Abstract
True sawflies (Tenthredinoidea) have a substantial fossil record but are rarely encountered in Eocene ambers. Here we describe three new taxa in this superfamily from late Eocene ambers. †Rovnotaxonus aristovi gen. et sp. nov. is the first true sawfly reported from Rovno amber. Based primarily on characters in the fore wing venation, we assign the new taxon to Tenthredinidae: Allantinae: Allantini. We also describe two new species of Diprionidae from Baltic amber: †Eodiprion pectinatus sp. nov. from a female specimen and †Monodiprion gladius gen. et sp. nov. from a male previously reported as †Eodiprion sp. by Schedl (Citation2008); we redescribe †Eodiprion Schedl, Citation2007 and provide emended diagnoses of the genus and of †Eodiprion groehni Schedl, Citation2007. We integrate all these fossils as well as †Sambia Vilhelmsen and Engel, Citation2012 (Tenthredinidae: Tenthredininae) previously described from Baltic amber in a combined data set assembled from previously published morphological and molecular data sets. We analyse the combined data set in a Bayesian framework and implement RoguePlots to evaluate the positions of the fossils. The diprionid fossils are unequivocally placed inside Diprionidae, in a polytomy with extant members of Diprioninae; the Monocteninae, the other subfamily currently recognized in the family, is not retrieved as monophyletic. †Rovnotaxonus is placed inside Allantinae and shares some characters with Taxonus. †Sambia is placed near the base of Tenthredininae. The evaluation of the phylogenetic position of the fossils treated here will make them available for future dating analyses of Tenthredinoidea, helping to further elucidate the evolutionary history of this significant lineage of herbivorous insects. Possible reasons for the comparatively low abundance of true sawflies in late Eocene ambers are discussed. http://zoobank.org/urn:lsid:zoobank.org:pubF2095774-CF02-4851-8D73-250B5718EF6F.
Introduction
Tenthredinoidea or true sawflies is the most diverse clade of non-apocritan wasps, comprising some 7500 described species. It is also one of the major herbivorous hymenopteran clades, having radiated during the Cretaceous in parallel with the angiosperms (Isaka & Sato, Citation2015; Nyman et al., Citation2019). Traditionally, six families have been recognized within Tenthredinoidea: Argidae, Blasticotomidae, Cimbicidae, Diprionidae, Pergidae, and Tenthredinidae (e.g. Taeger et al., Citation2010). Of these, Tenthredinidae is the largest by far, encompassing approx. three-quarters of the species in the superfamily. It has also been the most difficult family to diagnose and retrieve in recent phylogenetic analyses, e.g. Malm and Nyman (Citation2015), Niu et al. (Citation2022), Ronquist, Klopfstein et al. (Citation2012), Vilhelmsen (Citation2015), Wutke et al. (Citationin review). Attempts to resolve this have resulted in raising Athaliidae (Niu et al., Citation2022) and Heptamelidae (Malm & Nyman, Citation2015) to family status, leaving a putatively monophyletic Tenthredinidae sensu stricto. In addition, Zenargidae was raised to family status by Malagón-Aldana et al. (Citation2021) as it was placed as sister to Argidae + Pergidae in their analyses.
The fossil record of Tenthredinoidea is substantial (e.g. Taeger et al., Citation2010), although comparatively poor for late Eocene ambers. Menge (Citation1856), according to Larsson (Citation1978), mentions an Emphytus (= Allantus; Tenthredinidae). Brischke (Citation1886) lists five adult tenthredinoid specimens from the Menge and Helm amber collections – one Lophyrus sp. (i.e. a diprionid), one Selandria sp. (Tenthredinidae) and three Tenthredo spp. – as well as two larval Tenthredo spp. specimens, but does not provide any further information. Schedl (Citation2007) was unable to access this material and suggested that it might be lost; apparently no formal descriptions have been associated with any of these specimens. Larsson (Citation1978) reported a Lophyrus and a tenthredinid specimen from the amber collections of the Geological and Zoological Museums in Copenhagen (both currently part of the Natural History Museum of Denmark); we have only been able to find the diprionid which we describe in the present paper.
More recent, confirmable records of Tenthredinoidea from Eocene ambers comprise †Eodiprion groehni Schedl, Citation2007 (based on a female specimen), †Eodiprion sp. Schedl (Citation2008; based on a male specimen) and †Sambia succinica Vilhelmsen and Engel, Citation2012 (based on a female specimen). Schedl (Citation2007, Citation2008) noted variation in some potentially diagnostic features (length of inner hind tibial apical spur in male, configuration of anal cell) in the two specimens he placed in †Eodiprion but did not suggest subfamily placement, or placing the specimens in different genera, despite the substantial character differences (see also Nel et al., Citation2023). †Sambia was assigned to the subfamily Tenthredininae without being subjected to a cladistic analysis by Vilhelmsen and Engel (Citation2012). The analyses involving dating of the radiation of basal lineages of Hymenoptera, including Tenthredinoidea, have so far mostly employed stem-group fossils for calibration, and no tenthredinoid fossils from Eocene ambers have been included in previous efforts to date the radiation of the superfamily (e.g. Nyman et al., Citation2019; Ronquist, Klopfstein et al., Citation2012).
In the present paper, we describe a new fossil tenthredinid genus and species, based on a female specimen from the Schmalhausen Institute of Zoology, Kyiv, Ukraine. We also reexamine the †Eodiprion spp. previously reported and describe a female specimen from the Natural History Museum of Denmark (NHMD) as a new species of this genus, and the previously reported male †Eodiprion as a new genus and species. We incorporate the five Eocene amber fossil Tenthredinoidea available to us in a data set combined from the morphological data set of Vilhelmsen (Citation2015) and the nine-gene molecular data set from Malm and Nyman (Citation2015) to evaluate the phylogenetic positions of the fossil taxa and make them available as calibration points for future studies implementing total-evidence and/or node-dating approaches.
Material and methods
Localities
Baltic amber is the oldest known Lagerstätte with fossil arthropods of late Eocene age (Iakovleva et al., Citation2022). Rovno amber is its southern coeval (Jenkins Shaw et al., Citation2023) that is found mostly in north-western Ukraine in the Rovno (Mitov et al., Citation2021), Zhitomir (Melnitsky et al., Citation2021) and Volyn (Legalov et al., Citation2023) regions.
Microscopy and imaging
Specimens were examined with a Leica M205 C dissection microscope. Digital images were produced with a Visionary Digital imaging set-up with flash lightning and P-51 Camlift Driver v. 2.6.1 to control the camera. A cylinder of semitransparent paper was placed around the specimen to disperse the light. Some images of †Eodiprion sp. were taken with the specimen immersed in maple syrup. Images were stored in Adobe Lightroom 2 and composite images were compiled from stacks with the software Zerene Stacker v. 1.04 by implementing the Pyramidal Stacking Method (Pmax).
Phylogenetic analysis
We explored the phylogenetic placement of the three fossils by including them in the most comprehensive total-evidence phylogeny (TEP) of the Tenthredinidae published so far. Our total-evidence analysis was based on previously published morphological and molecular phylogenies which had significant overlap in taxon sampling. Despite this and to maximize overlap in taxon sampling, it was necessary to create taxon chimeras between the morphological and molecular data (Supplemental material Table S1). Morphological data from the three fossils were scored into the matrix of Vilhelmsen (Citation2015) in Mesquite v. 3.70 (Maddison & Maddison, Citation2021). The morphological data set was then combined with the molecular data set of Malm and Nyman (Citation2015). Specifically, we used molecular data from the ‘12 + CAD3 + GLN3_hym’ data set (appendix S3 in Malm & Nyman Citation2015), which includes data from nine protein-coding genes (eight nuclear, one mitochondrial), including fragments of: CAD, gelsolin (GLN), glycogen synthase (GS), isocitrate dehydrogenase (IDH), sodium-potassium adenosine triphosphatase (NAK), phosphogluconate dehydrogenase (PGD), DNA polymerase, and triose-phosphate isomerase (TPI), and a fragment of the mitochondrial Cytochrome c oxidase I. For details of sequence generation, alignment and partitioning, readers are referred to Malm and Nyman (Citation2015). Our final matrix included 191 taxa, 5164 base pairs of molecular data from nine protein-coding genes and 146 morphological characters; the data set can be downloaded from https://doi.org/10.6084/m9.figshare.25623588.
For the Bayesian inference of the TEP data set, we used the same parameters as Malm and Nyman (Citation2015), with the addition that the morphological partition was analysed under the Mk + G (Gamma) model (Lewis, Citation2001) and the analysis was run for 80 million generations. Urocerus gigas (Siricidae) was set as the outgroup. Computation for the TEP using MrBayes (Ronquist, Teslenko et al., Citation2012) was performed on the National Life Science Supercomputing Center – Computerome 2.0 (www.computerome.dk). We used R/Rogueplots (Klopfstein & Spasojevic, Citation2019; R Core Team, Citation2021) to explore phylogenetic placement of the three fossil taxa in all output trees of the Bayesian analysis. The online blog post by Mario Coiro (https://mariocoiro.blog/2020/12/02/how-to-represent-uncertainty-in-phylogenies-rogueplots-to-the-rescue/) was also useful when implementing RoguePlots. Statistical support for placements of the three fossil taxa was summarized using a majority-rule consensus tree and the post-burn-in combined runs from the Bayesian analyses.
Terminology
Morphological terminology follows Huber and Sharkey (Citation1993).
Results
Bayesian analysis
The Bayesian analysis reached convergence with the average standard deviation of split frequencies of 0.004. All effective sample sizes (ESS) were greater than 200 for all parameters. Average potential scale reduction factor (PSRF) was 1.000 with a maximum value of 1.012. Convergence was also checked visually using Tracer v. 1.7.2 (Rambaut et al., Citation2018). The 50% majority rule consensus tree with posterior probabilities at the respective nodes is shown in full in Supplemental material Fig. S1; a condensed version of the topology excluding all non-tenthredinoid taxa is shown in .
Figure 1. Phylogeny of Tenthredinoidea derived from Bayesian analyses, part 1 (all families except Athaliidae and Tenthredinidae). All families except Diprionidae collapsed, number of included taxa indicated in parentheses. For complete tree, see Supplemental material Fig. S1. Support values given at nodes. Positions of †Eodiprion spp. and †Monodiprion indicated in red.
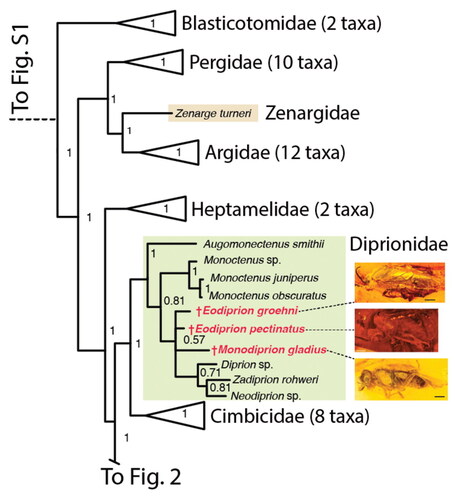
Figure 2. Phylogeny of Tenthredinoidea derived from Bayesian analyses, part 2 (Athaliidae and Tenthredinidae). All subfamilies except Allantinae and Tenthredininae collapsed, number of included taxa indicated in parentheses. For complete tree, see Supplemental material Fig. S1. Support values given at nodes. Positions of †Rovnotaxonus and †Sambia indicated in red.
Unsurprisingly, the topology of the tree retrieved here is highly similar to that presented by Malm and Nyman (Citation2015). Tenthredinoidea is monophyletic, Blasticotomidae is sister to all other Tenthredinoidea, Zenargidae is sister to Argidae, and these two together are the sister to Pergidae (); Heptamelidae is sister to a clade comprising ((Cimbicidae + Diprionidae) + remaining Tenthredinidae). Nematinae, Selandriinae and Tenthredininae are monophyletic; Allantinae is divided into Athalia (Athaliidae) and Eriocampa ovata (Linné, Citation1760), placed basal to the remaining Tenthredinidae, and ‘core’ Allantinae which is monophyletic and sister to Tenthredininae (); Blennocampinae is paraphyletic, including two clades classified as Heterarthrinae, and Eusunoxa sp. which has previously been classified in Allantinae.
†Eodiprion spp. and †Monodiprion are nested inside Diprionidae, in a polytomy with extant Diprioninae (). Monocteninae are retrieved as paraphyletic with regard to the clade comprising the fossil diprionids and the Diprioninae, Augomonoctenus being sister to all other Diprionidae. The RoguePlots for the two †Eodiprion spp. (Supplemental material Figs S2, S3) show that they are most likely sister groups; a range of alternative positions inside Diprionidae with low probability are also suggested. †Monodiprion is most likely the sister to all Diprioninae including †Eodiprion, or just the extant Diprioninae; this fossil also floats widely across the Diprionidae in the RoguePlot (Supplemental material Fig. S4), with a freak low-probability occurrence as sister to Chrysis sp. (Chrysididae).
†Rovnotaxonus is placed in a basal polytomy in ‘core’ Allantinae (; only Eopsis is outside the clade including the fossil taxon); however, this position has low probability. Taxonus epicera Say, Citation1836, the sole representative of Taxonus included, is sister to a clade comprising Allantus, Macroemphytus and Monostegia. The RoguePlot (Supplemental material Fig. S5) indicates alternative positions with very low probability inside ‘core’ Allantinae (including as sister to Taxonus epicera), inside Blennocampinae, or as sister to a clade comprising core Allantinae and Tenthredininae; none of these alternatives have significantly higher support than any other.
†Sambia is placed in the basal trichotomy of Tenthredininae, with Siobla spp. and the remaining representatives of the subfamily. The RoguePlot (Supplemental material Fig. S6) reveals high probability that the fossil is either sister to Siobla or all other Tenthredininae; there is less support for †Sambia being sister to all Tenthredininae except Siobla, and very low probability of being sister to Eusunoxa sp., an ‘allantine’ nested deeply inside Blennocampinae. †Sambia is the fossil displaying the least ‘rogue’ behaviour in the analyses conducted here.
Systematic palaeontology
Order Hymenoptera Linné, Citation1758
Family Tenthredinidae (Allantinae Rohwer, Citation1911)
Genus †Rovnotaxonus Vilhelmsen and Perkovsky gen. nov.
Type species
†Rovnotaxonus aristovi Vilhelmsen and Perkovsky gen. et sp. nov.
Diagnosis
As per the type species, see below.
Derivation of name
The genus name is a combination of Rovno, the provenance of the fossil, and Taxonus Hartig, Citation1837, the extant genus the fossil resembles the most.
†Rovnotaxonus aristovi Vilhelmsen and Perkovsky gen. et sp. nov.
()
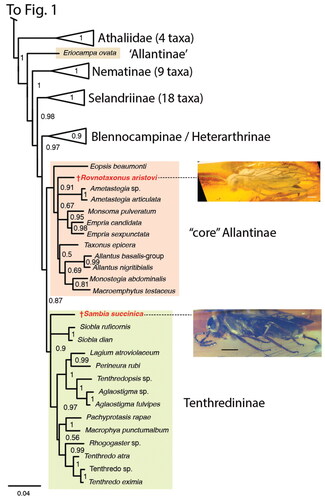
Figure 3. †Rovnotaxonus aristovi gen. et sp. nov., female holotype (SIZK-ZH-69). A, habitus, dorsal; B, habitus, lateral; C, head, ventral; D, mesonotum. Black arrow = abcissa of Rs; red arrow = occipital carina; cyan arrow = median mesoscutal sulcus; green arrow = notaulus. Abbreviations: md, mandible; sc2, mesoscutellum. Remaining abbreviations (R, M, Rs-M, etc.) refer to wing veins, see main text.
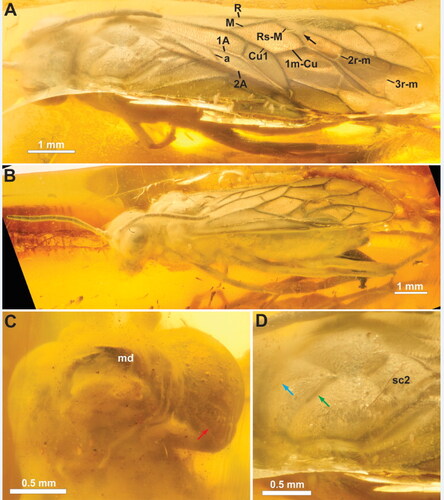
Figure 4. †Rovnotaxonus aristovi gen. et sp. nov., female holotype (SIZK ZH-69). A, antenna; B, fore legs, ventral; C, hind leg and tip of abdomen, ventral. Red arrows = calcar; green arrow = 3rd valvula; black arrows = hind tibial apical spurs. Abbreviations: 3vv, 3rd valvula; fe1, fore femur; tb1, fore tibia; tb3, hind tibia. Numbers in A refers to antennomeres 1–9.
Holotype
Deposited as SIZK ZH-69 in the collection of the I.I. Schmalhausen Institute of Zoology of the National Academy of Sciences of Ukraine, Kiev (SIZK).
Diagnosis
Occipital carina developed at least laterally (). Pedicellus longer than wide, 3rd antennomere longer than 4th (). Right mandible with at least one tooth, left mandible with at least two teeth (). Anterior margin of mesoscutellum convex, rounded (). Hind basitarsus approx. equal in length to remaining tarsomeres (); tarsal claws bifid, inner tooth long, basal lobe present. Fore wing veins () M and Rs + M join R at approx. the same point; veins M and 1m-cu of approx. same length, parallel; angle between 1m-cu and Cu1 more than 120°; abscissa of Rs present; anal cell fully developed, veins 1A and 2A not merging except distally, 2A with incurvation proximally, vein a situated distally, oblique.
Derivation of name
The species epithet honours the memory of the late palaeoentomologist Daniil S. Aristov.
Locality and horizon
From the Zhovkini locality which can be dated to the Priabonian (late Eocene, 37.7 to 33.9 Ma).
Description
Female () (male unknown). Body length (excluding wing tips) 8.7 mm; fore wing length 7.1 mm. Specimen almost complete, a few leg extremities were cut during preparation (cf. ). In clear piece of Rovno amber without any stellate hairs included (weight: 12 g after treatment); parts of surface of inclusion occluded by milky secretions and/or cracks.
Head
Head capsule broader than high, widely covered with short setae; compound eyes () oval, higher than long, converge at most slightly ventrally, setation not observed; ocelli placed in approx. equilateral triangle; gena widening dorsally; occipital carina present at least laterally (); epistomal sulcus and clypeal shape could not be observed. Antenna () inserted approx. at mid-height of eyes, approx. 1.6× longer than width of head, with nine antennomeres; pedicellus slightly longer than wide; 3rd antennomere the longest, antennomeres progressively shorter and narrower distally, three distalmost antennomeres combined shorter than 3rd antennomere. Right mandible with at least one tooth, left mandible with at least two teeth, teeth pointing towards each other (); maxillary and labial palps difficult to observe.
Thorax
Pronotum short, with many short hairs, closely abutting mesopleuron, with incurvation for anterior thoracic spiracle laterally; foreleg calcar () bifid, curved, slightly longer than other apical fore tibial spur. Mesonotum covered with short, curved hairs and scattered small punctures; median mesoscutal sulcus and converging notauli well developed (); notauli widening and deepening posteriorly, meeting medially well anteriorly to mesoscuto-scutellar sulcus; mesoscuto-scutellar sulcus triangular pit, anterior margin of mesoscutellum convex, rounded, posterior margin acutely triangular. Mesopleuron with short, curved hairs anteroventrally and scattered small punctures throughout; prepectus not observed, no apparent subdivisions of mesopleuron anterolaterally; posterior thoracic spiracle visible, in small incurvation in posterodorsal margin of mesopleuron. Metanotum and metapleuron difficult to observe due to wing position and occlusion; hind basitarsus () approx. same length as remaining hind tarsomeres (difficult to measure); hind tarsal claws bifid, subequal in length, with basal lobe.
Wings
Wings in normal resting position folded above abdomen, making observation of hind wing venation impossible. Fore wing () covered with short, erect setae both on and between veins; veins C and R diverging distally, costal cell widest at junction of veins M and Rs + M to R; Sc not observed, R without distinct bend distally; veins M and Rs + M join R at approx. the same point; veins M and 1m-cu of approx. same length, parallel; angle between 1m-cu and Cu1 more than 120°; abscissa of Rs, veins 2r-m and 3r-m all present; anal cell fully developed, veins 1A and 2A not merging except distally, 2A with incurvation proximally, vein a situated distally, oblique.
Abdomen
Abdomen widely covered with short posteriorly pointing setae. Abdominal tergum 1 not observable in dorsal view (see above). Saw sheath length 1.7 mm, 3rd valvula () with rounded apex in lateral view, not expanded distally in ventral view, darker than 2nd valvifer. Cerci short, less than twice as long as wide.
Remarks
We assign †Rovnotaxonus aristovi gen. et sp. nov. to Allantinae: Allantini, primarily based on the fore wing venation (see Diagnosis). The presence of the abscissa of the Rs indicates affinity with Taxonus Hartig, Citation1837 rather than the other two extant genera placed in Allantini (Smith, Citation1979), Allantus Panzer, Citation1801 and Macremphytus MacGillivray, Citation1908, in which this vein is absent. Indeed, when comparing the amber specimen with the diagnosis of Taxonus in Smith (Citation1979, p. 133), there seem to be few significant differences. Unfortunately, due to occlusions of the fossil specimen it was not possible to observe whether the clypeus is deeply incurved and the mandibles asymmetrical, the right mandible with one tooth, and the left with two, which could have provided further corroboration of the association with Taxonus. One difference we did observe when comparing with specimens of e.g. Taxonus agrorum (Fallén, Citation1808) concerns the anterior margin of the mesoscutellum, which is acutely triangular and with the mesoscuto-scutellar sulcus of equal width in T. agrorum, whereas in †R. aristovi the anterior margin of the mesoscutellum is more evenly rounded and the mesoscuto-scutellar sulcus is lengthened into a triangular pit medially and tapering laterally. Furthermore, in the cladistic analyses †Rovnotaxonus does not come out closely related to extant Taxonus, and there is considerable uncertainty as to the exact position of the fossil (see above).
†Rovnotaxonus aristovi is the first true sawfly described from Rovno amber. Zhovkini is one of the recently discovered Rovno amber localities from Varash district (Rovno Region) (Telnov et al., Citation2023), that already yielded an Eocene representative of Diapriidae: Ambositrinae (Chemyreva et al., Citation2024), a monotypic genus of Encyrtidae (Simutnik et al., Citation2022) and a monotypic genus of Cicadellidae: Typhlocybinae (Dietrich et al., Citation2023). Rosaceae, the host plants of Taxonus, were reported from Rovno amber, e.g. the oldest flower of Prunus outside North America was described from this Lagerstätte (Sokoloff et al., Citation2018).
Family Diprionidae Rohwer, Citation1911
Genus †Eodiprion Schedl, Citation2007
Type species
†Eodiprion groehni Schedl, Citation2007.
Additional species
†Eodiprion pectinatus Vilhelmsen sp. nov.
Emended diagnosis
Gena and anterodorsal mesopleuron finely punctate. Various numbers of antennomeres with lateral extensions of various extent. Third labial palpomere broadly triangular (), with 4th palpomere inserted medially on distal margin. Mesoscuto-scutellar sulcus boomerang-shaped. Hind tibial apical spurs shorter than hind basitarsus. Fore wing vein 2A interrupted in middle, anal cell constricted ().
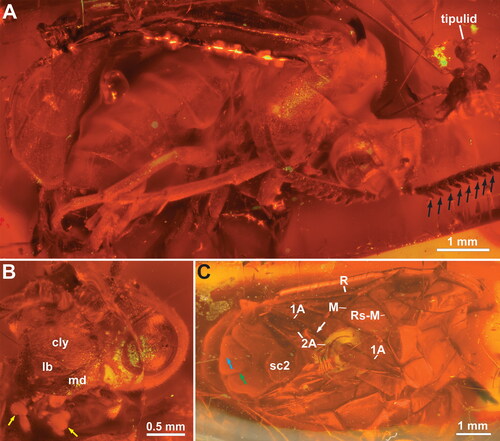
Figure 5. †Eodiprion groehni, female holotype (GPIH 4498). A, habitus, lateral; B, habitus, dorsal; C, antenna; D, tip of abdomen. White arrow = constriction of anal cell; black arrow = abcissa of Rs; cyan arrow = median mesoscutal sulcus; green arrow = notauli; yellow arrow = cercus. Abbreviations: 3vv, 3rd valvula; sc2, mesoscutellum; T9, abdominal tergum 9. Remaining abbreviations (M, Rs-M, etc.) refer to wing veins, see main text.
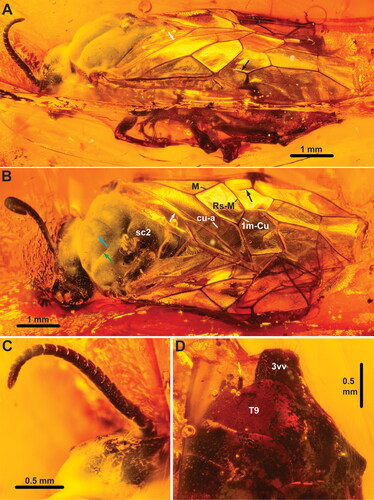
Figure 6. †Eodiprion pectinatus, female holotype (NHMD-115402). A, habitus, lateral; B, head, anterior; C, habitus, dorsal. Black arrows = lateral extensions of antennomeres; yellow arrows = 3rd labial palpomere; cyan arrow = median mesoscutal sulcus; green arrow = notaulus; white arrow = constriction of anal cell. Abbreviations: cly, clypeus; lb, labrum; md, mandible; sc2, mesoscutellum. Remaining abbreviations (R, M, Rs-M, etc.) refer to wing veins, see main text.
Redescription
Based on both †E. groehni and †E. pectinatus; differences between specimens or missing information reported in square brackets ([]) below.
Head
Head capsule () broader than high, covered with short setae; compound eyes oval, higher than long, slightly converging ventrally, setation not observed; ocelli placed in obtuse triangle, posterior margin of median ocellus in line with anterior margins of lateral ocelli; distance between lateral ocelli and posterior margin of head approx. 0.8× †E. groehni [1.2× in †E. pectinatus] distance between lateral ocelli; gena slightly widening dorsally; occipital carina entirely absent; epistomal sulcus present, anterior clypeal margin slightly incurved [not observed in †E. groehni]. Antenna () inserted just below mid-height of eyes, slightly longer than width of head, with 18 antennomeres; pedicellus slightly wider than long, approx. half length of 3rd antennomere; antennomeres 4–8 with lateral extensions shorter than basal antennomere width in †E. groehni [lateral extensions present on antennomeres 3–12 in †E. pectinatus; at least some longer than basal antennomere width; extensions of decreasing extent distally]. Labrum short, with rounded anterior margin, covered with elongate setae; both right and left mandibles with at least two teeth, pointing towards each other; maxillary palp with six palpomeres, labial palp with four, 3rd labial palpomere broadly triangular (), with 4th palpomere inserted medially on distal margin [mouthparts difficult to observe in †E. groehni].
Thorax
Pronotum short and flattened, covered with short hairs, with incurvation medially in dorsal margin, closely abutting mesopleuron, with incurvation for anterior thoracic spiracle laterally; medioventral corners of propleura widely separated, katepisterna small transverse sclerites not connected to propleura or prosternum; prosternum lozenge-shaped anteriorly, with prominent lateral corners, narrowing posteriorly [propleura and prosternum not observed †E. pectinatus]; foreleg calcar straight, not bifid; apical tibial spurs with small membraneous globule distally. Mesonotum covered with short hairs, sculpture indistinct; median mesoscutal sulcus narrow line, notauli broader grooves, meeting medially well anteriorly to mesoscuto-scutellar sulcus (); mesoscuto-scutellar sulcus boomerang-shaped depression, anterior and posterior margins of mesoscutellum slightly triangular, postscutellum narrow strip. Mesopleuron covered with short hairs; prepectus and posterior thoracic spiracle not observed. Metanotum mostly concealed by wings, cenchrus approx. 4× wider than long; hind tibial apical spurs shorter than basitarsomere, inner spur longer than outer; hind basitarsus shorter than remaining tarsomeres (), hind tarsal claws bifid, inner tooth significantly shorter, basal lobe absent.
Wings
Wings in normal resting position folded above abdomen, making observation of hind wing venation impossible. Fore wings () covered with evenly distributed, short hairs; anterior branch of subcostal vein complete, tubular, situated proximal to M – Sc + R junction; vein R with bend distally; veins M and Rs + M join R at bend, close to each other in †E. groehni [M and Rs + M insertion on R more separate in †E. pectinatus]; abcissa of Rs incomplete, does not reach Rs + M proximally; veins M and 1m-cu converging, angle between 1m-cu and Cu1 less than 90°; abcissa of vein Rs incomplete, not reaching vein M; cross vein 2r absent; veins 2r-m and 3r-m present; vein cu-a insert on Cu slightly closer to 1m-cu than M in †E. groehni [inserted more proximally in †E. pectinatus; slightly closer to M than 1m-cu]; vein 2A interrupted in middle, anal cell constricted ().
Abdomen
Abdomen widely covered with short, posteriorly pointing setae. Abdominal tergum 1 not observable in dorsal view (see above). Ovipositor with 3rd valvula rectangular with straight posterior margin in lateral view () and not expanded distally in ventral view, 3rd valvula shorter than 2nd valvifer [ovipositor less well observed in †E. pectinatus]. Cerci slender, approx. twice as long as wide (), with short hairs.
Remarks
As the two †Eodiprion spp. are very similar and the holotypes complement each other in which features can be observed, rather than prepare two almost identical but also partly incomplete descriptions, we have produced above a more comprehensive redescription for the genus combining observations from the two specimens, and provide short diagnoses for the species below. The most prominent characters separating †Eodiprion groehni Schedl, Citation2007 and †Eodiprion pectinatus sp. nov. are on the head, especially the distinct lateral extensions on the antennae in †E. pectinatus (see above); the differences in wing venation are perhaps less convincing and might be subject to intraspecific variation. Schedl (Citation2007, p. 66) mentions a ‘keilformigen Endglied’, i.e. a wedge-shaped end segment in the labial palp of †E. groehni, whereas we observe the 3rd palpomere in †E. pectinatus to be triangular and the 4th palpomere to be small and slender; we were unable to confirm the exact condition in the holotype of †E. groehni, so it remains unclear whether Schedl overlooked the smaller apical (4th) labial palpomere in †E. groehni, or whether this might be another difference between the two †Eodiprion spp. Among extant taxa of Diprionidae, we observed the 3rd palpomere in Neodiprion sertifer (Geoffroy, Citation1785) to be flared distally and having the 4th palpomere inserting medially on the distal margin, but the configuration is not as striking as in †E. pectinatus.
The constriction of the anal cell in the fore wing indicates that †Eodiprion should be placed in Monocteninae; the other diprionid subfamily, Diprioninae, has the anal cell unconstricted, as in the †Monodiprion male described below. In the phylogenetic analyses, †Eodiprion spp. are retrieved in a polytomy with †Monodiprion and the extant Diprioninae (), with Monocteninae being paraphyletic, even when †Eodiprion is excluded, as Augomonoctenus is sister to all other Diprionidae included. The RoguePlots (Supplemental material Figs S2, S3) indicate that †Eodiprion might be monophyletic (see above).
†Eodiprion groehni Schedl, Citation2007
()
Holotype
Holotype GPIH 4498 in Museum der Natur Hamburg, Germany (Geological and Paleontological Institute).
Emended diagnosis
Distance between lateral ocelli and posterior head margin shorter than distance between lateral ocelli. Antennomeres 4–8 with lateral extensions shorter than basal antennomere width (). Fore wing veins M and Rs + M join vein R very close to each other; vein cu-a insert on Cu slightly closer to 1m-cu than M ().
Locality and horizon
From Yantarnyi mine in the Kaliningrad Region, Russia (C. Gröhn, pers. comm., 2024).
Description
Female (male unknown). Body length approx. 7.4 mm; fore wing length approx. 6.4 mm. Specimen complete, with some characters obscured by refraction plane extending across specimen across head and below wings. Amber piece otherwise reasonably clear with a few small air bubbles. Body held straight, legs folded under body, fore wings folded over hind wings and abdomen.
†Eodiprion pectinatus Vilhelmsen sp. nov.
()
Holotype
Specimen NHMD-115402 from the amber collection of the Natural History Museum of Denmark; ‘Lophyridae/Danzig/O. Jacobsen/24-3 1916’.
Diagnosis
Distance between lateral ocelli and posterior head margin longer than distance between lateral ocelli. Antennomeres 3–12 with prominent lateral extensions, some of them longer than basal width of antennomere; extensions progressively shorter distally (). Fore wing veins M and Rs + M join vein R more separated (); vein cu-a insert on Cu slightly closer to M than 1m-cu.
Derivation of name
The species epithet refers to the pectinate antennae, the most prominent diagnostic trait of the new species.
Locality and horizon
Collected at Danzig (now Gdańsk, Poland), i.e. from the Vistula estuary.
Description
Female (male unknown). Body length approx. 8.4 mm; fore wing length approx. 7.3 mm. Specimen complete except for tip of left antenna and distal part of right hind tarsus. Body slightly curved up, legs folded under body, fore wings folded over hind wings and abdomen. Amber piece somewhat darkened and with some cracks and occlusions. Syninclusion: Cranefly (Diptera, Tipulidae) situated dorsally and left of thorax of †E. pectinatus specimen ()].
Remarks
Amber from Gdańsk where the †E. pectinatus holotype was collected is characterized by containing the smallest share of cryophobic (earlier named tropical; see Jenkins Shaw et al., Citation2023 and references therein) elements known from European Eocene ambers (Perkovsky, Citation2016; Radchenko & Perkovsky, Citation2021). This indicates the coldest environment in the European amber forest, so the two †Eodiprion spp. could be slightly separated both geographically and temporally.
Genus †Monodiprion Vilhelmsen, gen. nov.
Type species
†Monodiprion gladius Vilhelmsen, gen. et sp. nov.
Diagnosis
As per type species, see below.
Derivation of name
The genus name refers both to a combination of Monoctenus and Diprion, the nominal taxa for the two diprionid subfamilies some of the diagnostic features of which †Monodiprion combines (see Remarks below), and to the unpaired lateral extensions on most antennomeres.
†Monodiprion gladius Vilhelmsen, gen et sp. nov.
()
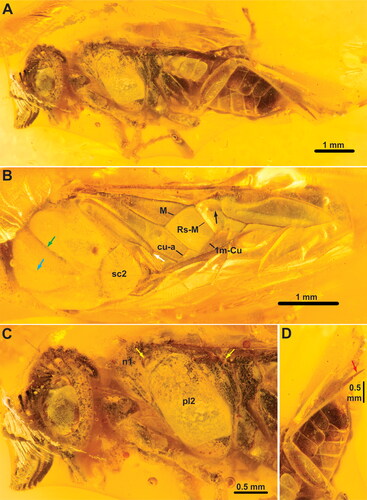
Figure 7. †Monodiprion gladius gen. et sp. nov., male holotype (GPIH 4001). A, habitus, lateral; B, habitus, dorsal; C, head and thorax, lateral; D, hind leg. White arrow = vein a; black arrow = abcissa of Rs; cyan arrow = median mesoscutal sulcus; green arrow = notauli; yellow arrows = spiracles; red arrow = inner hind tibial spur. Abbreviations: n1, pronotum; pl2, mesopleuron; sc2, mesoscutellum. Remaining abbreviations (M, Rs-M, etc.) refer to wing veins, see main text.
Holotype
Symphyta GPIH 4001 from Museum der Natur Hamburg, Germany.
Diagnosis
Gena and anterodorsal mesopleuron with prominent coarse punctation (). Third to penultimate antennomere with one distinct lateral extension each, longest extensions more than 4× longer than basal width of antennomere (). Mesoscuto-scutellar sulcus triangular. Inner apical hind tibial spur elongate, approx. equal in length to hind basitarsus (). Fore wing vein 2A entire, short proximally placed vein a connecting veins 1A and 2A, anal cell complete ().
Derivation of name
The species epithet is the Latin word for sword and refers to the prominent inner apical hind tibial spur which is diagnostic for the new taxon.
Locality and horizon
Not recorded.
Description
Male (female unknown). Body length approx. 7.5 mm; fore wing length approx. 5.3 mm. Specimen complete, preserved in clear piece of amber with few small air bubbles embedded in epoxy resin; diffraction plane extends for part of length inclusion at level of wings, mouthparts partly occluded by milky substance. Body held straight, legs folded under body, fore wings folded over hind wings and abdomen.
Head
Head capsule broader than high, covered with short setae, with minute punctation unless otherwise noticed; compound eyes oval, higher than long, slightly converging ventrally, setation not observed; ocelli placed in obtuse triangle, posterior margin of median ocellus in line with anterior margins of lateral ocelli; distance between lateral ocelli and posterior margin of head approx. 0.85× distance between lateral ocelli; median ocellus situated dorsally in shallow, lozenge-shaped depression; gena slightly widening dorsally, with coarse punctures/foveae (); occipital carina entirely absent; epistomal sulcus not observed, anterior clypeal margin slightly incurved. Antenna () inserted just below mid-height of eyes, slightly longer than width of head, with approx. 20 antennomeres; pedicellus much wider than long; 3rd to penultimate antennomere with one distinct lateral extension each, longest extensions more than 4× longer than basal width of antennomere, extensions of decreasing extent distally, each extension with two rows of elongate setae set in V-shaped pattern; apical antennomere without lateral extension but covered with setae, several times longer than wide. Labrum with rounded anterior margin, covered with short setae; mandibles closed, left mandible overlapping right, number of teeth not observable; maxillary palp and labial palp difficult to observe, exact palpomere count not possible.
Thorax
Pronotum developed medially, curved, covered with short hairs, with incurvation medially in dorsal margin, closely abutting mesopleuron, with incurvation for anterior thoracic spiracle laterally (); propleura with well-developed propleural sulcus, medioventral corners apparently widely separated, katepisterna not observed; prosternum apparently lozenge-shaped anteriorly, with prominent lateral corners, narrowing posteriorly; fore leg calcar straight, not bifid; apical tibial spurs with small membraneous globule distally. Mesonotum covered with short hairs, sculpture indistinct; median mesoscutal sulcus narrow line, notauli broader grooves, meeting medially in broadened depression well anteriorly to mesoscuto-scutellar sulcus (); mesoscuto-scutellar sulcus triangular, anterior and posterior margins of mesoscutellum rounded, postscutellum well-developed; mesopleuron coarsely punctate/foveate anterodorsally (), covered with short hairs; prepectus not observed, anterior margin of mesopleuron not raised or otherwise modified; posterior thoracic spiracle situated in shallow incurvation at posterodorsal corner of mesopleuron. Metanotum mostly concealed by wings, cenchri not observed; posterior part of metapleuron rectangular flange; inner hind tibial spur elongate, approx. twice as long as outer, of approx. equal length with basitarsus; hind basitarsus shorter than remaining tarsomeres (), hind tarsal claws bifid, inner tooth significantly shorter, basal lobe absent.
Wings
Fore wings () covered with evenly distributed, short hairs; anterior branch of subcostal vein complete, tubular, situated proximal to M – Sc + R junction; vein R bent distally; veins M and Rs + M join R at bend, close to each other; abcissa of Rs incomplete, does not reach Rs + M proximally; veins M and 1m-cu converging, angle between 1m-cu and Cu1 approx. 90°; cross vein 2r absent; veins 2r-m and 3r-m present; vein cu-a insert on Cu much closer to 1m-cu than M; anal cell complete, vein 2A continuous, bent towards 1A in middle, with short, straight vein a in distal part of bend ().
Abdomen
Abdomen without prominent sculpture, mostly covered with short hairs. Abdominal terga 1–8 of similar proportions, much wider than long (); abdominal sterna 1–7 of similar proportions, much wider than long; sternum 8 with deeply incurved posterior margin, median part concealed by sternum 7; tergum 9 much wider than long, with short, cylindrical cerci with elongate hairs protruding from posterior margin; sternum 9 approx. as long as wide, ovoid.
Remarks
†Monodiprion gladius can most easily be separated from †Eodiprion spp. by the foveate gena and mesopleuron, the elongate hind tibial inner apical spur, and the complete anal cell in the fore wing. Schedl (Citation2008) noted some of these differences and stated that they exceeded what would normally be observed within an extant species, but he refrained from placing the male specimen in a different species or genus due to the limited material of diprionid Baltic amber fossils at his disposal. The combination of having male antennomeres with only one lateral extension each and a complete anal cell seems to be unique among Diprionidae, not being observed in any extant genera; see e.g. keys in Goulet (Citation1992) and Lacourt (Citation2020) and Rohwer (Citation1918). For this reason, we have decided to erect †Monodiprion as a separate genus, even if the relationships with †Eodiprion spp. are not fully resolved.
Discussion
Phylogenetic position of fossils
The results of our analyses corroborate the recognition of Heptamelidae as a family (Malm & Nyman, Citation2015), and Athaliidae is retrieved as sister to the remaining Tenthredinidae, thus not contradicting the family level status assigned by Niu et al. (Citation2022). This is also the case for Zenargidae, which comes out as sister to Argidae, in contrast to the results of Malagón-Aldana et al. (Citation2021) which placed them as sister to Argidae + Pergidae. The remainder of the Tenthredinidae as traditionally circumscribed is retrieved as a monophylum. This clade might be characterized by the presence of nine antennomeres, at least in the ground plan. Within the Tenthredinidae sensu stricto, our results, should they be confirmed in future analyses, indicate that substantial changes will have to be made at the subfamily level; e.g. Heterarthrinae should probably be subsumed in Blennocampinae, while Eriocampini: Eriocampa might have to be raised to subfamily status. Some errant taxa, e.g. Allantinae: Eusunoxa, will have to be transferred to a subfamily placement more consistent with their phylogenetic position. We postpone any formal taxonomic changes for now, awaiting more comprehensive phylogenetic treatments of the Tenthredinoidea in the pipeline (Wutke et al., Citationin review).
The phylogenetic placement of †Eodiprion in a clade with Diprioninae is at odds with the constricted fore wing anal cell that indicates affinity with Monocteninae. Similarly, the presence of one lateral extension on each antennomere in the male (diagnostic of Monocteninae) of †Monodiprion contrasts with its phylogenetic position – which is, however, supported by the entire, unconstricted anal cell with a short and straight cross vein a (diagnostic of Diprioninae). Having the male antennomeres initially with one lateral extension each and subsequently evolving two extensions (Diprioninae) seems to be a plausible scenario for character evolution but provides character support only for Diprionidae (presence of one or two lateral antennal extensions in males) and Diprioninae (having two lateral extensions). Having the fore wing anal cell unconstricted (e.g. ) is plesiomorphic for Tenthredinoidea and Hymenoptera as a whole (Vilhelmsen, Citation2001); however, in most Cimbicidae (three out of four subfamilies, i.e. all except Cimbicinae; see Vilhelmsen, Citation2019, figs 15, 16), the sister group of Diprionidae, and many other Tenthrediniodea (Vilhelmsen, Citation2015, figs 11, 12), the anal cell is constricted. Furthermore, in Diprioninae the cross vein a is straight and placed proximally in the anal cell, as opposed to oblique and placed distally in the hymenopteran ground plan (compare and ), indicating that these two conditions are probably not truly homologous. Given the character distribution, the common ancestor of Cimbicidae and Diprionidae probably had the fore wing anal cell constricted, with independent reversals in Cimbicinae and Diprioninae.
Malm and Nyman (Citation2015) retrieved Monocteninae as paraphyletic, a result replicated in our analyses. The results of the phylogenetic analyses and the distribution of the diagnostic characters in the fossils presented here do not corroborate the current classification of Diprionidae in two subfamilies. Monocteninae is apparently characterized only by plesiomorphic traits and is paraphyletic with regard to Diprioninae. A more comprehensive phylogenetic treatment with a denser taxon sampling among the extant members of the family is needed to establish a more natural subfamily classification.
The placement of †Rovnotaxonus in ‘core’ Allantinae probably reflects its wing venation which suggests strong affinity with Allantinae: Allantini. The presence of the abscissa of the Rs in the fore wing, presumably a plesiomorphic trait, excludes †Rovnotaxonus from extant genera of Allantini other than Taxonus. However, our inability to observe diagnostic traits in the clypeus and mandibles (see above) prevent us from confirming a placement in this genus, and we therefore place the fossil in its own genus for now. The RoguePlot for †Rovnotaxonus (Supplemental material Fig. S3) suggests a number of alternative placements, also outside Allantinae, and all with poor support.
Vilhelmsen and Engel (Citation2012) compared †Sambia to a range of extant genera of Tenthredininae and concluded that none of these were a good fit for the fossil, hence placing it in a new genus. This is corroborated by the results of the analyses presented here, with †Sambia in the basal trichotomy of Tenthredininae. The RoguePlot (Supplemental material Fig. S6) suggests a few alternative positions, the ones at the base of Tenthredinidae being the best supported and the one outside this subfamily having very low probability.
Preservation of ‘Symphyta’ in Eocene ambers
Despite having a substantial fossil record (Taeger et al., Citation2010), Tenthredinoidea are surprisingly rare in Eocene ambers, with only four genera described so far, including †Rovnotaxonus and †Monodiprion. This is comparable to Orussoidea (Vilhelmsen & Zimmermann, Citation2014); Vilhelmsen et al. (Citation2024) reports four specimens in three genera of Orussidae from Baltic amber), a wood-living ‘symphytan’ taxon which is much rarer in extant faunas than the true sawflies. The fraction of Tenthredinoidea caught in extant conifer resins was reported to be 5.9% of all solitary wasps by Zherikhin et al. (Citation2009); Darling and Packer (Citation1988) found ‘Symphyta’ (probably mostly tenthredinoids) to constitute 4% of all Hymenoptera collected in a range of Malaise traps. Archibald et al. (Citation2010, Citation2018) reported the occurrence of a range of families and subfamilies of tenthredinoids in two compression fossil localities of Ypresian age: 13% of all Hymenoptera in the Okanagan Highlands, British Columbia (early Eocene, 53.2–49.6 Ma; Archibald, pers. comm., 2024); approx. 30% in Tadushi Formation, Russian Far East (late early Eocene; Makarkin, Citation2014). In comparison with both the present-day fauna and fossil faunas closer to them in time, Tenthredinoidea seems to be substantially underrepresented in the late Eocene amber faunas.
Several factors might reduce the chances of a true sawfly becoming embedded in resin and preserved in amber. Comparatively large body size, restricted home range and low affinity for the woody habitat (again, comparison with Orussoidea, parasitoids of wood-living insect larvae, is instructive) are probably among them. It might not be a coincidence that of the five fossils treated in this paper, three belong to Diprionidae, a family with larvae feeding on conifer needles, an unusual host plant choice within Tenthredinoidea (Nyman et al., Citation2019). Larsson (Citation1978) suggested that the diprionids in the Baltic amber forest might have fed on the trees that produced the resin, trapping a few of them. Whereas Diprionidae have yet to be recorded in Rovno amber, their presence is indicated by the presence of the parasitoid wasp †Dipriocampe bouceki Gumovsky and Perkovsky, Citation2005 from this locality (Gumovsky & Perkovsky, Citation2005); extant members of this genus are known parasitoids of Diprionidae.
Another factor could be off-set timing between the occurrence of sawflies and the peak of resin production. Stellate hairs, the signature inclusion of late Eocene ambers, were probably primarily produced by staminate flowers of red oaks (Quercus sect. Lobatae) and Quercus sect. Protobalanus (Sadowski et al., Citation2020). These are today distributed in North, Central and northern South America and flower in early spring in a Mediterranean-like climate with warm summers; if the climate in the Eocene amber forests was partially similar (Jenkins Shaw et al., Citation2023) to that in the present range of these trees (i.e. warmer in winter and spring than the current climate in northern and central Europe as well as in the temperate regions of eastern USA; this applies in particular to the Rovno forest; see Kirichenko-Babko and Perkovsky Citation2023 and references therein), the abundance of stellate hairs in many amber pieces might indicate that peak resin flow was also in early spring (Borkent & Grogan, Citation1995). In contrast, peak season for tenthredinoids in the Northern Hemisphere is currently in late spring–early summer (Roller, Citation2006). Interestingly, the amber piece in which the holotype of †Rovnotaxonus aristovi is included is devoid of stellate hairs, indicating that it might have been caught in a resin flow in late spring after the peak of resin production earlier in the season.
Conclusions
In this paper, we have established the approximate phylogenetic positions of the five known Eocene amber fossil taxa of true sawflies (Tenthredinoidea). †Eodiprion + †Monodiprion, †Rovnotaxonus and †Sambia provide minimum ages for the clades Diprioninae, ‘core’ Allantinae, and Tenthredininae, respectively. The phylogenetic framework provided here will facilitate future dating endeavours in true sawflies by establishing new calibration nodes across the superfamily. We stress the importance of fully integrating fossils in data sets assembled for exploring the patterns and timing of insect evolution in a phylogenetic context.
Author contributions
LV conceived the study, produced the descriptions and the images, and scored the fossil taxa for phylogenetic analyses. JJS assembled the combined data set, performed the phylogenetic analyses and produced the trees and RoguePlots. EEP curated the material and provided background information on collecting circumstances. LV wrote the first draft of the paper. All authors contributed to the final version of the paper.
Associate Editor: Vincent Perrichot
Supplemental Material
Download Zip (2 MB)Acknowledgements
Carsten Gröhn, Glinde, Germany, and Ulrich Kotthoff, Museum der Natur Hamburg, Germany, provided access to images of the holotype of †Eodiprion groehni. Tobias Malm and Tommi Nyman provided information about the analytical settings for their 2015 paper. Thanks to Mario Coiro for helpful suggestions regarding RoguePlots, and to Alexandr P. Rasnitsyn, Paleontological Institute, Moscow, Russia, for the discussion of sawfly seasonality. Mykola R. Khomich (Rovno, Ukraine) helped obtain the specimens from Zhovkini; Anatoly P. Vlaskin (SIZK) cut and polished the piece. Two anonymous reviewers provided useful input on the first submitted version of the paper. Evgeny Perkovsky is supported by a grant from the Scholars at Risk Ukraine (SARU) programme jointly funded by the Villum Foundation, Carlsberg Foundation and Novo Nordisk Foundation; Josh Jenkins Shaw is supported by the Villum Experiment grant ‘Baltic amber enigma’, managed by Alexey Solodovnikov.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental material
Supplemental material for this article can be accessed here: https://doi.org/10.1080/14772019.2024.2348774.
References
- Archibald, S. B., Bossert, W. H., Greenwood, D. R., & Farrell, B. D. (2010). Seasonality, the latitudinal gradient of diversity, and Eocene insects. Paleobiology, 36, 374–398. https://doi.org/10.1666/09021.1
- Archibald, S. B., Rasnitsyn, A. P., Brothers, D. J., & Mathewes, R. W. (2018). Modernisation of the Hymenoptera: Ants, bees, wasps, and sawflies of the early Eocene Okanagan Highlands of western North America. The Canadian Entomologist, 150, 205–257. https://doi.org/10.4039/tce.2017.59
- Borkent, A., & Grogan, W. L. (1995). A revision of the genus Ceratopogon Meigen (Diptera: Ceratopogonidae). Memoirs of the Entomological Society of Washington, 15, 122–130.
- Brischke, C. G. A. (1886). Die Hymenopteren des Bernsteins. Schriften der Naturforschenden Gesellschaft Danzig, 6, 278–279.
- Chemyreva, V. G., Vasilenko, D. V., & Perkovsky, E. E. (2024). ‘Where there are many cattle' in the Eocene of Ukraine: Review of Ambositra Masner (Hymenoptera, Diapriidae, Ambositrinae) from the Rovno amber, with the description of three new species. Zootaxa, 5446, 499–516. https://doi.org/10.11646/ZOOTAXA.5446.4.3
- Darling, D. C., & Packer, L. (1988). Effectiveness of Malaise traps in collecting Hymenoptera: The influence of trap design, mesh size and location. The Canadian Entomologist, 120, 787–796. https://doi.org/10.4039/Ent120787-8
- Dietrich, C. H., Simutnik, S. A., & Perkovsky, E. E. (2023). Typhlocybinae leafhoppers (Hemiptera: Cicadellidae) from Eocene Rovno amber reveal a transition in wing venation and a defensive adaptation. Journal of Paleontology, 97, 366–379. https://doi.org/10.1017/jpa.2023.3
- Fallén, C. F. (1808). Försok till uppställning och beskrifning å de i Sverige fundne Arter af Insect-Slägtet Tenthredo Linn. Kongl. Vetenskaps Academiens nya Handlingar, 29, 39–64.
- Geoffroy, E. L. (1785). In A. F. Fourcroy (Ed.), de Entomologia Parisiensis, sive catalogus Insectorum quae in agro parisiensi reperiuntus (vol. 1–2, pp. i–viii + 1–231 + [232]–544).
- Goulet, H. (1992). The genera and subgenera of the sawflies of Canada and Alaska. The Insects and Arachnids of Canada, 20 (pub. 1876), 1–235.
- Gumovsky, A. V., & Perkovsky, E. E. (2005). Taxonomic notes on Tetracampidae (Hymenoptera: Chalcidoidea) with description of a new fossil species of Dipricocampe from Rovno amber. Entomological Problems, 35, 123–130.
- Hartig, T. (1837). Die Aderflügler Deutschlands mit besonderer Berücksichtigung ihres Larvenzustandes und ihres Wirkens in Wäldern und Gärten für Entomologen, Wald- und Gartenbesitzer. In Die Familien der Blattwespen und Holzwespen nebsteiner allgemeinen Einleitung zur Naturgeschichte der Hymenopteren. Erster Band (pp. i–xiv + 1–416). Haude und Spener.
- Huber, J. T., & Sharkey, M. J. (1993). Structure. In H. Goulet & J. T. Huber (Eds.), Hymenoptera of the world: An identification guide to families (pp. 13–59). Research Branch, Agriculture Canada, Publ. 1894/E.
- Iakovleva, A. I., Aleksandrova, G. N., & Mychko, E. V. (2022). Late Eocene (Priabonian) dinoflagellate cysts from Primorsky quarry, southeast Baltic coast, Kaliningrad Oblast, Russia. Palynology, 46, 1–40. https://doi.org/10.1080/01916122.2021.1980743
- Isaka, Y., & Sato, T. (2015). Was species diversification in Tenthredinoidea (Hymenoptera: Symphyta) related to the origin and diversification of angiosperms? The Canadian Entomologist, 147, 443–458. https://doi.org/10.4039/tce.2014.60
- Jenkins Shaw, J., Perkovsky, E. E., Ślipiński, A., Escalona, H., & Solodovnikov, A. (2023). An extralimital fossil of the genus Diagrypnodes (Coleoptera: Salpingidae: Inopeplinae). Historical Biology. https://doi.org/10.1080/08912963.2023.2206858
- Kirichenko-Babko, M., & Perkovsky, E. E. (2023). The first Neotropical Carabidae (Coleoptera) from the Eocene of Ukraine: Finding the first Old World ant nest beetle related to Eohomopterus in the Rovno amber. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 114, 115–124. https://doi.org/10.1017/S1755691023000105
- Klopfstein, S., & Spasojevic, T. (2019). Illustrating phylogenetic placement of fossils using RoguePlots: An example from ichneumonid parasitoid wasps (Hymenoptera, Ichneumonidae) and an extensive morphological matrix. PLoS One, 14, e0212942. https://doi.org/10.1371/journal.pone.0212942
- Lacourt, J. (2020). Sawflies of Europe. N.A.P. Editions.
- Larsson, S. G. (1978). Baltic amber – a palaeobiological study. Entomonograph, 1, 1–192.
- Legalov, A. A., Vasilenko, D. V. & Perkovsky, E. E. (2023). New proxy for Moraceae in Priabonian of Europe: First record of the genus Demimaea Pascoe, 1870 (Coleoptera: Curculionidae) from Eocene Rovno amber. Historical Biology, 35, 1322–1328. https://doi.org/10.1080/08912963.2022.2089983
- Lewis, P. O. (2001). A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology, 50, 913–925. https://doi.org/10.1080/106351501753462876
- Linné, C. (1758). Systema Naturae, per regna tria naturae secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Editio Decima Reformata (10th ed., Vol. 1, pp. 1–824). Laurentius Salvius.
- Linné, C. (1760). Fauna Svecica sistens animalia Sveciae regni: Mammalia, Aves, Amphibia, Pisces, Insecta, Vermes. Distributa per classes & ordines, genera & species, cum differentiis specierum, synonymis auctorum, nominibus incolarum, locis natalium, descriptionibus insectorum. Editio altera, auctior (p. 578). Laurentius Salvius.
- MacGillivray, A. D. (1908). Emphytinae—new genera and species and synonymical notes. The Canadian Entomologist, 40(10), 365–369. https://doi.org/10.4039/Ent40365-10
- Maddison, W. P., & Maddison, D. R. (2021). Mesquite: A modular system for evolutionary analysis. Version 3.70. Available from: http://www.mesquiteproject.org (version released August 2021).
- Makarkin, V. M. (2014). A new fossil green lacewing (Neuroptera: Chrysopidae) from the Eocene Tadushi Formation, eastern Sikhote-Alin. Far Eastern Entomologist, 272, 1–7.
- Malagón-Aldana, L. A., Smith, D. R., Shinohara, A., & Vilhelmsen, L. (2021). From Arge to Zenarge: Adult morphology and phylogenetics of argid sawflies (Hymenoptera: Argidae). Zoological Journal of the Linnean Society, 193, 880–938. https://doi.org/10.1093/zoolinnean/zlaa170
- Malm, T., & Nyman, T. (2015). Phylogeny of the symphytan grade of Hymenoptera: New pieces into the old jigsaw (fly) puzzle. Cladistics, 31(1), 1–17. https://doi.org/10.1111/cla.12069
- Melnitsky, S. I., Ivanov, V. D., & Perkovsky, E. E. (2021). A new species of the fossil genus Electrotrichia (Insecta: Trichoptera: Hydroptilidae) from Rovno amber (Zhytomyr region, Olevsk amber locality). Palaeoentomology, 4, 421–424. https://doi.org/10.11646/palaeoentomology.4.5.4
- Menge, A. (1856). Lebenszeichen vorweltlicher, im Bernstein eingeschlossener Thiere. Programm Petrischule, Danzig, 1–32.
- Mitov, P. G., Perkovsky, E. E., & Dunlop, J. A. (2021). Harvestmen (Arachnida: Opiliones) in Eocene Rovno amber (Ukraine). Zootaxa, 4984, 43–72. https://doi.org/10.11646/zootaxa.4984.1.6
- Nel, A., Wei, M., Niu, G., Garrouste, R., & Jouault, C. (2023). Description of a new fossil genus of conifer sawfly (Hymenoptera: Diprionidae) revealed by UV light. Palaeoentomology, 6, 313–320. https://doi.org/10.11646/palaeoentomology.6.3.14
- Niu, G., Budak, M., Korkmaz, E. M., Doğan, Ö., Nel, A., Wan, S., Cai, C., Jouault, C., Li, M., Wei, M. (2022). Phylogenomic analyses of the Tenthredinoidea support the familial rank of Athaliidae (Insecta, Tenthredinoidea). Insects, 13, 858. https://doi.org/10.3390/insects13100858
- Nyman, T., Onstein, R. E., Silvestro, D., Wutke, S., Taeger, A., Wahlberg, N., Blank, S. M., Malm, T. (2019). The early wasp plucks the flower: Disparate extant diversity of sawfly superfamilies (Hymenoptera: ‘Symphyta’) may reflect asynchronous switching to angiosperm hosts. Biological Journal of the Linnean Society 128, 1–19. https://doi.org/10.1093/biolinnean/blz071
- Panzer, G. W. F. (1801). Faunae Insectorum Germanicae initia oder Deutschlands Insecten (vol. 7, pp. 1–24). Felssecker.
- Perkovsky, E. E. (2016). Tropical and Holarctic ants in Late Eocene ambers. Vestnik zoologii, 50, 111–122. https://doi.org/10.1515/vzoo-2016-0014
- R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
- Radchenko, A. G., & Perkovsky, E. E. (2021). Wheeler’s dilemma revisited: First Oecophylla-Lasius syninclusion and other ants syninclusions in the Bitterfeld amber (late Eocene). Invertebrate Zoology, 18, 47–65. https://doi.org/10.15298/invertzool.18.1.02
- Rambaut, A., Drummond, A. J., Xie, D., Baele, G., & Suchard, M. A. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67, 901–904. https://doi.org/10.1093/sysbio/syy032
- Rohwer, S. A. (1911). A classification of the suborder Chalastogastra of the Hymenoptera. Proceedings of the Entomological Society of Washington, 13, 215–226.
- Rohwer, S. A. (1918). New sawflies in the subfamily Diprioninae. Proceedings of the Entomological Society of Washington, 20, 79–90.
- Roller, L. (2006). Seasonal flight activity of sawflies (Hymenoptera, Symphyta) in submontane region of the Western Carpathians, Central Slovakia. Biologia, 61, 193–205. https://doi.org/10.2478/s11756-006-0030-z
- Ronquist, F., Klopfstein, S., Vilhelmsen, L., Schulmeister, S., Murray, D. L., & Rasnitsyn, A. P. (2012). A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera. Systematic Biology, 61, 973–999. https://doi.org/10.1093/sysbio/sys058
- Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., Larget B., Liu L., Suchard M. A., & Huelsenbeck J. P. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029
- Sadowski, E.-M., Schmidt, A. R., & Denk, T. (2020). Staminate inflorescences with in situ pollen from Eocene Baltic amber reveal high diversity in Fagaceae (oak family). Willdenowia, 50, 405–517. https://doi.org/10.3372/wi.50.50303
- Say, T. (1836). Descriptions of new species of North American Hymenoptera, and observations on some already described. Boston Journal of Natural History, 1, 209–305
- Schedl, W. (2007). Eidonomische und taxonomische Beschreibung einer Diprionidae aus dem baltischen Bernstein (Hymenoptera: Symphyta: Diprionidae). Zeitschrift der Arbeitsgemeinschaft Österreichischer Entomologen, 59, 65–69.
- Schedl, W. (2008). Nachweis eines Männchens von Eodiprion sp. aus dem baltischen Bernstein (Hymenoptera: Symphyta: Diprionidae). Berichte des naturwissenschaftlichen-medizinischen Verein Innsbruck, 95, 77–80.
- Simutnik, S. A., Perkovsky, E. E., & Vasilenko, D. V. (2022). Protaphycus shuvalikovi Simutnik gen. et sp. n. (Chalcidoidea, Encyrtidae, Encyrtinae) from Rovno amber. Journal of Hymenoptera Research, 91, 1–9. https://doi.org/10.3897/jhr.91.81957
- Smith, D. R. (1979). Nearctic Sawflies IV. Allantinae: Adults and larvae (Hymenoptera: Tenthredinidae). US Department of Agriculture Technical Bulletin, 1595, 1–172.
- Sokoloff, D. D., Ignatov, M. S., Remizowa, M. V., Nuraliev, M. S., Blagoderov, V., Garbout, A., & Perkovsky, E. E. (2018). Staminate flower of Prunus s. l. (Rosaceae) from Eocene Rovno amber (Ukraine). Journal of Plant Research, 131, 925–943. https://doi.org/10.1007/s10265-018-1057-2
- Taeger, A., Blank, S. M., & Liston, A. D. (2010). World catalog of Symphyta (Hymenoptera). Zootaxa, 2580, 1–1064. https://doi.org/10.11646/zootaxa.2580.1.1
- Telnov, D., Perkovsky, E. E., Kundrata, R., Kairišs, K., Vasilenko, D. V., & Bukejs, A. (2023). Revealing Paleogene distribution of the Ptilodactylidae (Insecta: Coleoptera): The first Ptilodactyla Illiger, 1807 records from Rovno amber of Ukraine. Historical Biology, 35, 2171–2180. https://doi.org/10.1080/08912963.2022.2136034
- Vilhelmsen, L. (2001). Phylogeny and classification of the extant basal lineages of the Hymenoptera (Insecta). Zoological Journal of the Linnean Society, 131, 393–442. https://doi.org/10.1111/j.1096-3642.2001.tb01320.x
- Vilhelmsen, L. (2015). Morphological phylogenetics of the Tenthredinidae (Insecta: Hymenoptera). Invertebrate Systematics, 29, 164–190. https://doi.org/10.1071/IS14056
- Vilhelmsen, L. (2019). Giant sawflies and their kin: Morphological phylogeny of Cimbicidae (Hymenoptera). Systematic Entomology, 44, 103–127. https://doi.org/10.1111/syen.12314
- Vilhelmsen, L., Boudinot, B., Jenkins Shaw, J., Hammel, J.U., & Perrichot, V. (2024). Echoes from the Cretaceous: New fossils shed light on the evolution of host detection and concealed ovipositor apparatus in the parasitoid wasp superfamily Orussoidea (Hymenoptera). Zoological Journal of the Linnean Society, [2024], 1–19. (published online 28 February 2024). https://doi.org/10.1093/zoolinnean/zlae021
- Vilhelmsen, L., & Engel, M. S. (2012). Sambia succinica, a crown group tenthredinid from Eocene Baltic amber (Hymenoptera: Tenthredinidae). Insect Systematics & Evolution, 43, 271–281. https://doi.org/10.1163/1876312X-04303003
- Vilhelmsen, L., & Zimmermann, D. (2014). Baltorussus total makeover: Rejuvenation and sex change in an ancient parasitoid wasp lineage. PLoS ONE, 9, e98412. https://doi.org/10.1371/journal.pone.0098412
- Wutke, S., Blank, S. M., Boevé, J.-L., Faircloth, B. C., Koch, F., Linnen, C., Malm, T., Niu, G., Prous, M., Schiff, N., Schmidt, S., Taeger, A., Vilhelmsen, L., Wahlberg, N., Wei, M., & Nyman, T. (in review). Phylogenomics and biogeography of sawflies and woodwasps. Molecular Phylogenetics and Evolution, [2024], xx–xx.
- Zherikhin, V. V., Sukacheva, I. D., & Rasnitsyn, A. P. (2009). Arthropods in contemporary and some fossil resins. Paleontological Journal, 43, 987–1005. https://doi.org/10.1134/S0031030109090019
