ABSTRACT
Background
FCS significantly affects health-related quality of life (HRQOL). Legacy patient-reported outcome measures are often not sensitive to FCS’s impact. NIH PROMIS and Neuro-QoL measures may accurately capture HRQOL in FCS patients. This study assessed a broad range of PROMIS and Neuro-QoL measures covering physical, mental, and social HRQOL to determine their suitability for the FCS population.
Methods
Adult FCS patients in the United States (N = 25) were recruited to an online survey study and completed several PROMIS short forms and Neuro-QoL computer adaptive tests.
Results
Scores were more than 0.5 standard deviations (SD) worse than the normative mean on 10 of 16 normed measures, and more than 0.75 SDs worse than the normative mean on two measures. Responses at the floor and ceiling were occasionally observed, marginal reliabilities were strong, and significant differences across performance status (ps < 0.05) provided preliminary support for construct validity. The measures correlated with each other strongly and as expected.
Conclusion
Results support the ability of PROMIS and Neuro-QoL measures to detect HRQOL impairment among patients with FCS. PROMIS and Neuro-QoL measures captured the functional impact and symptom burden associated with FCS, and the broad range of symptom severity experienced by patients with FCS.
1. Introduction
Familial Chylomicronemia Syndrome (FCS), also known as type 1 hyperlipoproteinemia or lipoprotein lipase deficiency, is a rare genetic disorder wherein the body is not able to break down fats appropriately. Lipoprotein lipase activity is decreased or absent, which interferes with the hydrolysis of triglycerides in chylomicrons, the largest lipoprotein particle [Citation1,Citation2]. Although the precise prevalence of FCS is not confidently known, it has been estimated to occur in one of every one million individuals [Citation3]. The elevated plasma chylomicrons observed in FCS contribute to a number of clinically relevant manifestations, such as severe abdominal pain and increased risk for acute pancreatitis. Acute pancreatitis is generally viewed as the most concerning consequence of FCS, presenting recurrently in greater than 50% of patients [Citation2]. As plasma triglyceride levels increase, so does risk of both acute pancreatitis and persistent organ failure [Citation4]. Specifically, risk of acute pancreatitis increases 3 to 4% for every 100 mg/dL increase in plasma triglycerides [Citation5,Citation6]. Given that healthy triglyceride levels are generally accepted as values below 150 mg/dL, and patients with FCS often present with values greater than 1,500 mg/dL [Citation7], the risk of acute pancreatitis among patients with FCS is likely more than 40% greater than the risk in the general population. This is particularly concerning given that acute pancreatitis has been linked with mortality rates as high as 30% [Citation8], and can be a cause of chronic fear and anxiety.
Unfortunately, there are no FDA-approved therapies for the treatment of FCS. Traditional lipid-lowering interventions such as fibrates and statins are ineffective in the context of FCS because they rely on at least some degree of lipoprotein lipase enzyme functionality. Therefore, disease management is predominantly behavioral in nature, with a focus on diet. Global dietary guidelines for patients with FCS include: (1) limiting total fat intake to no more than 15–20 g per day, representing less than 10–15% of total daily energy intake, (2) meeting recommendations for essential fatty acids, (3) increasing consumption of complex carbohydrate foods while limiting consumption of simple and refined carbohydrate foods, (4) supplementing with fat-soluble vitamins, minerals, and medium-chain triglyceride oils as needed, and (5) adjust calories to maintain an overall healthy weight [Citation9]. In addition, patients are required to avoid alcohol and medications known to increase triglyceride levels. The restrictive nature of this diet is highly burdensome, and has been hypothesized to negatively impact health-related quality of life (HRQOL).
Given the low prevalence of FCS, the extent to which dietary restriction and other components of FCS impact patient functioning is poorly understood [Citation10]. To date fewer than ten known studies have been conducted specifically focusing on symptom burden and HRQOL associated with FCS [Citation11–Citation17]. The limited data that are available suggest significant burden related to both the disease itself and the management thereof, including major effects on both physical and mental health. For example, a recent online survey study identified bloating, generalized abdominal pain, asthenia, anxiety about potential pain attacks, and anxiety about overall health as the five most commonly reported symptoms associated with FCS [Citation12,Citation14]. A large portion of patients also endorsed experiencing difficulty concentrating and ‘brain fog.’ Another qualitative study reported dietary restriction, anxiety, and stress as primary drivers of the psychosocial burden of FCS [Citation11]. However, the available literature remains sparse.
The most appropriate measures of HRQOL for FCS patients have not yet been identified. The NIH Patient-Reported Outcomes Measurement Information System (PROMIS) was originally developed to provide a comprehensive measurement evaluation system for use with any health condition and in the general population to assess physical, mental, and social health among adults and children. It was developed as part of an NIH Roadmap project to improve measurement of multiple dimensions of HRQOL by bringing together clinical experts, leaders in patient-reported outcome (PRO) science, and NIH partners to develop a system of psychometrically sound, flexible, and universal HRQOL measures [Citation18]. It is included with Neuro-QoL (Quality of Life in Neurological Disorders), a parallel effort in neurological disorders, under the HealthMeasures umbrella (www.healthmeasures.net).
PROMIS and other HealthMeasures systems take an innovative approach to the development and evaluation of PROs through the use of item response theory (IRT) and computer adaptive testing (CAT), drawing from banks of items to generate efficient and precise measures of patients’ HRQOL. This approach yields greater precision than most conventional measures, making such measures particularly useful among patients with rare diseases because power is enhanced without requiring a larger sample size. PROMIS and Neuro-QoL measures may be ideal for assessing HRQOL among patients with FCS; however, they have not previously been used with this population. As such, the ability of these measures to detect elevated symptom burden and reduced HRQOL among patients with FCS has not been established. The present study therefore aimed to evaluate the appropriateness of PROMIS and Neuro-QoL measures for use among patients with FCS in research.
2. Patients and methods
2.1. Recruitment and patient sample
Patients with FCS were recruited to the study online via two research pools of patients living in the United States who had previously volunteered themselves for participation in research. An online survey was developed and a specific URL was disseminated to FCS patients via targeted e-mails. In addition, social media advertisements were placed via outlets including Facebook and Twitter. All e-mails and social media advertisements included a direct link to the survey. Participants were required to confirm eligibility by answering two forced‐choice multiple‐choice questions to confirm a prior diagnosis of FCS and that they were 18 years of age or older. Participants who met eligibility criteria automatically continued to the survey. Participants who did not meet eligibility criteria were informed of this via notification within the survey platform, and thanked for their time. Participants who completed the survey received a 25 USD virtual gift card as compensation for their time.
2.2. Measures
Measures were selected for inclusion in the survey based on literature review and expert opinion. These instruments were chosen to cover health domains hypothesized to be most germane to FCS patients, and to cover a broad spectrum of physical, mental, and social health domains. Thus, the final online survey contained those measures likely to be most useful for future research related to HRQOL in FCS. Though the majority of measures selected for inclusion were drawn from PROMIS, measures from the other systems housed under the HealthMeasures umbrella were also considered for inclusion to ensure evaluation of tools able to assess all domains identified as central to the FCS experience. Ultimately, two assessments from Neuro-QoL were also administered. Additional questions, such as sociodemographic information and a small number of self‐reported clinical questions regarding the FCS diagnosis trajectory, were included to ensure that the study sample sufficiently reflected the broader FCS patient population. Additionally, participants reported their level of functioning according to Eastern Cooperative Oncology Group (ECOG) status [Citation19] to enable evaluation of PROMIS measures across differing levels of functional impairment.
With the exception of the measure of pain intensity, as described below, PROMIS and Neuro-QoL instruments were scored using IRT and yielded T‐scores that have been standardized so a score of 50 represents the average (mean) for the population with which the measure was developed and the standard deviation around that mean is 10 points. Measures were developed with the general population unless otherwise indicated below. The final online survey included the following measures.
2.2.1. PROMIS global health short form v1.1 [Citation20]
This 10‐item self‐report questionnaire provides broad information regarding general self‐perceptions of health. Items assess physical health, pain, fatigue, mental health, and social health. All items except for the assessment of pain intensity are rated using a five‐category response scale, while the single‐item assessment of pain intensity includes a numerical rating scale with response options ranging from 0 (no pain) to 10 (worst imaginable pain). The measure has two factor‐analytically derived subscales reflecting Global Physical Health and Global Mental Health. Higher scores reflect better overall health.
2.2.2. PROMIS‐57 profile v2.0 [Citation21–Citation28]
The 57‐item adult profile includes a single item assessing pain intensity and eight items assessing each of the following domains: depression, anxiety, physical function, pain interference, fatigue, sleep disturbance, and ability to participate in social roles and activities. All items assess symptoms and functioning over the past seven days, except for Physical Function, which includes no specified timeframe. T‐scores are provided for each of the seven domains assessed with multiple items, while a raw score from 0‐10 is provided for the single-item pain intensity measure, with higher scores reflecting more of the construct being assessed. For most domains, these T‐scores were calculated so that 50 is the average for the general population. However, two domains (i.e. sleep disturbance and ability to participate in social roles and activities) were developed with a sample generally more enriched for chronic illness. Therefore, on these two domains a score of 50 represents the average for individuals slightly more impaired than the general population.
2.2.3. PROMIS cognitive function short form v2.0, form 4a [Citation29]
This four‐item fixed form assesses patient‐perceived cognitive deficits. Items assess perceived mental acuity, mental function, and concentration. Higher scores reflect better perceived cognitive functioning.
2.2.4. PROMIS self‐efficacy for managing social interactions short form v1.0, form 4a [Citation30]
This four-item fixed form assesses patients’ confidence in participating in social activities and getting help when necessary, as well as managing communication with others, including health professionals, about their medical condition. The development sample for this measure was enriched for chronic illness. Higher scores reflect greater self-efficacy for participating in social activities.
2.2.5. PROMIS self‐efficacy for managing symptoms short form v1.0, form 4a [Citation30]
This four‐item fixed form assesses patients’ confidence generally managing symptoms, managing symptoms in different settings, and keeping symptoms from interfering with functioning in work, sleep, relationships, or recreational activities. The development sample for this measure was enriched for chronic illness. Higher scores reflect greater self‐efficacy for managing symptoms.
2.2.6. Neuro‐QoL positive affect and well‐being CAT [Citation31]
Items from the Neuro‐QoL Positive Affect and Well‐Being item bank v1.0 were delivered as a CAT, with respondents answering a custom subset of the 23 items in the bank. The measure evaluates aspects of a person’s life that relate to a general sense of well‐being, satisfaction with life, or an overall sense of purpose and meaning. Higher scores reflect more positive affect.
2.2.7. PROMIS gastrointestinal belly pain scale v1.0, form 5a [Citation32,Citation33]
This five‐item fixed form assesses the intensity, nature, frequency, bothersomeness, and predictability of belly pain severity. Higher scores reflect more severe belly pain.
2.2.8. PROMIS social isolation short form v2.0, form 8a [Citation28]
This eight‐item fixed form assesses patient perceptions of being avoided by, excluded by, detached from, disconnected from, or unknown by others. The development sample for this measure was enriched for chronic illness. Higher scores reflect more isolation.
2.2.9. Neuro‐QoL stigma CAT [Citation34]
Items from the Neuro‐QoL Stigma item bank v1.0 were delivered as a CAT, with respondents answering a custom subset of the 24 items in the bank. The measure evaluates perceptions of self‐ and publically‐enacted negativity, prejudice, and discrimination as a result of disease‐related manifestations. The development sample for this measure was enriched for neurologic conditions. Higher scores reflect greater perceived disease‐related stigma.
2.3. Data analysis
The following descriptive statistics were calculated to evaluate the ability of these measures to assess HRQOL and symptom burden among patients with FCS,: mean, standard deviation, number and percentage at floor (i.e. the lowest possible score), number and percentage at ceiling (i.e. the highest possible score), number and percentage at the lowest and highest observed scores, and the 25th, 50th (median), and 75th percentiles. Mean values on each measure were also calculated for each ECOG performance status group separately and compared across groups. Marginal reliability coefficients were estimated with graded response models (IRT). Nunnally’s rule of thumb was used for reliability cutoffs: acceptable: 0.70–0.79; good: 0.80–0.89; excellent: ≥0.90 [Citation35]. In addition, the inter‐correlations among all administered measures were evaluated. For all statistical tests, a p-value of 0.05 was considered statistically significant.
3. Results
Twenty-five patients with FCS met inclusion criteria and completed the online survey. Patient characteristics are in . The majority of respondents were female. On average respondents were middle aged, had experienced FCS symptoms for nearly 20 years, and had received a diagnosis of FCS approximately 10 years prior to study participation. The mean completion time for the survey was 11.26 minutes (standard deviation [SD] = 5.79, range = 5–32).
Table 1. Patient characteristics (N = 25).
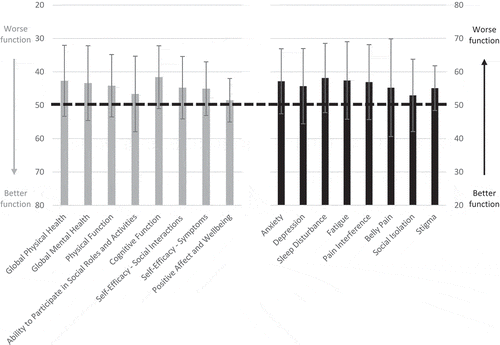
The administered measures yielded 17 scores. On average, patients with FCS demonstrated worse outcomes on all measures administered relative to the norm-referenced mean. Scores were more than half a standard deviation worse than the normative mean, indicating worse functioning, on assessments of global physical and mental health, anxiety, depression, physical function, fatigue, pain interference, self‐efficacy for managing social interactions, belly pain, and stigma. Scores were more than three‐quarters of a standard deviation worse than the normative mean on assessments of sleep disturbance and cognitive function (see and ).
Table 2. Descriptives of PROMIS and Neuro-QoL assessment T-scores among patients with FCS.
For tools administered as fixed forms, responses at the floor (i.e. lowest possible score) were observed for nine of the 15 resultant scores. Responses at the floor were observed for anxiety, depression, sleep disturbance, fatigue, ability to participate in social roles and activities, pain interference, pain intensity, cognitive function, and social isolation. Responses at the floor were not observed for global physical health, global mental health, physical function, and self‐efficacy for managing social interactions and symptoms. In addition, responses at the floor were not observed for belly pain among participants who endorsed pain within the past week, although they were observed for belly pain among participants who did not endorse recent pain. Responses at the ceiling (i.e. highest possible score) were observed for seven of the 15 scores resulting from tests administered as fixed forms. Responses at the ceiling were observed for global physical health, global mental health, physical function, sleep disturbance, ability to participate in social roles and activities, cognitive function, and self-efficacy for managing social interactions. Responses at the ceiling were not observed for anxiety, depression, fatigue, pain interference, pain intensity, self‐efficacy for managing symptoms, social isolation, and belly pain. For the two tools administered as CATs, only one individual (4.0%) demonstrated a score at the minimum observed value and one individual (4.0%) demonstrated a score at the maximum observed value on each test (see ).
Table 3. Floor and ceiling effects of PROMIS and Neuro-QoL assessments among patients with FCS.
Marginal reliabilities are shown in . Reliabilities were estimated for 16 of the 17 total scores, with Pain Intensity excluded due to it consisting of a single item. Among the remaining measures, reliabilities were often excellent (seven of 16 measures) or good (seven of 16 measures). Only two measures had estimated reliabilities in the acceptable range: PROMIS Pain Interference and PROMIS Self-Efficacy for Managing Chronic Conditions – Manage Social Interactions. Both measures administered as CATs, Neuro-QoL Positive Affect and Well-Being and Neuro-QoL Stigma, had reliabilities exceeding the cutoff for excellent.
Differences in scores across ECOG performance status were statistically significant (ps < 0.05) for numerous physical health outcomes, including global physical health, physical function, fatigue, pain interference, pain intensity, and cognitive function, across individuals reporting normal activity without symptoms and those endorsing some symptoms but not requiring bed rest during the waking day. These groups also significantly differed in reported ability to participate in social roles and activities (see ). Comparisons were not made for ECOG performance status groups representing more severe disability due to low sample size. Finally, the administered tools related to each other strongly and consistently (see ).
Table 4. Comparisons of PROMIS and Neuro-QoL assessments by ECOG performance status among patients with FCS.
Table 5. Correlations of all PROMIS and Neuro-QoL assessments among patient with FCS.
4. Discussion
This study’s results provide preliminary support for the appropriateness of PROMIS and Neuro-QoL measures to evaluate HRQOL among patients with FCS. The literature suggests that patients with FCS experience decreased HRQOL and elevated symptom burden (e.g. pain, fatigue, brain fog, stigma) relative to the general population. However, prior research has been limited by a lack of validated measurement tools to assess PROs within FCS. The present study aimed to fill this gap, and found evidence of both reliability and validity for several PROMIS and Neuro-QoL measures among FCS patients.
To our knowledge, this is the first study to implement PROMIS and Neuro-QoL measures in patients with FCS. Prior work identified sources of increased symptom burden among patients with FCS, providing insight into which symptoms are likely to be elevated in this population, including physical, emotional, and cognitive concerns [Citation14]. Given this, it was anticipated that patients with FCS would demonstrate elevations on all of the measures administered relative to the normative samples. Consistent with expectations, the measures effectively captured the anticipated worse global physical and mental health, anxiety, depression, physical function, fatigue, pain interference, cognitive function, and belly pain experienced by patients with FCS relative to the general population, as well as the anticipated worse sleep disturbance, self‐efficacy for managing social interactions, and stigma experienced by these patients relative to other chronic illness populations. Moreover, given that a difference in T‐scores of one half of a standard deviation can be interpreted as reflective of a meaningful difference, the present results provide support for the utility of PROMIS and Neuro-QoL measures in capturing clinically relevant distress among patients with FCS.
The identification of values at both the ceiling and floor of most of the evaluated measures suggests that these tools are able to capture the broad range of symptom severity experienced within the context of FCS. In their recent qualitative work, Neelamekam and colleagues [Citation16] demonstrated that different patients report highly variable disease burden and HRQOL outcomes. Thus, not only were the PROMIS and Neuro-QoL measures able to reflect the average poorer outcomes among patients with FCS relative to the general population, they were also able to capture the wide range of symptom experiences reported by different individuals.
Significant differences in scores on PROMIS and Neuro-QoL measures across ECOG functional status groups provided preliminary support for the validity of the administered measures. Moreover, scores on all assessment tools were strongly related to each other, as expected. Although participants needed an average of only approximately 11 minutes to complete the full survey, future applications may benefit from a more parsimonious approach. For example, the 10-item PROMIS Global Health Short Form effectively captured general impairment among patients with FCS, and thus may be a good option when only a measure of broad functioning is needed. Identifying how these tools relate to each other can help inform which instruments to include and which instruments may be redundant in future studies with patients with FCS.
Participants in this study were demographically similar to patients with FCS included in prior studies focused on HRQOL. These results may therefore generalize to the broader FCS patient population. For example, in the APPROACH study [Citation36], the largest study population to date of patients with FCS (n = 66), the mean age was 46 years, and the majority was female (55%) and either White (80%) or Asian (17%). The proportion of White participants in the present study was greater, and the proportion of Asian participants was less, than that observed in the APPROACH study. However, this may be because the present sample was smaller, and the APPROACH study recruited participants globally while the present study was only conducted within the United States. Notably, these were the only two racial categories represented in the present sample, accurately reflecting the increased prevalence of FCS among these groups and further supporting the likely representativeness of the present sample relative to the broader FCS population.
4.1. Limitations
There are limitations to this study. The sample size was small and there was no adjustment for multiple comparisons. Future work with these measures in FCS patients should be conducted with larger sample sizes and formal hypothesis testing. Patient clinical data were self-reported and were not verified. Moreover, patients were only recruited from the United States. While PROMIS and Neuro-QoL measures were specifically designed for use across diverse cultures and are available in numerous languages, the measures might function differently among patients with FCS from other countries.
5. Conclusions
Despite these limitations, the present results provide a valuable contribution to the literature. This was the first study to explore the appropriateness of PROMIS and Neuro-QoL measures for use among patients with FCS. Given the rare nature of this disease, assessment tools that prioritize parsimony are particularly attractive as they allow for the assessment of a larger number of domains without elevating response burden. Given the present findings, it is recommended that PROMIS and Neuro-QoL measures be used in future larger-scale studies to evaluate HRQOL and symptom burden among patients with FCS.
6. Expert Opinion
Valid and reliable patient-reported outcome measures should be used to assess HRQOL and symptom burden among patients with FCS. PROMIS and Neuro-QoL measures can be used to meet this need. Pending additional evaluation with larger samples, these measures are likely to be ideally suited for monitoring symptoms and side effects in research with this patient population.
Author Contributions
Rina S. Fox: Study design, analysis and interpretation of data, drafting of paper, revising of paper for intellectual content, final approval of the version to be published
John Devin Peipert: Study conception and design, analysis and interpretation of data, revising of paper for intellectual content, final approval of the version to be published
Montserrat Vera Llonch: Revising of paper for intellectual content, final approval of the version to be published
Glenn Philips: Study conception and design, revising of paper for intellectual content, final approval of the version to be published
David Cella: Study conception and design, revising of paper for intellectual content, final approval of the version to be published
All authors agree to be accountable for all aspects of the work
Declaration of interest
The authors were supported by Akcea Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have served on Speaker Faculty and advisory committees for Akcea. They have also disclosed that they were a PI for Akcea. Another reviewer on this manuscript has disclosed that they received payment for talks and advisory boards by Amgen, Fresenius medical care, Sanofi, Alexion and Daichii Sankyo. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
Funding for this work was provided to Northwestern University by Akcea Therapeutics. Montserrat Vera Llonch and Glenn Phillips contributed to this work as part of their employment with Akcea Therapeutics. Rina S. Fox, John Devin Peipert, and David Cella contributed to this work at Northwestern University as contracted by Akcea Therapeutics. We thank Maria Corona for facilitating project management. We also thank the study participants for their time and contributions.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Brahm AJ, Hegele RA. Chylomicronaemia–current diagnosis and future therapies. Nat Rev Endocrinol. 2015;11(6):352–362.
- Gaudet D, Blom D, Bruckert E, et al. Acute pancreatitis is highly prevalent and complications can be fatal in patients with Familial Chylomicronemia: results from a survey of lipidologist. J Clin Lipidol. 2016;10(3):680–681.
- Pouwels E, Blom D, Firth J, et al. Severe hypertriglyceridaemia as a result of Familial Chylomicronaemia. South Afr Med J. 2008;98(2):105–108.
- Nawaz H, Koutroumpakis E, Easler J, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol. 2015;110(10):1497–1503.
- Rashid N, Sharma PP, Scott RD, et al. Severe hypertriglyceridemia and factors associated with acute pancreatitis in an integrated health care system. J Clin Lipidol. 2016;10(4):880–890.
- Murphy MJ, Sheng X, MacDonald TM, et al. Hypertriglyceridemia and acute pancreatitis. JAMA Intern Med. 2013;173(2):162–164.
- Chokshi N, Blumenschein SD, Ahmad Z, et al. Genotype-phenotype relationships in patients with type I hyperlipoproteinemia. J Clin Lipidol. 2014;8(3):287–295.
- Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354(20):2142–2150.
- Williams L, Rhodes KS, Karmally W, et al. Familial chylomicronemia syndrome: bringing to life dietary recommendations throughout the life span. J Clin Lipidol. 2018;12(4):908–919.
- Ahmad Z, Halter R, Stevenson M. Building a better understanding of the burden of disease in familial chylomicronemia syndrome. Expert Rev Clin Pharmacol. 2017;10(1):1–3.
- Gelrud A, Williams KR, Hsieh A, et al. The burden of familial chylomicronemia syndrome from the patients’ perspective. Expert Rev Cardiovasc Ther. 2017;15(11):879–887.
- Davidson M, Stevenson M, Hsieh A, et al. The burden of familial chylomicronemia syndrome: interim results from the IN-FOCUS study. Expert Rev Cardiovasc Ther. 2017;15(5):415–423.
- Arca M, Hsieh A, Soran H, et al. The effect of volanesorsen treatment on the burden associated with familial chylomicronemia syndrome: the results of the ReFOCUS study. Expert Rev Cardiovasc Ther. 2018;16(7):537–546.
- Davidson M, Stevenson M, Hsieh A, et al. The burden of familial chylomicronemia syndrome: results from the global IN-FOCUS study. J Clin Lipidol. 2018;12(4):898–907.
- Wilson LM, Cross RR, Duell PB. Reduced psychological distress in familial chylomicronemia syndrome after patient support group intervention. J Clin Lipidol. 2018;12(1):240–242.
- Neelamekam S, Kwok S, Malone R, et al. The impact of lipoprotein lipase deficiency on health-related quality of life: a detailed, structured, qualitative study. Orphanet J Rare Dis. 2017;12(1):156.
- Salvatore V, Gilstrap A, Williams KR, et al. Evaluating the impact of peer support and connection on the quality of life of patients with familial chylomicronemia syndrome. Expert Opin Orphan Drugs. 2018;6(8):497–505.
- Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–11.
- Zubord C, Scheiderman M, Frei E. Appraisal of methods for the study of chemotherapy in man: comparative therapeutic trial of nitrogen mustard and triethylene thiopphosphoramide. J Chron Dis. 1960;11:7–33.
- Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–880.
- Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18(3):263–283.
- Pilkonis PA, Yu L, Dodds NE, et al. Validation of the depression item bank from the Patient-Reported Outcomes Measurement Information System (PROMIS®) in a three-month observational study. J Psychiatr Res. 2014;56:112–119.
- Lai J-S, Cella D, Choi S, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil. 2011;92(10):S20–S7.
- Rose M, Bjorner JB, Becker J, et al. Evaluation of a preliminary physical function item bank supported the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS). J Clin Epidemiol. 2008;61(1):17–33.
- Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150(1):173–182.
- Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781–792.
- Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2011;10(1):6–24.
- Hahn EA, DeWalt DA, Bode RK, et al. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. 2014;33(5):490–499.
- Lai JS, Wagner LI, Jacobsen PB, et al. Self‐reported cognitive concerns and abilities: two sides of one coin? Psycho‐Oncology. 2014;23(10):1133–1141.
- Gruber-Baldini AL, Velozo C, Romero S, et al. Validation of the PROMIS® measures of self-efficacy for managing chronic conditions. Qual Life Res. 2017;26(7):1915–1924.
- Salsman JM, Victorson D, Choi SW, et al. Development and validation of the positive affect and well-being scale for the neurology quality of life (Neuro-QOL) measurement system. Qual Life Res. 2013;22(9):2569–2580.
- Spiegel BM, Hays RD, Bolus R, et al. Development of the NIH patient-reported outcomes measurement information system (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109(11):1804–1814.
- Khanna D, Hays RD, Shreiner AB, et al. Responsiveness to change and minimally important differences of the patient-reported outcomes measurement information system gastrointestinal symptoms scales. Dig Dis Sci. 2017;62(5):1186–1192.
- Gershon RC, Lai JS, Bode R, et al. Neuro-QOL: quality of life item banks for adults with neurological disorders: item development and calibrations based upon clinical and general population testing. Qual Life Res. 2012;21(3):475–486.
- Nunnally JC. Psychometric theory. 2nd ed. New York: McGraw-Hill; 1978.
- Blom DJ, O’Dea L, Digenio A, et al. Characterizing familial chylomicronemia syndrome: baseline data of the APPROACH study. J Clin Lipidol. 2018;12(5):1234–1243.