ABSTRACT
Introduction: Primary percutaneous coronary intervention (PCI) represents the gold-standard treatment for patients presenting with an ST-elevation myocardial infarction (STEMI). Acute myocardial infarction is a complex clinical scenario, and an appropriate therapeutic approach could be represented by a balanced integration between healthcare system and medical competence.
Areas covered: In this review we discuss how a primary PCI network, and the new therapeutic options could be coupled in order to obtain improved clinical outcomes. The present report will focus on three main issues related to STEMI patients, namely, out of hospital management, primary PCI and pharmacological treatment.
Expert commentary: A possible correct approach to a patient presenting a STEMI could be considered as a stepwise process, given by 5 steps: reducing the time to reperfusion; dual antiplatelet administration; radial access; new generation drug eluting stent implantation; long term management.
1. Introduction
Despite a recently reported reduction in the incidence of ST elevation myocardial infarction (STEMI), probably due to some effective preventive strategies [Citation1], STEMI remains one of the most important causes of morbidity and mortality in developed countries [Citation1]. STEMI is due to an abrupt thrombotic occlusion of a main epicardial coronary vessel and the main clinical and therapeutic goal is represented by a prompt and timely coronary reperfusion that could be summarized in a ‘rapid, radial, reperfusion’ ().
In this setting, primary percutaneous coronary intervention (PCI) is the gold standard treatment [Citation2,Citation3]. In patients presenting with STEMI, the most effective way to reduce mortality and morbidity is a short reperfusion time established by a narrow interval between first medical contact (FMC) and recanalization of the culprit lesion [Citation2–Citation6]. A reduction of door-to-balloon (D-to-B) time < 90 min could be of minor relevance [Citation7]. Menees et al. observed that a significant reduction of average D-to-B time (from 83 to 67 min; p < 0.001) did not impact in-hospital mortality in an observational registry of 96,738 STEMI patients (more than 80% presented with a D-to-B time ≤ 90 min) treated with primary PCI [Citation8]. These observational results suggest STEMI as complex clinical conundrum with early coronary revascularization as a key element, even though not the only one relevant to achieve an optimal treatment [Citation2,Citation3].
The aim of the present review is to summarize the recent insights in out-of-hospital management, primary PCI, and pharmacological treatment resulting in an effective primary PCI network ().
2. Out-of-hospital management and therapy
Primary PCI is the core treatment of patients with STEMI. A rate of 600 primary PCI procedures per 1 million inhabitants is considered to be the cutoff value to examine a STEMI network, according to the international standard [Citation9,Citation10]. Anyway, large disparities in reperfusion treatment still exist globally. In Eastern Europe, USA, and China, an extensive number of STEMI patients are not receiving any reperfusion therapy [Citation11,Citation12]. Kristensen et al. observed a considerable variety in primary PCI adoption in 37 European countries ranging from 23 to 884 primary PCI procedures per 1,000,000 inhabitants. Conversely, thrombolysis rate was 100 per 1,000,000 inhabitants, suggesting that primary PCI is the preferred revascularization strategy in Europe [Citation11].
Implementation of a primary PCI network is paramount to realize timely reperfused STEMI [Citation1,Citation2]. Nevertheless also in geographies with a well-performing primary PCI network, STEMI patients not presenting to a hospital with on-site PCI capacity will often not receive primary PCI within guidelines suggested times [Citation13]. In this setting, several pharmacological strategies were proposed and tested in order to reduce the total ischemia time and improve clinical outcomes. In the ‘Strategic Reperfusion Early after Myocardial Infarction (STREAM) study,’ which enrolled 1892 STEMI patients who presented within 3 h after symptom onset and unable to undergo primary PCI within 1 h, Armostrong et al. demonstrated that prehospital fibrinolysis coupled with timely coronary angiography is as effective as primary angioplasty alone, in terms of composite of death from any cause, shock, congestive heart failure, or reinfarction within 30 days (fibrinolysis + primary PCI 116 [12.4%] vs. primary PCI 135 [14.3%] ; relative risk in the fibrinolysis group, 0.86; 95% confidence interval [CI], 0.68–1.09; p = 0.21). This data demonstrated, for the first time, that a reperfusion strategy through prehospital fibrinolysis, combined with a timely coronary angiography is effective in early-presenter STEMI who could not be treated by primary PCI within 1 h after the FMC. The main explanation of this result is attributable to a significant reduction of overall reperfusion time (100 min in the fibrinolysis group vs. 178 min in the primary PCI group) and consequently to a fall of the total ischemia time [Citation14]. However, a significant increase in intracranial hemorrhages in patients treated with fibrinolysis (1.0% vs. 0.2%; p = 0.04), mainly in patients >75 years of age, was observed.
In order to increase the benefits in terms of efficacy and to reduce the risk of hemorrhagic events, different strategies with prehospital administration of antiplatelet agents were experienced [Citation15]. The in-ambulance administration of direct-acting platelet P2Y12 receptor antagonist was tested in the ‘Administration of Ticagrelor in the Cath Lab or in the Ambulance for New ST Elevation Myocardial Infarction to Open the Coronary Artery (ATLANTIC) study,’ an international, multicenter, randomized, double-blind study that enrolled 1862 STEMI patients with chest pain duration ≥30 min but ≤6 h. Patients were randomized to prehospital (ambulance) or in-hospital (catheterization laboratory) treatment with 180-mg loading dose Ticagrelor, in addition to aspirin and standard care. The ATLANTIC trial demonstrated that a prehospital administration of Ticagrelor in the setting of primary PCI is not associated with significant benefit in term of percentage of patients with ST-segment elevation resolution >70% before primary PCI (prehospital group 672 [86.8%] vs. in-hospital group 722 [87.6%], 0.93; 95% CI: 0.69–1.25; p = 0.63), percentage of patients with Thrombolysis in Myocardial Infarction flow grade 3 in the infarct-related artery at initial coronary-angiography (prehospital group 681 [82.6%] vs. 711 [83.1%] in-hospital group, 0.97; 95% CI, 0.75–1.25; p = 0.82) and in term of clinical composite secondary end points (death, myocardial infarction [MI], stroke, urgent coronary revascularization, or stent thrombosis) [Citation16]. However, pretreated patients experienced less definite stent thrombosis events, both at 24 h (prehospital group 0 [0%] vs. in-hospital group 8 [0.8%], p = 0.008) and at 30 days (2 [0.2%] vs. 11 [1.2%], p = 0.02) [Citation16]. It should be highlighted that in this trial, the median time between the two loading doses (prehospital group vs. in-hospital group) was only 31 min and with an optimal average from randomization to angiography time of 48 min.
These data suggest that, in the out-of-hospital setting, the best ‘therapy’ is the reduction of the time to reperfusion, reasonably through an optimization of the primary PCI networks.
Other strategies, such as prehospital thrombolysis, could be considered as adjunctive to primary PCI, but they do not represent an equally valid alternative. According to ESC guidelines, prehospital thrombolysis is justified in early presenting patients with a large infarct and low bleeding risk with a predictable time from FMC to balloon inflation greater than 90 min and in all patients with an expected time from FMC to percutaneous reperfusion more than 120 min [Citation3]. Finally, as suggested by ESC guidelines, Class IIB Level of evidence B, upstream use of a glycoprotein IIb/IIIa inhibitor should be considered in high-risk STEMI patients undergoing transfer for primary PCI [Citation3].
3. Primary PCI
3.1. Access site
The large body of evidence produced in last 10 years regarding the importance of an invasive coronary revascularization associated with more powerful anti-aggregation and anticoagulation, had markedly reduced mortality in STEMI patients, nevertheless it was associated to an increased risk of bleeding [Citation3,Citation17–Citation19]. One of the most common sites of bleeding is the femoral artery, if used as coronary angiography access. For this reason, several attempts were carried out to reduce the risk of access-site bleeding. One of the most investigated and effective approaches was the shift from femoral to radial access [Citation20–Citation24]. The Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome (RIFLE-STEACS) study enrolled 1001 patients and compared radial versus femoral in terms of 30-day incidence of net adverse clinical events (NACE: cardiac death, MI, stroke, target lesion revascularization, and non-coronary artery bypass graft [non-CABG]-related bleeding) [Citation17]. RIFLE-STEACS showed a significant reduction of the primary end point (13.6% vs. 21.0%; 95% CI: 2.7–12.0%; p = 0.003), mainly due to a reduction of bleeding events (7.8% vs. 12.2%; 95% CI: 2.7–12.0%; p = 0.026) and access site-related bleedings (2.6% vs. 6.8%; 95% CI: 1.6–7.0%; p = 0.002) in the radial group [Citation17]. These results were later confirmed by the STEMI treated by RADIAL or femoral approach (STEMI-RADIAL) that reported a significant reduction in terms of bleedings and access site complications with radial access [Citation22]. In the ‘Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL)’ trial, 7021 acute coronary syndrome patients were enrolled. No significant difference in term of primary end point (defined as composite of death, MI, stroke, or non-CABG-related major bleeding at 30 days) between patients randomized to radial access or femoral access was observed [Citation23]. Nevertheless, the RIVAL trail showed that in patients with STEMI, a prespecified subgroup, radial artery access reduced the incidence of primary outcome ([STEMI] 3.1% vs. [NSTEMI] 5.2%; HR: 0.60; p = 0.026) and mortality ([STEMI] 1.4% vs. [NSTEMI] 3.1%; HR: 0.46; p = 0.041) [Citation24].
The recent Minimizing Adverse Hemorrhagic Events by TRansradial Access Site and Systemic Implementation of angioX (MATRIX Access), enrolling 8404 acute coronary syndrome patients (STEMI = 4010) allocated to radial access or femoral access is the largest published randomized trial, powered to test the superiority of radial approach versus femoral in term of two co-primary 30-day composite end points: major adverse cardiovascular events [MACE]: all-cause mortality, MI, or stroke; NACE: major bleeding not related to CABG surgery (Bleeding Academic Research Consortium [BARC] type 3 or 5) or MACE. The MATRIX trial demonstrated that radial approach is superior to femoral access in terms of MACE (369 [8.8%] vs. 429 [10.3%]; 95% CI: 0.85 [0.74–0.99]; p = 0.0307) and NACE (410 [9.8%] vs. 486 [11.7%]; 95% CI: 0.83 [0.73–0.96]; p = 0.00092), through a reduction BARC major bleeding unrelated to CABG surgery (66 [1.6%] vs. 94 [2.3%]; 95% CI: 0.68 [0.49–0.92]; p = 0.013) and all-cause mortality (66 [1.6%] vs. 91 [2.2%]; 95% CI: 0.72 [0.53–0.99]; p = 0.0450) [Citation25]. These data suggested that the radial approach, right or left, should be the preferred vascular access during emergency PCI.
Recently, comparing a cohort of 10,137 consecutive patients (from 2006 to 2008), where both radial access (n = 4.663) and femoral access (n = 5.474) were performed with a historical control cohort (from 1996 to 1998) where only femoral access was used (n = 6.922), Azzalini et al. described a significant increased rate of vascular access site complications when femoral access is performed in the contemporary era (adjusted rates: 4.19% vs. 1.98%; OR: 2.16; 95% CI: 1.67–2.81; p < 0.001) [Citation26]. These findings, known as ‘radial paradox,’ suggest the importance of femoral access and expertise in femoral access should be preserved also in the radial era. Femoral access maintains a central role especially in patients presenting in cardiogenic shock, where it can be difficult to obtain access and circulatory support is needed.
3.2. Manual thrombectomy
In order to improve microvascular reperfusion avoiding the no-reflow phenomenon during primary PCI, several attempts were performed. The incidence of no-reflow ranges from 5% to 20% and is associated with worse clinical outcomes [Citation27,Citation28]. An optimal epicardial and myocardial reperfusion represents a primary aim of primary PCI, being related to late remodeling and long-term outcomes [Citation29]. Distal embolization during primary PCI is a fearsome event and represents the major mechanism for impaired myocardial reperfusion, mechanical capillary obstruction, endothelial dysfunction, and inflammation.
Microvascular impairment strongly correlates with a poor long-term prognosis [Citation30]. In order to limit microvascular impairment, mechanical and pharmacologic approaches have been matched to conventional primary PCI. Stone et al. showed that the infusion of 0.25 mg/kg bolus of abciximab at the site of the infarct lesion via a drug delivery balloon catheter in patients presenting with large anterior STEMI is associated to a reduction of 30 days infarct size [Citation31]. In this setting, several thrombectomy devices, manual or mechanical, were tested to prevent distal embolization and microvascular impairment [Citation32,Citation33]. In particular, during primary PCI, manual thrombectomy is associated to a better myocardial reperfusion, a reduction of distal embolization and microvascular impairment [Citation33–Citation39] that could theoretically translate into an improved long-term survival [Citation39,Citation40]. The Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS) trial was designed in order to assess the superiority of manual aspiration thrombectomy during primary PCI, compared with standard PCI, in terms of the primary end point defined as the post-procedural frequency of a myocardial blush grade of 0 or 1 [Citation36]. In the TAPAS trial, a histopathological examination of the aspirated material confirmed the efficacy manual thrombectomy. The TAPAS trial reported manual aspiration to be associated with a better myocardial reperfusion, when considering myocardial blush grade 0 or 1 (84 [17.1%] vs. 129 [26.3%]; 95% CI: 0.65 [0.51–0.83]; p < 0.001); and ST-segment resolution (275 [56.6%] vs. 219 [44.2%]; 95% CI: 1.28 [1.13–1.45]; p < 0.001) [Citation36].
The optimal reperfusion achieved with the manual aspiration was followed by a 1 year clinical benefit with a reduction of cardiac death (19 [3.6%] vs. 36 [6.7%]; 95% CI: 1.93 [1.11–3.37]; p = 0.020) and the composite of cardiac death or nonfatal reinfarction (30 [5.6%] vs. 53 [9.9%]; 95% CI: 1.81 [1.16–2.84]; p = 0.020) [Citation40]. These results were acknowledged by guidelines giving recommendation II level A: ‘manual aspiration thrombectomy is reasonable for patients undergoing primary PCI’ [Citation2].
Nevertheless, two recent trials, the Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia (TASTE) trial and the ‘The Trial of Routine Aspiration Thrombectomy with PCI versus PCI Alone in Patients with STEMI (TOTAL)’ [Citation41–Citation44], showed no benefits in terms of clinical outcomes of thrombus aspiration during primary PCI, despite manual thrombectomy was associated to an improvement of ST resolution and a reduced distal embolization [Citation41–Citation44]. As consequence of this large randomized trial, ACC/AHA/SCAI guidelines reported that the ‘usefulness of selective and bailout aspiration thrombectomy in patients undergoing primary PCI is not well established (Class II; level of evidence B)’ and the routine use of manual aspiration during primary PCI is not useful (Class III; level of evidence A) [Citation2].
Finally, in order to reduce the incidence and to treat no-reflow phenomenon, several attempts have been tested using intracoronary adenosine; however, still conflicting data were obtained [Citation3].
3.3. Stent type
Several published data demonstrated that drug-eluting stents (DES) are superior to bare-metal stents (BMS) in terms of target vessel revascularization in the setting of STEMI [Citation45].
However, concerns about the safety of first-generation DES in terms of stent thrombosis mainly in STEMI patients were raised [Citation46,Citation47].
Second-generation DES were designed to achieve efficacy and long-term safety, mainly reducing stent thrombosis [Citation48]. Using a network metanalysis methodology, Palmerini et al. demonstrated that cobalt–chromium everolimus-eluting stent (CoCr-EES) is superior to BMS in efficacy and safety. If compared to BMS, CoCr-EES reduces the incidence of 1-year and 2-year definite stent thrombosis [Citation48]. Moreover, the great efficacy and safety of CoCr-EES was also confirmed in STEMI setting, compared to BMS, with a significant lower risk of 1-year cardiac death/MI, and stent thrombosis [Citation49].
The Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare-Metal Stents in Acute ST-Elevation Myocardial Infarction ‘COMFORTABLE AMI’ trial, in which 1161 randomized STEMI patients were enrolled to receive either BMS or to biolimus-eluting stents (BES) with a biodegradable polymer, showed a significant lower rate of the composite primary end point (cardiac death, target-vessel MI, and target-lesion revascularization; 4.3% vs. 8.7%; HR 0.49; 95% CI: 0.30–0.80; p = 0.004) in the BES group [Citation49]. These results positively clarified the role of new-generation DES during primary PCI, rising to class IA recommendation in ESC guidelines on myocardial revascularization [Citation50].
Recently, fully bioresorbable scaffolds were tested in STEMI patients [Citation51]. The non-inferiority randomized ABSORB-STEMI TROFI II trial was designed to compare arterial healing response between the Absorb BVS and the metallic EES in STEMI [Citation52]. The authors hypothesized that the use of bioresorbable vascular scaffolds could be associated with recovery of coronary vessel physiology reducing the incidence of late events [Citation53] and plaque stabilization due to the formation of a neo-cap on the unstable plaque [Citation54].
The TROFI II trial showed an arterial healing after Absorb BVS implantation non-inferior to CoCr-EES in STEMI patients. No significant differences were observed also in clinical secondary end points, confirming previous observational results [Citation52–Citation55].
3.4. Type of revascularization: complete versus incomplete
In the setting of primary PCI, almost 50% of STEMI patients show a multivessel disease. The aim of the primary PCI is to treat the culprit lesion and restore a normal myocardial perfusion. Three different revascularization strategies could be adopted in order to treat non-culprit lesions: (1) Culprit artery – only primary PCI. PCI of non-culprit arteries only for spontaneous ischemia or intermediate- or high-risk findings on predischarge noninvasive testing; (2) Multivessel PCI at the time of primary PCI; and (3) culprit artery – only primary PCI followed by staged PCI of non-culprit arteries [Citation3].
Previous guidelines strongly suggested to treat only the culprit lesion in the setting of primary PCI and to differ the non-culprit lesions to a second ischemia-driven procedure. Patients presenting with cardiogenic shock and multivessel critical coronary artery disease represent the only exception where complete coronary revascularization is justified [Citation3]. Recently, the Preventive Angioplasty in Acute Myocardial Infarction (PRAMI) trial has tested the hypothesis that a complete coronary revascularization at the time of primary PCI is superior to a culprit lesion-only primary PCI. The PRAMI trial, enrolling 465 STEMI patients, observed that a complete coronary revascularization at the time of primary PCI is superior to a culprit only strategy with regard of the composite primary end point (cardiac death, nonfatal MI, or refractory angina; [21 pts (9%) vs. 53 pts (22%); HR: 0.35; 95% CI: 0.21–0.58; p < 0.001]) [Citation56]. Similarly, the CvLPRIT (Complete Versus Culprit-Lesion Only Primary PCI) trial showed that a complete coronary revascularization during index hospitalization is superior to a culprit only strategy in terms of the composite primary end point (death, reinfarction, heart failure, and ischemia-driven revascularization at 12 months) [Citation57].
These results were confirmed by the DANAMI 3 PRIMULTI (Third Danish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction) trial reporting a complete coronary revascularization, guided by fractional flow reserve to be superior (primary end point composite of death, nonfatal MI or ischemia-driven revascularization of non-culprit artery disease) to the culprit only revascularization strategy (13% vs. 22%; HR: 0.56; 95% CI: 0.38–0.83; p = 0.004) [Citation58]. These evidences were recognized by guidelines that for the first time suggested: ‘PCI of a non-infarct artery may be considered in selected patients with STEMI and multivessel disease who are hemodynamically stable, either at the time of primary PCI or as a planned staged procedure (Class IIb)’ [Citation2].
4. Pharmacological treatment
4.1. Antiplatelet therapy
Dual antiplatelet therapy, aspirin plus P2Y12 receptor inhibitors, represents the gold standard in the setting of primary PCI. The Study of Platelet Inhibition and Patient Outcomes (PLATO) trial, conducted in ACS with or without ST elevation patients, showed that Ticagrelor as compared with Clopidogrel is associated to a reduction of death from vascular causes (4.0% vs. 5.1%; p = 0.001) and MI (5.8% vs. 6.9%; p = 0.005) without increasing the rate of overall major bleeding but with an increase of non-CABG-related major bleeding (4.5% vs. 3.8%; p = 0.03) [Citation59]. In patients presenting with an acute coronary syndrome and planned percutaneous coronary revascularization, the Trial to assess Improvement in Therapeutic Outcomes by optimizing platelet inhibition with Prasugrel – Thrombolysis In Myocardial Infarction (TRITON-TIMI 38) trial demonstrated the superiority of Prasugrel respect to Clopidogrel in terms of primary composite end point (defined as rate of: death from cardiovascular causes, nonfatal MI, or nonfatal stroke; 9.9% vs. 12.1%; p < 0.001). However, an increased risk of major bleeding (2.4% vs. 1.8%; p = 0.003), CABG-related TIMI major bleeding (13.4% vs. 3.2%; p < 0.001) and fatal bleeding (0.4% vs. 0.1%; p = 0.002) was observed [Citation60]. Ticagrelor and Prasugrel, as compared to Clopidogrel, have a more powerful antiplatelet effect, with a faster and strongest platelet inhibition. The great efficacy of these two drugs respect to Clopidogrel, in terms of ischemic events reduction, is partially balanced by an increased risk of bleedings, which is mainly evident for Prasugrel.
According to European guidelines, in the setting of STEMI, the use of Ticagrelor or Prasugrel should be preferred to Clopidogrel: ‘dual antiplatelet therapy with a combination of aspirin and prasugrel or aspirin and ticagrelor is recommended (over aspirin and clopidogrel) in patients treated with PCI (Class I level A)’ [Citation3].
A substudy of PLATO trial evaluating the effect of Ticagrelor in STEMI patients undergoing primary PCI demonstrated no significant difference with respect of clinical events between Ticagrelor and Clopidogrel (composite of death from vascular causes, MI, or stroke; Ticagrelor 7.9% vs. Clopidogrel 8.6%; 95% CI: 0.91 [0.75–1.12]; p = 0.38). However, a significant reduction of definite stent thrombosis (HR 0.58, 95% CI: 0.37–0.89; p = 0.013) was observed. Moreover, this clinical benefit was not associated to an increased risk of bleeding events (Ticagrelor 6.7% vs. Clopidogrel 6.8%; 95% CI: 0.97 [0.77–1.22]; p = 0.79) [Citation61].
The prespecified STEMI substudy of the TRITON-TIMI 38 STEMI patients were divided according to time symptoms onset: those enrolled within 12 h (primary PCI); and [Citation2] those others enrolled between 12 h and 14 days (secondary PCI). Prasugrel demonstrated superiority to Clopidogrel (primary end point defined as the composite of cardiovascular death, nonfatal MI, or nonfatal stroke) in the overall cohort (Clopidogrel 166 [9.5%] vs. Prasugrel 115 [6.5%]; 95% CI: 0.68 [0.54–0.87]; p = 0.0017) and in the secondary PCI (Clopidogrel 65 [12.3%] vs. Prasugrel 36 [6.4%]; 95% CI: 0.50 [0.34–0.76]; p = 0.0008) [Citation62]. However, Prasugrel showed no benefits in STEMI patients treated with primary-PCI in terms of primary end point (Clopidogrel 101 [8.2%] vs. Prasugrel 79 [6.6%]; 95% CI: 0.80 [0.60–1.08]; p = NS) and TIMI major bleeding unrelated to CABG surgery.
The two substudy of the TRITON-TIMI 38 and PLATO trials showed that the use of Prasugrel as well as of Ticagrelor is not associated to a clear benefit in setting of primary PCI [Citation61,Citation62].
In acute coronary syndrome patients, an important issue of ongoing debate is the need for P2Y12 receptor inhibitors pretreatment at the time of diagnosis.
The ACCOAST (the Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention [PCI] or as Pretreatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction) trial, that randomized 4033 non-STEMI patients to a pretreatment group (Prasugrel 30-mg loading dose before the angiography and additional 30 mg of Prasugrel at the time of PCI) or to control group (placebo before the angiography and 60 mg of Prasugrel at the time of PCI), is the only trial designed in order to evaluate the superiority of pretreatment as compared with no pretreatment in this clinical scenario. ACCOAST failed to show any benefit for a pretreatment approach with Prasugrel in terms of major ischemic events up to 30 days. Moreover, ACCOAST showed that pretreatment, as compared with control group, is associated to an increased rate of bleeding events suggesting that this strategy could be harmful [Citation63].
Regarding STEMI patients undergoing to primary PCI, to avoid the issue between pretreatment or no pretreatment, two recent trials demonstrated that crushed pills (Ticagrelor or Prasugrel), as compared with whole tablets, maximize and speed antiplatelet effect during primary PCI limiting the need for pretreatment [Citation64–Citation66].
In order to explore the potential benefits of dual antiplatelet therapy beyond 1 year after a MI, in this subgroup the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin – Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial was designed. The PEGASUS-TIMI 54 enrolled 21,162 patients with a former (1 and 3 years earlier) MI diagnosis and randomized patients to three arms: aspirin and Ticagrelor at a dose of 90 mg twice daily; aspirin and Ticagrelor at a dose of 60 mg twice daily; aspirin and placebo. The aim of the PEGASUS-TIMI 54 was to assess the superiority of Ticagrelor in terms of composite primary efficacy, end point defined as the occurrence of cardiovascular death, MI, or stroke. Compared to placebo, Ticagrelor at any dosage was more effective in terms of the primary end point reduction; nevertheless, major rates of TIMI major bleeding were observed on the Ticagrelor group [Citation67].
Cangrelor is an intravenous reversible P2Y12 inhibitor and has been recently approved in Europe and USA to reduce ischemic events in patients not pretreated with an oral P2Y12 inhibitor and undergoing PCI. In literature, several important studies were published about the clinical effects of Cangrelor [Citation67–Citation73]. In particular, the Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) PHOENIX enrolling 11,145 patients undergoing PCI (Stable angina: 6.140 [55.1%]; Non-STEMI:2.810 [25.2%]; STEMI: 1.992 [17.9%]) demonstrated that Cangrelor is reducing the composite of death, MI, ischemia-driven revascularization, or stent thrombosis at 48 h compared with Clopidogrel (Cangrelor 4.7% vs. Clopidogrel 5.9%; 95% CI: 0.78 [0.66–0.93]; p = 0.005) and the rate of stent thrombosis Stent thrombosis (Cangrelor 0.8% vs. Clopidogrel 1.4%; 95% CI: 0.62 [0.43–0.90]; p = 0.01). The effect of Cangrelor was analogous among patients presenting with STEMI, NSTEMI, and those presenting with stable angina [Citation71]. No safety issues were raised.
The use of GP IIb/IIIa inhibitors is not routinely recommended, European guidelines propose bailout use of GP IIb/IIIa inhibitors if a visible thrombus is present in the basal angiogram with Class II and level of evidence A. Upstream use of a GP IIb/IIIa inhibitor may be considered in patients presenting a high-risk profile, who should be transferred in a primary PCI capable center [Citation3].
4.2. Anticoagulants
As suggested by guidelines, intravenous anticoagulants should be administered during primary PCI [Citation3]. Anticoagulant options for primary PCI include unfractionated heparin, enoxaparin, and bivalirudin. In the last years, several trials tested the role of bivalirudin in the setting of acute coronary syndrome and in particular during primary PCI [Citation74–Citation78]. The Harmonizing Outcomes with RevascularIZatiON and Stents in Acute Myocardial (HORIZONS-AMI) trial randomized 3.602 ST-segment elevation MI patients undergoing to primary PCI to heparin plus a glycoprotein IIb/IIIa inhibitor or to bivalirudin alone. The HORIZONS-AMI presented two prespecified primary 30-day end points: major bleeding (not related to coronary artery bypass grafting); combined adverse clinical events, defined as the combination of major bleeding or a composite of MACE, including death, reinfarction, target vessel revascularization for ischemia, and stroke [Citation75]. This trial demonstrated that the use of bivalirudin was associated with a reduction of NACE and major bleedings, as compared to heparin plus glycoprotein IIb/IIIa inhibitor.
In this setting, the MATRIX trial was designed to test the superiority of bivalirudin to heparin plus discretionary use of glycoprotein IIb/IIIa inhibitors in term of two co-primary 30-day composite clinical outcomes (MACE: all-cause mortality, MI, or stroke; NACE: major bleeding not related to CABG surgery (BARC type 3 or 5) or MACE [Citation77,Citation78].
The study at variance of the HORIZONS-AMI demonstrated that bivalirudin is associated with no benefit when considering the rate of MACE respect to heparin (Bivalirudin group, 10.3% vs. heparin group 10.9%; 95% CI: 0.94 [0.81–1.09]; p = 0.44) and of NACE (Bivalirudin group 11.2% and heparin group 12.4%; 95% CI: 0.89 [0.78–1.03]; p = 0.12).
These data seem to state that the heparin represents the ideal anticoagulant in patients who underwent to percutaneous revascularization.
5. Intra-aortic balloon counterpulsation and STEMI
The use of intra-aortic balloon counterpulsation (IABP) in the setting of STEMI was recently debated, two important trials failed to show any benefits of IABP usage in STEMI patients [Citation79–Citation82]. The Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction ‘CRISP AMI’ trial, that enrolled 337 patients with acute anterior STEMI without cardiogenic shock, failed to demonstrate the hypothesis that the use of IABP was associated to a reduction of infarct-size to cardiac magnetic resonance imaging [Citation79]. In a substudy of the CRISP AMI trial, the authors observed that the use of IABP, in large anterior MI complicated by persistent ischemia after PCI, is associated with a decreased 6-month mortality [Citation80]. The IABP-SHOCK II trial, that randomized 600 patients with cardiogenic shock complicating acute MI to IABP (n = 301) or no IABP (n = 299 patients), failed to demonstrate that the use of IABP is associated in a reduction of 30-day and 1-year mortality [Citation81,Citation82]. ‘The IMPella versus IABP REduceS infarct Size IN STEMI patients treated with primary PCI’ (IMPRESS in STEMI) trial compared IABP and Impella 2.5 in patients presenting with STEMI and cardiogenic pre-shock, undergoing to primary PCI. Due to a slow patient enrollment, the study was stopped prematurely [Citation83]. The IMPRESS in STEMI trial confirms the difficulties to conduct trial in the setting of cardiogenic shock [Citation83]. New devices are developing in order to reduce the time of positioning. In this setting, the new generation of PulseCath iVAC 2L left ventricular assist device, a percutaneous transfemoral membrane pump connected to a IABP console and able to generate a pulsatile blood flow up to 2 L/min, seems extremely promising [Citation84]. Ultimately, it is important to underline the importance of IABP in stabilizing patients with mechanical complications after STEMI, such as acute mitral valve regurgitation or ventricular septal rupture, waiting for surgery or percutaneous treatments [Citation3].
6. STEMI and atrial fibrillation
Special considerations should be made for patients presenting with STEMI and non-valvular atrial fibrillation. In patients with MI, atrial fibrillation is common and is associated to impaired prognosis [Citation85]. Between January 2000 and December 2009, among 155,071 patients survived to MI and enrolled in the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies register (SWEDEHEART), Batra et al. reported an atrial fibrillation incidence of 15.5%. In this setting, the main clinical issue is represented by the needs of anticoagulation and dual-antiplatelet therapies after primary PCI.
According to guidelines: new oral anticoagulants are indicated as first-line therapy in atrial fibrillation patients needing anticoagulation [Citation86]; new generation DES should be preferred over BMS [Citation50]; Prasugrel and Ticagrelor in association with oral anticoagulation should be avoided until solid data will be published [Citation87]. Dual antiplatelet therapy (aspirin plus Clopidogrel) plus oral anticoagulation (new oral anticoagulants or vitamin K antagonists), known as triple therapy, is recommended in patients with atrial fibrillation needing anticoagulation and coronary stent implantation. Triple therapy is associated to an increased risk of bleeding; consequently, this therapeutic regimen should be adjusted according to patient’s bleeding risk. HAS-BLED risk score [hypertension, abnormal renal and liver function (1 point each), stroke, bleeding history or predisposition, labile INR, elderly (>65 years), drugs and alcohol (1 point each)] is crucial in order to plan the duration of triple therapy. In patients with HAS-BLED ≤ 2, triple therapy is recommended for 6 months followed by a dual therapy (anticoagulation and aspirin or Clopidogrel) until 12 months and, then, by monotherapy with oral anticoagulant. In patients with HAS-BLED ≥ 2, triple therapy is recommended for 1 month followed by a dual therapy (anticoagulation and aspirin or Clopidogrel) until 12 months and, then, by monotherapy with oral anticoagulant [Citation87]. Nowadays, several ongoing clinical trials are evaluating the safety and the efficacy of new oral anticoagulants in patients with atrial fibrillation undergoing PCI needing antiplatelet therapy [Citation88–Citation90].
7. Expert commentary
A possible correct approach to a patient presenting a STEMI could be considered as a stepwise process, given in five steps ():
Figure 2. STEMI treatment An integrated stepwise process. Therapeutic approach to STEMI patients should rely on pharmacotherapy and invasive management integrated in an effective primary PCI network.
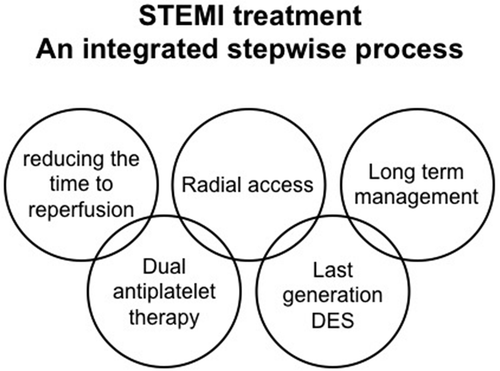
The first step is of paramount importance and it aims at reducing the time to reperfusion. It is characterized by an integration of health care system, availability of reperfusion strategies and primary PCI. Without the first step, all the other parts of the treatment of a STEMI patient are absolutely secondary. In this step, the health-care system could foresee coupled reperfusion strategies such as prehospital fibrinolysis in order to reduce the time of reperfusion.
The second step is characterized by administration of antiplatelet therapies; Ticagrelor or Prasugrel should be the preferred ones. The data are not definitive in the setting if the STEMI, nevertheless the evidence of a benefit of these two P2Y12 receptor inhibitors in acute coronary syndrome is unequivocal.
The third important moment is the choice of the arterial access for coronary intervention, recent evidences strongly support that a radial approach in the majority of the cases, reduces bleeding complications.
The fourth step is characterized by the primary PCI procedure, and by the two major aspects recently investigated, namely the adoption of new generation of DES that appear to be safe and effective; there is an open discussion on the appropriateness of manual thrombectomy that should be probably not performed routinely but reserved to selected patients with evidence of large thrombus.
Fifth, novel medical strategies comprising long-term dual antiplatelet therapy could be considered after the acute phase in low bleeding risk patients.
8. Five-year view
The research over the next years in the setting of STEMI patients will be probably focused on several topics comprising the role of novel devices such as bioresorbable vascular scaffold during primary PCI, the role of novel antiplatelet agents (i.e. Cangrelor) in clinical practice and the impact of new oral anticoagulants in STEMI patients, presenting comorbidity requiring long-term anticoagulation such as atrial fibrillation, ventricular apical thrombus, or mechanical valves.
The management of the possible MI complications, like cardiogenic shock, acute mitral regurgitation, and ventricular remodeling, represents additional fields of increasing scientific interest.
Key issues
Primary PCI is the gold standard revascularization strategy in patients presenting with ST elevation myocardial infarction.
The presence of an effective health care system is the key of revascularization strategy in STEMI patients in order to obtain excellent results in terms of clinical outcomes.
The reduction of total ischemia time is the main goal of primary PCI network.
The Primary PCI is a complex procedure and represents the mix of operator’s technical skills and culture.
New-generation of drug eluting stents, thanks to the their efficacy and safety, raised a recommendation of Class I level of evidence A and represent the ideal implantable stents.
Complete coronary revascularization is attractive and should be completed during index hospitalization. Intermediate coronary non-culprit lesions should be functionally evaluated.
DAPT aspirin plus Prasugrel or Ticagrelor is mandatory in STEMI patients. Clopidogrel should be reserved only to patients with contraindication to Ticagrelor or Prasugrel.
Heparin represents the standard of treatment in STEMI patients.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165.
- Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67(10):1235–1250.
- Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569–2619.
- McNamara RL, Wang Y, Herrin J, et al. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2006;47:2180–2186.
- Berger PB, Ellis SG, Holmes DR Jr, et al. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction: results from the Global Use of Strategies to Open Occluded arteries in acute coronary syndromes (GUSTO-IIb) trial. Circulation. 1999;100:14–20.
- De Luca G, Suryapranata H, Ottervanger JP, et al. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–1225.
- Zijlstra F, Patel A, Jones M, et al. Clinical characteristics and outcome of patients with early (<2 h), intermediate (2-4 h) and late (>4 h) presentation treated by primary coronary angioplasty or thrombolytic therapy for acute myocardial infarction. Eur Heart J. 2002;23:550–557.
- Menees DS, Peterson ED, Wang Y, et al. Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;369:901–909.
- Widimsky P, Fajadet J, Danchin N, et al. ‘Stent 4 Life’ targeting PCI at all who will benefit the most. A joint project between EAPCI, Euro-PCR, EUCOMED and the ESC Working Group on acute cardiac care. EuroIntervention. 2009;4:555–557.
- Fedele F, Mancone M. Perspectives: how to evaluate healthcare systems in primary angioplasty. Eur Heart J Supplements. 2014;16:A45–A47.
- Kristensen SD, Laut KG, Fajadet J, et al.; European Association for Percutaneous Cardiovascular Interventions. Reperfusion therapy for ST elevation acute myocardial infarction 2010/2011: current status in 37 ESC countries. Eur Heart J 2014;35(29):1957–1970.
- Li J, Li X, Wang Q, et al.; China PEACE Collaborative Group. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet 2015;385(9966):441–451.
- Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010;56:254–263.
- Armstrong PW, Gershlick AH, Goldstein P, et al.; STREAM Investigative Team. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368(15):1379–1387.
- Koul S, Andell P, Martinsson A, et al. A pharmacodynamic comparison of 5 anti-platelet protocols in patients with ST-elevation myocardial infarction undergoing primary PCI. BMC Cardiovasc Disord. 2014;14:189.
- Montalescot G, van ‘t Hof AW, Lapostolle F, et al.; ATLANTIC Investigators. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med 2014;371(11):1016–1027.
- Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol. 2012;60:2481–2489.
- Sinnaeve PR, Danays T, Bogaerts K, et al. Drug treatment of STEMI in the elderly: Focus on fibrinolytic therapy and insights from the STREAM trial. Drugs Aging. 2016;33:109–118.
- Fach A, Bünger S, Zabrocki R, et al. Comparison of outcomes of patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention analyzed by age groups (<75, 75 to 85, and >85 Years); (Results from the Bremen STEMI Registry). Am J Cardiol. 2015;116(12):1802–1809.
- Sciahbasi A, Mancone M, Cortese B, et al. Transradial percutaneous coronary interventions using sheathless guiding catheters: a multicenter registry. J Interv Cardiol. 2011;24(5):407–412.
- Iqbal MB, Arujuna A, Ilsley C, et al.; London Heart Attack Centre (HAC) Group Investigators. Radial versus femoral access is associated with reduced complications and mortality in patients with non-ST-segment-elevation myocardial infarction: an observational cohort study of 10,095 patients. Circ Cardiovasc Interv 2014;7(4):456–464.
- Bernat I, Horak D, Stasek J, et al. ST-segment elevation myocardial infarction treated by radial or femoral approach in a multicenter randomized clinical trial: the STEMI-RADIAL trial. J Am Coll Cardiol. 2014;63:964–972.
- Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420.
- Mehta SR, Jolly SS, Cairns J, et al. Effects of radial versus femoral artery access in patients with acute coronary syndromes with or without ST-segment elevation. J Am Coll Cardiol. 2012;60(24):2490–2499.
- Valgimigli M, Gagnor A, Calabró P, et al.; MATRIX Investigators. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385(9986):2465–2476.
- Azzalini L, Tosin K, Chabot-Blanchet M, et al. The benefits conferred by radial access for cardiac catheterization are offset by a paradoxical increase in the rate of vascular access site complications with femoral access: the campeau radial paradox. JACC Cardiovasc Interv. 2015;8(14):1854–1864.
- van ‘t Hof AW, Liem A, de Boer MJ, et al. Clinical value of 12-lead electrocardiogram after successful reperfusion therapy for acute myocardial infarction. Zwolle myocardial infarction study group. Lancet. 1997;350:615–619.
- Montalescot G, Barragan P, Wittenberg O, et al.; ADMIRAL Investigators. Abciximab before direct angioplasty and stenting in myocardial infarction regarding acute and long- term follow-up. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction”. N Engl J Med. 2001;344:1895–1903.
- Stone GW, Grines CL, Cox DA, et al. “Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Investigators. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction”. N Engl J Med. 2002;346:957–966.
- Henriques JP, Zijlstra F, Ottervanger JP, et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J. 2002;23(14):1112–1117.
- Stone GW, Maehara A, Witzenbichler B, et al. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA. 2012;307(17):1817–1826.
- Migliorini A, Stabile A, Rodriguez AE, et al. Comparison of AngioJet rheolytic thrombectomy before direct infarct artery stenting with direct stenting alone in patients with acute myocardial infarction. The JETSTENT trial. JACC. 2010;56:1298–1306.
- Burzotta F, De Vita M, Gu YL, et al. Clinical impact of thrombectomy in acute ST- elevation myocardial infarction: an individual patient-data pooled analysis of 11 trials. Eur Heart J. 2009;30(18):2193–2203.
- Sardella G, Mancone M, Nguyen BL, et al. The effect of thrombectomy on myocardial blush in primary angioplasty: the randomized evaluation of thrombus aspiration by two thrombectomy devices in acute myocardial infarction (RETAMI) trial. Catheter Cardiovasc Interv. 2008;71(1):84–91.
- Sardella G, Mancone M, Bucciarelli-Ducci C, et al. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. JACC. 2009;53(4):309–315.
- Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358(6):557–567.
- Burzotta F, Trani C, Romagnoli E, et al. Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (REMEDIA) trial. J Am Coll Cardiol. 2005;46(2):371–376.
- Galiuto L, Garramone B, Burzotta F, et al.; REMEDIA Investigators. Thrombus aspiration reduces microvascular obstruction after primary coronary intervention: a myocardial contrast echocardiography substudy of the REMEDIA trial. J Am Coll Cardiol 2006;48(7):1355–1360.
- Sardella G, Mancone M, Canali E, et al. Impact of thrombectomy with EXPort catheter in infarct-related artery during primary percutaneous coronary intervention (EXPIRA Trial) on cardiac death. Am J Cardiol. 2010;106(5):624–629.
- Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371(9628):1915–1920.
- Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369(17):1587–1597.
- Lagerqvist B, Fröbert O, Olivecrona GK, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371(12):1111–1120.
- Jolly SS, Cairns JA, Yusuf S, et al.; TOTAL Investigators. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet 2016;387(10014):127–135.
- Jolly SS, Cairns JA, Yusuf S, et al.; TOTAL Investigators. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med 2015;372(15):1389–1398.
- Daemen J, van Twisk PH, Kukreja N, et al. The relative safety and efficacy of bare-metal and drug-eluting stents in low and high-risk patient subsets. An epidemiological analysis of three sequential cohorts of consecutive all comers (n 1⁄4 6129). EuroIntervention. 2009;4:464–474.
- Merlini PA, Bauer KA, Oltrona L, et al. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation. 1994;90:61–68.
- Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–1402.
- Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2013;62:496–504.
- Raber L, Kelbaek H, Ostojic M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs.bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308(8):777–787.
- Windecker S, Kolh P, Alfonso F, et al. ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2014;2014(35):2541–2619.
- Diletti R, Karanasos A, Muramatsu T, et al. Everolimus-eluting bioresorbable vascular scaffolds for treatment of patients presenting with ST-segment elevation myocardial infarction: BVS STEMI first study. Eur Heart J. 2014;35(12):777–786.
- Sabate ́ M, Windecker S, In ̃ Iguez A, et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST- segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction—TROFI II trial. doi:10.1093/eurheartj/ehv50
- Brugaletta S, Heo JH, Garcia-Garcia HM, et al. Endothelial-dependent vasomotion in a coronary segment treated by ABSORB everolimus-eluting bioresorbable vascular scaffold system is related to plaque composition at the time of bioresorption of the polymer: indirect finding of vascular reparative therapy? Eur Heart J. 2012;33:1325–1333.
- Brugaletta S, Radu MD, Garcia-Garcia HM, et al. Circumferential evaluation of the neointima by optical coherence tomography after ABSORB bioresorbable vascular scaffold implantation: can the scaffold cap the plaque? Atherosclerosis. 2012;221:106–112.
- Cortese B, Ielasi A, Romagnoli E, et al. Clinical comparison with short-term follow-Upof bioresorbable vascular scaffold versus everolimus- eluting stent in primary percutaneous coronary interventions. Am J Cardiol. 2015;116:705e–710e.
- Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–1123.
- Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–972.
- Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI 3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665–671.
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015.
- Velders MA, Abtan J, Angiolillo DJ, et al.; PLATO Investigators. Safety and efficacy of ticagrelor and clopidogrel in primary percutaneous coronary intervention. Heart. 2016;102:617–625.
- Montalescot G, Wiviott SD, Braunwald E, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST- elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373:723–731.
- Montalescot G, Bolognese L, Dudek D, et al.; ACCOAST Investigators. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med 2013;12(369):999–1010.
- Parodi G, Xanthopoulou I, Bellandi B, et al. Ticagrelor crushed tablets administration in STEMI patients: the MOJITO study. J Am Coll Cardiol. 2015;65:511–512.
- Rollini F, Franchi F, Hu J, et al. Crushed prasugrel tablets in patients with STEMI undergoing primary percutaneous coronary intervention: the CRUSH study. J Am Coll Cardiol. 2016;67(17):1994–2004.
- Sardella G, Calcagno S, Mancone M. Different prasugrel administration in STEMI patients: go faster and no fear to crush! J Am Coll Cardiol. 2016;S0735. doi:10.1016/j.jacc.2016.02.046.
- Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800.
- Gargiulo G, Moschovitis A, Windecker S, et al. Developing drugs for use before, during and soon after percutaneous coronary intervention. Expert Opin Pharmacother. 2016 Jan 22;17:803–818.
- Bhatt DL, Lincoff AM, Gibson CM, et al. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361:2330–2341.
- Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361:2318–2329.
- Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368:1303–1309.
- Steg PG, Bhatt DL, Hamm CW, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet. 2013;382:1981–1992.
- Angiolillo DJ, Firstenberg MS, Price MJ, et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA. 2012;307:265–274.
- Mehran R, Lansky AJ, Witzenbichler B, et al. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet. 2009;374:1149–1159.
- Stone GW, Witzenbichler B, Guagliumi G, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–2230.
- Dangas GD, Caixeta A, Mehran R, et al. Frequency and predictors of stent thrombosis after percutaneous coronary intervention in acute myocardial infarction. Circulation. 2011;123:1745–1756.
- Valgimigli M, Frigoli E, Leonardi S, et al. Bivalirudin or unfractionated heparin in acute coronary syndromes. N Engl J Med. 2015;373:997–1009.
- Valgimigli M. Design and rationale for the minimizing adverse haemorrhagic events by TRansradial access site and systemic implementation of angioX program. Am Heart J. 2014;168(6):838–845.e6.
- Patel MR, Smalling RW, Thiele H, et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011;306(12):1329–1337.
- van Nunen LX, Van ‘T Veer M, Schampaert S, et al. Intra-aortic balloon counterpulsation reduces mortality in large anterior myocardial infarction complicated by persistent ischaemia: a CRISP-AMI substudy. EuroIntervention. 2015 Jul;11(3):286–292.
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296.
- Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382(9905):1638–1645.
- Ouweneel DM, Engstrom AE, Sjauw KD, et al. Experience from a randomized controlled trial with Impella 2.5 versus IABP in STEMI patients with cardiogenic pre-shock. Lessons learned from the IMPRESS in STEMI trial. Int J Cardiol. 2016;202:894–896.
- Van Mieghem NM, Daemen J, Lenzen MJ, et al. The PulseCath iVAC 2L left ventricular assist device: conversion to a percutaneous transfemoral approach. EuroIntervention. 2015;11(7):835–839.
- Batra G, Svennblad B, Held C, et al. All types of atrial fibrillation in the setting of myocardial infarction are associated with impaired outcome. Heart. 2016 Feb 29. doi:10.1136/heartjnl-2015-308678.
- Camm AJ, Lip GYH, De Caterina R, et al. Focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719–2747.
- Roffi M, Patrono C, Collet JP, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2015;2016(37):267–315.
- Gibson CM, Mehran R, Bode C, et al. An open-label, randomized, controlled, multicenter study exploring two treatment strategies of rivaroxaban and a dose-adjusted oral vitamin K antagonist treatment strategy in subjects with atrial fibrillation who undergo percutaneous coronary intervention (PIONEER AF-PCI). Am Heart J. 2015;169(4):472–478.
- Evaluation of dual therapy with dabigatran vs. triple therapy with warfarin in patients with AF that undergo a PCI with stenting (REDUAL-PCI). Available from: https://clinicaltrials.gov/ct2/show/NCT02164864?term=REDUAL+PCI&rank=1
- A study of apixaban in patients with atrial fibrillation, not caused by a heart valve problem, who are at risk for thrombosis (Blood Clots) due to having had a recent coronary event, such as a heart attack or a procedure to open the vessels of the heart. Available from: https://clinicaltrials.gov/ct2/show/NCT02415400?term=apixaban+stent&rank=1