1. Introduction
Left-sided heart failure (HF) is a complex chronic syndrome that affects multiple physiologic systems and continues to carry a poor prognosis. In addition to primary pathophysiology, a majority of patients with HF suffer from multimorbidity, defined as the presence of two or more chronic medical conditions; hypertension, dyslipidemia and cardiac arrhythmias are common comorbid conditions in the HF population [Citation1]. Multimorbidity worsens prognosis in patients with HF [Citation2] and new paradigms are needed to more effectively treat HF patients with multimorbidity to improve outcomes [Citation3,Citation4]. Ideally comorbid conditions are identified as early as possible with each condition addressed using guideline-based approaches, treating the ‘whole’ patient [Citation5].
2. Intracardiac multimorbidity in left-sided HF
Left-sided HF, as the name implies, originates from one or several conditions causing left ventricular (LV) dysfunction and diminished systemic cardiac output. As LV dysfunction (e.g., restrictive LV filling pattern, decreased stroke volume, etc.) progressively worsens, in addition to the parallel decline in systemic circulation, there is a retrograde increase in pressure in the pulmonary circulation system, which can lead to pulmonary hypertension (PH), a common occurrence in patients with HF [Citation6]. A highly undesirable consequence of PH in left-sided HF is right ventricular (RV) stress, dysfunction and ultimately failure. Pulmonary hypertension that leads to RV failure is a prognostically ominous development in patients with left-sided HF [Citation7]. Unfortunately there is often a delay in the diagnosis of PH in patients with left-sided HF, which contributes to poorer health outcomes as it allows for unchecked progression toward RV dysfunction and failure [Citation8,Citation9]. These conditions (i.e., LV and RV dysfunction) should each be routinely assessed, and treatment should be titrated to align with up-to-date guideline-based strategies. While there are currently limited treatment options for PH in left-sided HF [Citation7], identification of PH should change the approach to treatment, titrating pharmacology to optimize the balance between RV preload and afterload) [Citation10]. Perhaps we can learn from the concept of and desired approach to multimorbidity to improve clinical outcomes in patients with left-sided HF and PH – viewing LV and RV dysfunction as both intricately linked and separate conditions. In this context, we introduce the concept of intracardiac multimorbidity, treating the left and right ventricle as unique entities whose dysfunction should be viewed as separate conditions. The first step in addressing intracardiac multimorbidity is employing effective examinations that can provide insight into LV and RV function as well as pulmonary hemodynamics. Ideally, patients with left-sided HF are regularly monitored for the possibility of elevated PH, before RV dysfunction and ultimately failure manifests.
3. Diminished exercise capacity in left-sided HF
A hallmark characteristic of left-sided HF is a diminished cardiorespiratory fitness (CRF); as HF disease severity progresses CRF declines further. The gold standard assessment of CRF in patients with left-sided HF is cardiopulmonary exercise testing (CPX) [Citation11]. Peak oxygen consumption (VO2) and the minute ventilation/carbon dioxide production (VE/VCO2) slope are primary CPX measures that carry strong prognostic value as well as serve as gauges for disease severity and the response to therapy. The clinical precision of CPX, as well as its non-invasive nature and cost-efficacy, warrants consideration as a front-line assessment in patients with left-sided HF; the clinical value of information obtained from CPX justifies this assessment being performed annually in this patient population [Citation12]. Peak VO2, as defined by the Fick equation, is primarily driven by left-sided cardiac output. As such, the degree of reduction in peak VO2 seen in left-sided HF is a surrogate for the degree of LV dysfunction. The link between peak VO2 and LV function is a primary reason for the consistently demonstrated prognostic value this CPET measure holds in the HF population. A peak VO2 < 10 mlO2⋅kg−1⋅min−1, well below the age- and sex-predicted normal value across the lifespan, is particularly ominous in patients with left-sided HF. The VE/VCO2 slope, a primary measure of what has been described as ventilatory efficiency, reflects the matching between ventilation and perfusion during exercise. In the presence of elevated pulmonary pressure in left-sided HF, pulmonary perfusion lags pulmonary ventilation, resulting in an increase in the VE/VCO2 slope. A normal VE/VCO2 slope is defined as a value <30; this value is commonly well surpassed in patients with left-sided HF. In fact, a VE/VCO2 slope ≥45 portends a significant risk for adverse events and mortality in this patient population. The pathophysiologic mechanism most closely associated with a VE/VCO2 slope substantially elevated beyond the normal threshold, particularly values ≥45, is PH, which may foretell eminent or ongoing RV dysfunction/failure [Citation13,Citation14]. It should be noted that other abnormal CPX responses, such as a low partial pressure of end-tidal CO2 during exercise and exercise oscillatory ventilation, also are related to PH and may provide additional clinical insight [Citation15].
4. Using CPX to identify intracardiac multimorbidity
In the context of peak VO2 and the VE/VCO2 slope as central measures, CPX can provide insight into intracardiac multimorbidity; peak VO2 provides an indirect assessment of LV function while the VE/VCO2 slope provides an indirect assessment of RV function. These two CPX measures should be uniquely assessed and drive distinct diagnostic conclusions and clinical decisions moving forward. For example, a left-sided HF patient with a peak VO2 of 12 mlO2⋅kg−1⋅min−1 and a VE/VCO2 slope of 29 would indicate LV dysfunction without PH and concomitant RV dysfunction. This patient should be prescribed an individually tailored exercise program, similar to those recommended for PAH [Citation16] and have his/her pharmacologic regimen reviewed to ensure optimal evidence-based therapy is being prescribed to minimize stress placed upon the left ventricle [Citation17,Citation18]. Conversely, a left-sided HF patient with a peak VO2 of 12 mlO2⋅kg−1⋅min−1 and a VE/VCO2 slope of 49 is a markedly different clinical presentation. While LV dysfunction and reduced cardiac output to systemic circulation is clear, there is also a strong indication that PH is present, and the right ventricle is under concomitant excess stress. In such a patient, further assessment of pulmonary hemodynamics and RV function would be warranted (e.g., echocardiography and right-sided catheterization), and, if PH is confirmed, all efforts should be made to reduce pulmonary pressure with the goal of reducing excessive RV afterload [Citation10].
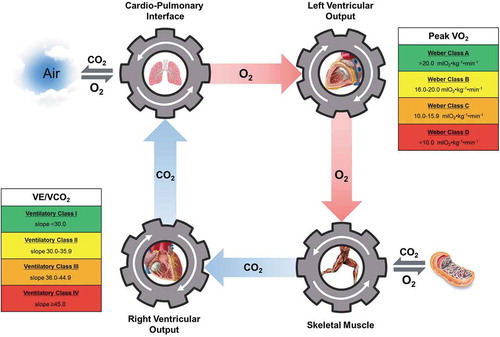
In current practice, clinicians often view CPX from a singular point of view, with all measures reflecting the primary pathophysiology of left-sided HF (i.e., LV dysfunction) and thus a singular condition. This results in an incomplete interpretation of CPX data with less than optimal clinical value. If an intracardiac multimorbidity paradigm was adopted and considered in the context of CPX, clinicians would more appropriately assess the unique responses of peak VO2 and the VE/VCO2 slope in the left-sided HF population; the former assessing LV function and the latter representing RV function. A reconceptualization of how the left and right side of the heart are viewed, as interwoven yet independent systems, and when compromised, as independent conditions, may help improve clinical interpretation of CPX and clinical decision-making processes thereafter. illustrates this reconceptualization, depicting the right and left sides of the heart as separate entities with the two primary CPX measures in left-sided HF distinctly reflecting function of these separate entities. Moreover, the Ventilatory and Weber Class Systems [Citation11] are also included in , placed near the side of the heart they assess. Clinicians responsible for assessing CPX findings and providing a final report to the referring cardiologist should consider the paradigm in , commenting on the VE/VCO2 slope in the context of pulmonary hemodynamics and its implications for RV afterload, dysfunction and failure.
5. Conclusion
Left-sided HF, initiated by LV dysfunction, precipitates a cascade of pathophysiologic events that worsen outcome. Pulmonary hypertension is part of this cascade, raising RV afterload and potentially leading to concomitant right-sided HF. In a sense, this scenario may be viewed as intracardiac multimorbidity, two linked but distinct conditions that results in a substantially poorer prognosis and clinical trajectory. All efforts should be made to assess for increased pulmonary pressure and excess RV stress. While several assessments are available and frequently utilized (e.g., echocardiography and right heart catheterization), CPX is the gold-standard exercise assessment as well as a standard of care in patients with left-sided HF. Presently, there is recognition of the value of assessing multiple key CPX variables that reflect unique aspects of exercise physiology particularly relevant to patients with left-sided HF. In the context of detecting intracardiac multimorbidity as described herein, CPX can play an important role in comprehensively evaluating CRF, including the detection of elevated pulmonary pressure and excess RV stress.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Review disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Chamberlain AM, St Sauver JL, Gerber Y, et al. Multimorbidity in heart failure: a community perspective. Am J Med. 2015 Jan;128(1):38–45.
- Kaur P, Saxena N, You AX, et al. Effect of multimorbidity on survival of patients diagnosed with heart failure: a retrospective cohort study in Singapore. BMJ open. 2018 May 20;8(5):e021291.
- Stewart S, Riegel B, Boyd C, et al. Establishing a pragmatic framework to optimise health outcomes in heart failure and multimorbidity (ARISE-HF): a multidisciplinary position statement. Int J Cardiol. 2016 Jun 1;212:1–10.
- Baron-Franco B, McLean G, Mair FS, et al. Comorbidity and polypharmacy in chronic heart failure: a large cross-sectional study in primary care. Br J Gen Pract. 2017 May;67(658):e314–e320. .
- Shaffer JA, Maurer MS. Multiple chronic conditions and heart failure: overlooking the obvious? JACC Heart Fail. 2015 Jul;3(7):551–553.
- Guazzi M, Labate V. Pulmonary hypertension in heart failure patients: pathophysiology and prognostic implications. Curr Heart Fail Rep. 2016 Dec;13(6):281–294.
- Rosenkranz S, Gibbs JS, Wachter R, et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016 Mar 21;37(12):942–954.
- Chung K, Strange G, Codde J, et al. Left heart disease and pulmonary hypertension: are we seeing the full picture? Heart Lung Circ. 2018 Mar;27(3):301–309.
- Strange G, Williams T, Kermeen F, et al. Pulmonary hypertension and breathlessness: is it a combination we can ignore? Intern Med J. 2014;44(2):114–123.
- Mercurio V, Palazzuoli A, Correale M, et al. Right heart dysfunction: from pathophysiologic insights to therapeutic options: a translational overview. J Cardiovasc Med (Hagerstown). 2018 Nov;19(11):613–623.
- Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012 Oct 30;126(18):2261–2274.
- Guazzi M, Arena R, Halle M, et al. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2016 Jun 14;133(24):e694–711.
- Lewis GD, Shah RV, Pappagianopolas PP, et al. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail. 2008 Nov;1(4):227–233.
- Methvin AB, Owens AT, Emmi AG, et al. Ventilatory inefficiency reflects right ventricular dysfunction in systolic heart failure. Chest. 2011 Mar;139(3):617–625.
- Guazzi M, Cahalin LP, Arena R. Cardiopulmonary exercise testing as a diagnostic tool for the detection of left-sided pulmonary hypertension in heart failure. J Card Fail. 2013 Jul;19(7):461–467.
- Morris NR, Kermeen FD, Holland AE. Exercise-based rehabilitation programmes for pulmonary hypertension. Cochrane Database Syst Rev. 2017 Jan 19;1:Cd011285.
- Torbicki A, Peacock A, Vonk Noordegraaf A, et al. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2015;37(1):67–119.
- Vachiery JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019 Jan;53. PubMed PMID: 30545974; eng. doi:10.1183/13993003.01897-2018