2022). Morphology and distribution of scales, dermal ossifications, and other non-feather integumentary structures in non-avialan theropod dinosaurs. Biological Reviews, 97, 960–1004. https://doi.org/10.1111/brv.12829
- Huene, F. von. (1914). The dinosaurs not a natural order. American Journal of Science (Series 4), 38, 145–146. https://doi.org/10.2475/ajs.s4-38.224.145
- Huene, F. von. (1932). Die fossil Reptil-Ordnung Saurischia,ihre Entwicklung und Geschichte. Monographien zur Geologie und Palaeontologie, 1, 1–361.
- Huxley, T. H. (1870). On the classification of the Dinosauria, with observations on the Dinosauria of the Trias. Quarterly Journal of the Geological Society, 26(1-2), 32–51. https://doi.org/10.1144/GSL.JGS.1870.026.01-02.09
- Irmis, R. B., Nesbitt, S. J., Padian, K., Smith, N. D., Turner, A. H., Woody, D., & Downs, A. (2007a). A Late Triassic dinosauromorph assemblage from New Mexico and the rise of dinosaurs. Science, 317, 358–361. https://doi.org/10.1126/science.1143325
- Irmis, R. B., Parker, W. G., Nesbitt, S. J., & Liu, J. (2007b). Early ornithischian dinosaurs: The Triassic record. Historical Biology, 19, 3–22. https://doi.org/10.1080/08912960600719988
- Kammerer, C. F., Nesbitt, S. J., Flynn, J. J., Ranivoharimanana, L., & Wyss, A. R. (2020). A tiny ornithodiran archosaur from the Triassic of Madagascar and the role of miniaturization in dinosaur and pterosaur ancestry. Proceedings of the National Academy of Sciences of the USA, 117, 17932–17936. https://doi.org/10.1073/pnas.1916631117
- Kellner, A. W. A., Holgado, B., Grillo, O., Pretto, F. A., Kerber, L., Pinheiro, F. L., Soares, M. B., Schultz, C. L., Lopes, R. T., Araújo, O., & Müller, R. T. (2022). Reassessment of Faxinalipterus minimus, a purported Triassic pterosaur from southern Brazil with the description of a new taxon. PeerJ, 10, e13276. https://doi.org/10.7717/peerj.13276
- Koch, N. M. & Parry, L. A. (2020). Death is on our side: Paleontological data drastically modify phylogenetic hypotheses. Systematic Biology, 69, 1052–1067. https://doi.org/10.1093/sysbio/syaa023
- Kubo, T., & Kubo, M. O. (2014). Dental microwear of a Late Triassic dinosauriform, Silesaurus opolensis. Acta Palaeontologica Polonica, 59, 305–312. https://doi.org/10.4202/app.2013.0027
- Kuhn, T. S. (1962). The structure of scientific revolutions. University of Chicago Press.
- Langer, M. C., & Benton, M. J. (2006). Early dinosaurs: A phylogenetic study. Journal of Systematic Palaeontology, 4, 309–358. https://doi.org/10.1017/S1477201906001970
- Langer, M. C., Ezcurra, M. D., Bittencourt, J. S., & Novas, F. E. (2010). The origin and early evolution of dinosaurs. Biological Reviews, 85, 55–110. https://doi.org/10.1111/j.1469-185X.2009.00094.x
- Langer, M. C., Ezcurra, M. D., Rauhut, O. W. M., Benton, M. J., Knoll, F., McPhee, B. W., Novas, F. E., Pol, D., & Brusatte, S. L. (2017). Untangling the dinosaur family tree. Nature, 551, E1–E3. https://doi.org/10.1038/nature24011
- Langer, M. C., & Ferigolo, J. (2013). The Late Triassic dinosauromorph Sacisaurus agudoensis (Caturrita Formation; Rio Grande do Sul, Brazil): Anatomy and affinities. Geological Society of London, Special Publications, 379, 353–392. https://doi.org/10.1144/SP379.16
- Langer, M. C., Nesbitt, S. J., Bittencourt, J. S., & Irmis, R. B. (2013). Non-dinosaurian Dinosauromorpha. Geological Society of London, Special Publications, 379, 157–186. https://doi.org/10.1144/SP379.9
- Lee, M. S. Y., Baron, M. G., Norman, D. B., & Barrett, P. M. (2019). Dynamic biogeographic models and dinosaur origins. Earth & Environmental Science Transactions of the Royal Society of Edinburgh, 110, 325. https://doi.org/10.1017/S1755691018000920
- Legendre, L. J., Rubilar-Rogers, D., Vargas, A. O., & Clarke, J. A. (2020). The first dinosaur egg remains a mystery. BioRxiv. https://doi.org/10.1101/2020.12.10.406678
- Mannion, P. D., & Upchurch, P. (2010). Completeness metrics and the quality of the sauropodomorph fossil record through geological and historical time. Paleobiology, 36, 283–302. https://doi.org/10.1666/09008.1
- Marsh, O. C. (1877). A new order of extinct Reptilia (Stegosauria) from the Jurassic of the Rocky Mountains. American Journal of Science (Series 3), 14, 513–514. https://doi.org/10.2475/ajs.s3-14.84.513
- Marsh, O. C. (1878). Principal characters of American Jurassic dinosaurs. Part I. American Journal of Science (Series 3), 16, 411–416 + pls IV–X. https://doi.org/10.2475/ajs.s3-16.95.411
- Marsh, O. C. (1881). Principal characters of American Jurassic dinosaurs. Part V. American Journal of Science (Series 3), 21, 417–423 + pls XII–XVIII. https://doi.org/10.2475/ajs.s3-21.125.417
- Marsh, O. C. (1882). Classification of the Dinosauria. American Journal of Science (Series 3), 23, 81–86. https://doi.org/10.2475/ajs.s3-23.133.81
- Marsicano, C. A., Domnanovich, N. S., & Mancuso, A. C. (2007). Dinosaur origins: Evidence from the footprint record. Historical Biology, 19, 83–91. https://doi.org/10.1080/08912960600866920
- Marsicano, C. A., Irmis, R. B., Mancuso, A. C., Mundil, R., & Chemale, F. (2016). The precise temporal calibration of dinosaur origins. Proceedings of the National Academy of Sciences of the USA, 113, 509–513. https://doi.org/10.1073/pnas.1512541112
- Marsola, J. C. A., Ferreira, G. S., Langer, M. C., Button, D. J., & Butler, R. J. (2019). Increases in sampling support the southern Gondwanan hypothesis for the origin of dinosaurs. Palaeontology, 62, 473–482. https://doi.org/10.1111/pala.12411
- Martínez, R. N., Sereno, P. C., Alcober, O. A., Colombi, C. E., Renne, P. R., Montañez, I. P., & Currie, B. S. (2011). A basal dinosaur from the dawn of the dinosaur era in southwestern Pangaea. Science, 331, 206–210. https://doi.org/10.1126/science.1198467
- Martz, J. W., Mueller, B., Nesbitt, S. J., Stocker, M. R., Parker, W. G., Atanassov, M., Fraser, N., Weinbaum, J., & Lehane, J. R. (2012). A taxonomic and biostratigraphic re-evaluation of the Post Quarry vertebrate assemblage from the Cooper Canyon Formation (Dockum Group, Upper Triassic) of southern Garza County, western Texas. Earth and Environmental Science Transactions of The Royal Society of Edinburgh, 103, 339–364. https://doi.org/10.1017/S1755691013000376
- Martz, J. W., & Small, B. J. (2019). Non-dinosaurian dinosauromorphs from the Chinle Formation (Upper Triassic) of the Eagle Basin, northern Colorado: Dromomeron romeri (Lagerpetidae) and a new taxon, Kwanasaurus williamparkeri (Silesauridae). PeerJ, 7, e7551. https://doi.org/10.7717/peerj.7551
- Maryanska, T., & Osmólska, H. (1985). On ornithischian phylogeny. Acta Palaeontologica Polonica, 30, 137–150.
- McPhee, B. W., Yates, A. M., Choiniere, J. N., & Abdala, F. (2014). The complete anatomy and phylogenetic relationships of Antetonitrus ingenipes (Sauropodiformes, Dinosauria): Implications for the origins of Sauropoda: The anatomy of Antetonitrus. Zoological Journal of the Linnean Society, 171, 151–205. https://doi.org/10.1111/zoj.12127
- Mestriner, G., Marsola, J. C. A., Nesbitt, S. J., Da-Rosa, Á. A. S., & Langer, M. (2023). Anatomy and phylogenetic affinities of a new silesaurid assemblage from the Carnian beds of south Brazil. Journal of Vertebrate Paleontology, e2232426. https://doi.org/10.1080/02724634.2023.2232426
- Meyer, H. von. (1832). Palaeologica zur Geschichte der Erde. S. Schmerber.
- Mounce, R. C. P., Sansom, R., & Wills, M. A. (2016). Sampling diverse characters improves phylogenies: Craniodental and postcranial characters of vertebrates often imply different trees. Evolution, 70, 666–686. https://doi.org/10.1111/evo.12884
- Müller, R. T., & Dias-da-Silva, S. (2019). Taxon sample and character coding deeply impact unstable branches in phylogenetic trees of dinosaurs. Historical Biology, 31, 1089–1092. https://doi.org/10.1080/08912963.2017.1418341
- Müller, R. T., Ezcurra, M. D., Garcia, M. S., Agnolín, F. L., Stocker, M. R., Novas, F. E., Soares, M. B., Kellner, A. W. A., & Nesbitt, S. J. (2023). New reptile shows dinosaurs and pterosaurs evolved among diverse precursors. Nature, 620, 589–594. https://doi.org/10.1038/s41586-023-06359-z
- Müller, R. T., & Garcia, M. S. (2020). A paraphyletic ‘Silesauridae’ as an alternative hypothesis for the initial radiation of ornithischian dinosaurs. Biology Letters, 16, 20200417. https://doi.org/10.1098/rsbl.2020.0417
- Müller, R. T., & Garcia, M. S. (2023). A new silesaurid from Carnian beds of Brazil fills a gap in the radiation of avian line archosaurs. Scientific Reports, 13, 4981. https://doi.org/10.1038/s41598-023-32057-x
- Müller, R., Langer, M., & Dias-da-Silva, S. (2018). Ingroup relationships of Lagerpetidae (Avemetatarsalia: Dinosauromorpha): A further phylogenetic investigation on the understanding of dinosaur relatives. Zootaxa, 4392, 149. https://doi.org/10.11646/zootaxa.4392.1.7
- Nesbitt, S. J. (2011). The early evolution of archosaurs: Relationships and the origin of major clades. Bulletin of the American Museum of Natural History, 352, 1–292. https://doi.org/10.1206/352.1
- Nesbitt, S. J., Barrett, P. M., Werning, S., Sidor, C. A., & Charig, A. J. (2013). The oldest dinosaur? A Middle Triassic dinosauriform from Tanzania. Biology Letters, 9, 20120949. https://doi.org/10.1098/rsbl.2012.0949
- Nesbitt, S. J., Butler, R. J., Ezcurra, M. D., Barrett, P. M., Stocker, M. R., Angielczyk, K. D., Smith, R. M. H., Sidor, C. A., Niedźwiedzki, G., Sennikov, A. G., & Charig, A. J. (2017a). The earliest bird-line archosaurs and the assembly of the dinosaur body plan. Nature, 544, 484–487. https://doi.org/10.1038/nature22037
- Nesbitt, S. J., Butler, R. J., Ezcurra, M. D., Charig, A. J., & Barrett, P. M. (2017b). The anatomy of Teleocrater rhadinus, an early avemetatarsalian from the lower portion of the Lifua Member of the Manda Beds (Middle Triassic). Memoir of the Society of Vertebrate Paleontology, 17, 142–177. https://doi.org/10.1080/02724634.2017.1396539
- Nesbitt, S. J., Langer, M. C., & Ezcurra, M. D. (2020). The anatomy of Asilisaurus kongwe, a dinosauriform from the Lifua Member of the Manda Beds (Middle Triassic) of Africa. The Anatomical Record, 303, 813–873. https://doi.org/10.1002/ar.24287
- Nesbitt, S. J., Patellos, E., Kammerer, C. F., Ranivoharimanana, L., Wyss, A. R., & Flynn, J. J. (2023). The earliest-diverging avemetatarsalian: A new osteoderm-bearing taxon from the Triassic (?earliest Late Triassic) of Madagascar and the composition of avemetatarsalian assemblages prior to the radiation of dinosaurs. Zoological Journal of the Linnean Society, 199, 327–353. https://doi.org/10.1093/zoolinnean/zlad038
- Nesbitt, S. J., Sidor, C. A., Irmis, R. B., Angielczyk, K. D., Smith, R. M. H., & Tsuji, L. A. (2010). Ecologically distinct dinosaurian sister group shows early diversification of Ornithodira. Nature, 464, 95–98. https://doi.org/10.1038/nature08718
- Nesbitt, S. J., Smith, N. D., Irmis, R. B., Turner, A. H., Downs, A., & Norell, M. A. (2009). A complete skeleton of a Late Triassic saurischian and the early evolution of dinosaurs. Science, 326, 1530–1533. https://doi.org/10.1126/science.1180350
- Niedźwiedzki, G., Brusatte, S. L., Sulej, T., & Butler, R. J. (2014). Basal dinosauriform and theropod dinosaurs from the mid–late Norian (Late Triassic) of Poland: Implications for Triassic dinosaur evolution and distribution. Palaeontology, 57, 1121–1142. https://doi.org/10.1111/pala.12107
- Niedźwiedzki, G., Sennikov, A. & Brusatte, S. L. (2016). The osteology and systematic position of Dongusuchus efremovi Sennikov, 1988 from the Anisian (Middle Triassic) of Russia. Historical Biology, 28, 550–570. https://doi.org/10.1080/08912963.2014.992017
- Nopcsa, F. (1901). Synopsis und Abstammung der Dinosaurier. Földtani Közlöny (Supplement), 31, 247–279.
- Norell, M. A., Wiemann, J., Fabbri, M., Yu, C., Marsicano, C. A., Moore-Nall, A., Varricchio, D. J., Pol, D., & Zelenitsky, D. K. (2020). The first dinosaur egg was soft. Nature, 583, 406–410. https://doi.org/10.1038/s41586-020-2412-8
- Norman, D. B. (1984). A systematic reappraisal of the reptile order Ornithischia. In W.-E. Reif & F. Westphal (Eds.), Third symposium on Mesozoic terrestrial ecosystems, Short Papers (pp.157–162). Attempto Verlag.
- Norman, D. B. (2020a). Scelidosaurus harrisonii (Dinosauria: Ornithischia) from the Early Jurassic of Dorset, England: Cranial anatomy. Zoological Journal of the Linnean Society, 188, 1–81. https://doi.org/10.1093/zoolinnean/zlz074
- Norman, D. B. (2020b). Scelidosaurus harrisonii from the Early Jurassic of Dorset, England: Postcranial skeleton. Zoological Journal of the Linnean Society, 189, 47–157. https://doi.org/10.1093/zoolinnean/zlz078
- Norman, D. B. (2020c). Scelidosaurus harrisonii from the Early Jurassic of Dorset, England: The dermal skeleton. Zoological Journal of the Linnean Society, 190, 1–53. https://doi.org/10.1093/zoolinnean/zlz085
- Norman, D. B., Baron, M. G., Garcia, M. S., & Müller, R. T. (2022). Taxonomic, palaeobiological and evolutionary implications of a phylogenetic hypothesis for Ornithischia (Archosauria: Dinosauria). Zoological Journal of the Linnean Society, 196, 1273–1309. https://doi.org/10.1093/zoolinnean/zlac062
- Norman, D. B., Crompton, A. W., Butler, R. J., Porro, L. B., & Charig, A. J. (2011). The Lower Jurassic ornithischian dinosaur Heterodontosaurus tucki Crompton & Charig, 1962: Cranial anatomy, functional morphology, taxonomy, and relationships. Zoological Journal of the Linnean Society, 163, 182–276. https://doi.org/10.1111/j.1096-3642.2011.00697.x
- Norman, D. B., Witmer, L. M., & Weishampel, D. B. (2004). Basal Ornithischia. In D. B. Weishampel, P. Dodson, & H. Osmólska (Eds.), The Dinosauria (Second Edition) (pp. 325–334). University of California Press. https://doi.org/10.1525/california/9780520242098.003.0017
- Novas, F. E. (1994). New information on the systematics and postcranial skeleton of Herrerasaurus ischigualastensis (Theropoda: Herrerasauridae) from the Ischigualasto Formation (Upper Triassic) of Argentina. Journal of Vertebrate Paleontology, 13, 400–423. https://doi.org/10.1080/02724634.1994.10011523
- Novas, F. E. (1996). Dinosaur monophyly. Journal of Vertebrate Paleontology, 16, 723–741. https://doi.org/10.1080/02724634.1996.10011361
- Novas, F. E., Agnolin, F. L., Ezcurra, M. D., Müller, R. T., Martinelli, A. G., & Langer, M. C. (2021). Review of the fossil record of early dinosaurs from South America, and its phylogenetic implications. Journal of South American Earth Sciences, 110, 103341. https://doi.org/10.1016/j.jsames.2021.103341
- Novas, F. E., Ezcurra, M. D., Chatterjee, S., & Kutty, T. S. (2010). New dinosaur species from the Upper Triassic Upper Maleri and Lower Dharmaram formations of Central India. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 101, 333–349. https://doi.org/10.1017/S1755691011020093
- Novas, F. E., Salgado, L., Suárez, M., Agnolín, F. L., Ezcurra, M. D., Chimento, N. R., de la Cruz, R., Isasi, M. P., Vargas, A. O., & Rubilar-Rogers, D. (2015). An enigmatic plant-eating theropod from the Late Jurassic period of Chile. Nature, 522, 331–334. https://doi.org/10.1038/nature14307
- O’Leary, M. A., Bloch, J. I., Flynn, J. J., Gaudin, T. J., Giallombardo, A., Giannini, N. P., Goldberg, S. L., Kraatz, B. P., Luo, Z.-X., Meng, J., Ni, X., Novacek, M. J., Perini, F. A., Randall, Z. S., Rougier, G. W., Sargis, E. J., Silcox, M. T., Simmons, N. B., Spaulding, M., Velazco, P. M., Weksler, M., Wible, J. R. & Cirranello, A. L. (2013). The placental mammal ancestor and the post–K-Pg radiation of placentals. Science, 339, 662–667. https://doi.org/10.1126/science.1229237
- Olsen, P. E., Kent, D. V., & Whiteside, J. H. (2010). Implications of the Newark Supergroup-based astrochronology and geomagnetic polarity time scale (Newark-APTS) for the tempo and mode of the early diversification of the Dinosauria. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 101, 201–229. https://doi.org/10.1017/S1755691011020032
- Owen, R. (1842). Report on British fossil reptiles. Part II. Reports of the British Association for the Advancement of Science, 11, 60–204.
- Pacheco, C., Müller, R. T., Langer, M., Pretto, F. A., Kerber, L., & da Silva, S. D. (2019). Gnathovorax cabreirai: A new early dinosaur and the origin and initial radiation of predatory dinosaurs. PeerJ, 7, e7963. https://doi.org/10.7717/peerj.7963
- Padian, K. (2012). The problem of dinosaur origins: Integrating three approaches to the rise of Dinosauria. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 103, 423–442. https://doi.org/10.1017/S1755691013000431
- Parry, L. A., Baron, M. G., & Vinther, J. (2017). Multiple optimality criteria support Ornithoscelida. Royal Society Open Science, 4, 170833. https://doi.org/10.1098/rsos.170833
- Paul, G. S. (1984). The segnosaurian dinosaurs: Relics of the prosauropod-ornithischian transition? Journal of Vertebrate Paleontology, 4(4), 507–515. https://doi.org/10.1080/02724634.1984.10012026
- Peecook, B. R., Sidor, C. A., Nesbitt, S. J., Smith, R. M. H., Steyer, J. S., & Angielczyk, K. D. (2013). A new silesaurid from the upper Ntawere Formation of Zambia (Middle Triassic) demonstrates the rapid diversification of Silesauridae (Avemetatarsalia, Dinosauriformes). Journal of Vertebrate Paleontology, 33, 1127–1137. https://doi.org/10.1080/02724634.2013.755991
- Peecook, B. R., Steyer, J. S., Tabor, N. J., & Smith, R. M. H. (2017). Updated geology and vertebrate paleontology of the Triassic Ntawere Formation of northeastern Zambia, with special emphasis on the archosauromorphs. Memoir of the Society of Vertebrate Paleontology, 17, 8–38, https://doi.org/10.1080/02724634.2017.1410484
- Pintore, R., Houssaye, A., Nesbitt, S. J., & Hutchinson, J. R. (2022). Femoral specializations to locomotor habits in early archosauriforms. Journal of Anatomy, 240, 867–892. https://doi.org/10.1111/joa.13598
- Pol, D., & Powell, J. (2007). New information on Lessemsaurus sauropoides (Dinosauria: Sauropodomorpha) from the Upper Triassic of Argentina. Special Papers in Palaeontology, 77, 223–243.
- Pollock, D. D., Zwickl, D. J., McGuire, J. A. & Hillis, D. M. (2002). Increased taxon sampling is advantageous for phylogenetic inference. Systematic Biology, 51, 664–671. https://doi.org/10.1080/10635150290102357
- Prendini, L. (2001). Species or supraspecific taxa as terminals in cladistic analysis? Groundplans versus exemplars revisited. Systematic Biology, 50, 290–300. https://doi.org/10.1080/10635150118650
- Prum, R. O., Berv, J. S., Dornburg, A., Field, D. J., Townsend, J. P., Lemmon, E. M. & Lemmon, A. R. (2015). A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature, 526, 569–573. https://doi.org/10.1038/nature15697
- Remes, K., & Rauhut, O. W. M. (2005). The oldest Indian dinosaur Alwalkeria maleriensis Chatterjee revised: A chimera including remains of a basal saurischian. In Anon. (Ed.), II Congresso Latino-Americano de Paleontologia de Vertebrados, Boletim de Resumos (p. 218). Museu Nacional, Rio de Janeiro.
- Rio, J. P. & Mannion, P. D. (2021). Phylogenetic analysis of a new morphological dataset elucidates the evolutionary history of Crocodylia and resolves the long-standing gharial problem. PeerJ, 9, e12094. https://doi.org/10.7717/peerj.12094
- Rogers, R. R., Swisher, C. C., Sereno, P. C., Monetta, A. M., Forster, C. A. & Martínez, R. N. (1993). The Ischigualasto tetrapod assemblage (Late Triassic, Argentina) and 40Ar/39Ar dating of dinosaur origins. Science, 260, 794–797. https://doi.org/10.1126/science.260.5109.794
- Romer, A. S. (1945). Vertebrate paleontology (Second Edition). University of Chicago Press.
- Sansom, R. S., Wills, M. A., & Williams, T. (2017). Dental data perform relatively poorly in reconstructing mammal phylogenies: Morphological partitions evaluated with molecular benchmarks. Systematic Biology, 66, 813–822. https://doi.org/10.1093/sysbio/syw116
- Seeley, H. G. (1888a). On the classification of the fossil animals commonly named Dinosauria. Proceedings of the Royal Society of London, 43, 165–171. https://doi.org/10.1098/rspl.1887.0117
- Seeley, H. G. (1888b). The classification of Dinosauria. Reports of the British Association for the Advancement of Science, 57, 698–699.
- Sen, K. (2005). A new rauisuchian archosaur from the Middle Triassic of India. Palaeontology, 48, 185–196. https://doi.org/10.1111/j.1475-4983.2004.00438.x
- Sereno, P. C. (1984). The phylogeny of the Ornithischia: A reappraisal. In W.-E. Reif & F. Westphal (Eds.), Third symposium on Mesozoic terrestrial ecosystems, short papers (pp. 219–226). Attempto Verlag.
- Sereno, P. C. (1986). Phylogeny of the bird-hipped dinosaurs (Order Ornithischia). National Geographic Research, 2, 234–256.
- Sereno, P. C. (1991). Lesothosaurus, ‘fabrosaurids’, and the early evolution of Ornithischia. Journal of Vertebrate Paleontology, 11, 168–197. https://doi.org/10.1080/02724634.1991.10011386
- Sereno, P. C. (1999). The evolution of dinosaurs. Science, 284, 2137–2147. https://doi.org/10.1126/science.284.5423.2137
- Sereno, P. C. (2007). Logical basis for morphological characters in phylogenetics. Cladistics, 23, 565–587. https://doi.org/10.1111/j.1096-0031.2007.00161.x
- Sereno, P. C., & Arcucci, A. B. (1994). Dinosaurian precursors from the Middle Triassic of Argentina: Marasuchus lilloensis, gen. nov. Journal of Vertebrate Paleontology, 14, 53–73. https://doi.org/10.1080/02724634.1994.10011538
- Sereno, P. C., Forster, C. A., Rogers, R. R., & Monetta, A. M. (1993). Primitive dinosaur skeleton from Argentina and the early evolution of Dinosauria. Nature, 361, 64–66. https://doi.org/10.1038/361064a0
- Sereno, P. C., Martínez, R. N., & Alcober, O. A. (2012). Osteology of Eoraptor lunensis (Dinosauria, Sauropodomorpha). Memoir of the Society of Vertebrate Paleontology, 12, 83–179. https://doi.org/10.1080/02724634.2013.820113
- Sereno, P. C., & Novas, F. E. (1992). The complete skull and skeleton of an early dinosaur. Science, 258, 1137–1140. https://doi.org/10.1126/science.258.5085.1137
- Shen, X.-X., Hittinger, C. T., & Rokas, A. (2017). Contentious relationships in phylogenomic studies can be driven by a handful of genes. Nature Ecology & Evolution, 1, 1–10. https://doi.org/10.1038/s41559-017-0126
- Steel, R. (1969). Ornithischia. Handbuch der Paläoherpetologie, 15. Gustav Fisher Verlag.
- Steel, R. (1970). Saurischia. In O. Kuhn (Ed.), Handbuch der Palaoherpetologie [Encyclopedia of Paleoherpetology], part 14. Gustav Fisher Verlag.
- Sues, H.-D., Nesbitt, S. J., Berman, D. S., & Henrici, A. C. (2011). A late-surviving basal theropod dinosaur from the latest Triassic of North America. Proceedings of the Royal Society B, 278, 3459–3464. https://doi.org/10.1098/rspb.2011.0410
- Suh, A. (2016). The phylogenomic forest of bird trees contains a hard polytomy at the root of Neoaves. Zoologica Scripta, 45, 50–62. https://doi.org/10.1111/zsc.12213
- Thulborn, R. A. (1975). Dinosaur polyphyly and the classification of archosaurs and birds. Australian Journal of Zoology, 23, 249–270. https://doi.org/10.1071/ZO9750249
- Tykoski, R. S. (2005). Anatomy, ontogeny, and phylogeny of coelophysoid theropods. [Unpublished PhD dissertation]. The University of Texas at Austin.
- Upchurch, P., Barrett, P. M., & Galton, P. M. (2007). A phylogenetic analysis of basal sauropodomorph relationships: Implications for the origin of sauropod dinosaurs. Special Papers in Palaeontology, 77, 57–90.
- Upchurch, P., Hunn, C. A., & Norman, D. B. (2002). An analysis of dinosaurian biogeography: Evidence for the existence of vicariance and dispersal patterns caused by geological events. Proceedings of the Royal Society B: Biological Sciences, 269, 613–621. https://doi.org/10.1098/rspb.2001.1921
- Upchurch, P., Mannion, P. D., Benson, R. B. J., Butler, R. J., & Carrano, M. T. (2011). Geological and anthropogenic controls on the sampling of the terrestrial fossil record: A case study from Dinosauria. Geological Society of London, Special Publications, 358, 209–240. https://doi.org/10.1144/SP358.14
- Walker, A. D. (1970). A revision of the Jurassic reptile Hallopus victor (Marsh), with remarks on the classification of crocodiles. Philosophical Transactions of the Royal Society of London, Series B, 257, 323–372.
- Wang, N., Braun, E. L., Liang, B., Cracraft, J. & Smith, S. A. (2022). Categorical edge-based analyses of phylogenomic data reveal conflicting signals for difficult relationships in the avian tree. Molecular Phylogenetics and Evolution, 174, 107550, https://doi.org/10.1016/j.ympev.2022.107550
- Weishampel, D. B., Dodson, P., & Osmólska, H. (eds). (1990). The Dinosauria. University of California Press.
- Weishampel, D. B., Dodson, P., & Osmólska, H. (eds). (2004). The Dinosauria (Second Edition). University of California Press.
- Wiemann, J., Menéndez, I., Crawford, J. M., Fabbri, M., Gauthier, J. A., Hull, P. M., Norell, M. A., & Briggs, D. E. G. (2022). Fossil biomolecules reveal an avian metabolism in the ancestral dinosaur. Nature, 606, 522–526. https://doi.org/10.1038/s41586-022-04770-6
- Wiemann, J., Yang, T.-R., & Norell, M. A. (2018). Dinosaur egg colour had a single evolutionary origin. Nature, 563, 555–558. https://doi.org/10.1038/s41586-018-0646-5
- Wu, S., Rheindt, F. E., Zhang, J., Wang, J., Zhang, L., Quan, C., Li, Z., Wang, M., Wu, F., Qu, Y., Edwards, S. V., Zhou, Z. & Liu, L. (2024). Genomes, fossils, and the concurrent rise of modern birds and flowering plants in the Late Cretaceous. Proceedings of the National Academy of Sciences, 121, e2319696121, https://doi.org/10.1073/pnas.2319696121
- Xu, X., Tang, Z., & Wang, X. (1999). A therizinosauroid dinosaur with integumentary structures from China. Nature, 399, 350–354. https://doi.org/10.1038/20670
- Xu, X., Zhou, Z., Wang, Y., & Wang, M. (2020). Study on the Jehol Biota: Recent advances and future prospects. Science China Earth Sciences, 63, 757–773. https://doi.org/10.1007/s11430-019-9509-3
- Yang, Z., Jiang, B., McNamara, M. E., Kearns, S. L., Pittman, M., Kaye, T. G., Orr, P. J., Xu, X., & Benton, M. J. (2019). Pterosaur integumentary structures with complex feather-like branching. Nature Ecology & Evolution, 3, 24–30. https://doi.org/10.1038/s41559-018-0728-7
- Yates, A. M. (2003). A new species of the primitive dinosaur Thecodontosaurus (Saurischia: Sauropodomorpha) and its implications for the systematics of early dinosaurs. Journal of Systematic Palaeontology, 1, 1–42. https://doi.org/10.1017/S1477201903001007
- Yates, A. M. (2004). Anchisaurus polyzelus (Hitchcock): The smallest known sauropod dinosaur and the evolution of gigantism among sauropodomorph dinosaurs. Postilla, 230, 1–58.
- Yates, A. M. (2007a). Solving a dinosaurian puzzle: The identity of Aliwalia rex Galton. Historical Biology, 19, 93–123. https://doi.org/10.1080/08912960600866953
- Yates, A. M. (2007b). The first complete skull of the Triassic dinosaur Melanorosaurus Haughton (Sauropodomorpha: Anchisauria). Special Papers in Palaeontology, 77, 9–55.
- Yates, A. M., & Kitching, J. W. (2003). The earliest known sauropod dinosaur and the first steps towards sauropod locomotion. Proceedings of the Royal Society B, 270, 1753–1758. https://doi.org/10.1098/rspb.2003.2417
- Zahner, M., & Brinkmann, W. (2019). A Triassic averostran-line theropod from Switzerland and the early evolution of dinosaurs. Nature Ecology & Evolution, 3, 1146–1152. https://doi.org/10.1038/s41559-019-0941-z
