ABSTRACT
Introduction
The presence of a chronic total occlusion (CTO) is associated with an increased risk of ventricular arrhythmias.
Areas covered
This review provides an overview of the relationship between CTO and ventricular arrhythmias, arrhythmogenic mechanisms, and the effect of revascularization.
Expert opinion
Studies in recipients of an implantable cardioverter-defibrillator (ICD) have shown that a CTO is an independent predictor of appropriate ICD therapy. The myocardial territory supplied by a CTO is a pro-arrhythmogenic milieu characterized by scar tissue, large scar border zone, hibernating myocardium, residual ischemia despite collaterals, areas of slow conduction, and heterogeneity in repolarization. Restoring coronary flow by revascularization might be associated with electrical homogenization as reflected by a decrease in QT(c) dispersion, decrease in T wave peak-to-end interval, reduction of late potentials, and decrease in scar border zone area. Future research should explore whether CTO revascularization results in a lower burden of ventricular arrhythmias. Furthermore, risk stratification of CTO patients without severe LV dysfunction is interesting to identify potential ICD candidates. Potential tools for risk stratification are the use of electrocardiographic parameters, body surface mapping, electrophysiological study, and close rhythm monitoring using an insertable cardiac monitor.
1. Introduction
The presence of a chronic total occlusion (CTO) is associated with an increased risk of sudden cardiac death (SCD) [Citation1–Citation2–Citation3–Citation4]. This seems to be mainly driven by a higher risk of ventricular arrhythmias (VAs) [Citation1,Citation5–Citation8]. There is emerging clinical evidence that the presence of a CTO has a proarrhythmic effect [Citation9–Citation11]. This observation is clinically relevant as a CTO is a common finding (approximately 20%) in patients with coronary artery disease [Citation1,Citation12–Citation13–Citation14–Citation15]. Technical advances in percutaneous CTO revascularization has resulted in higher success rates (>90%) and lower complication rates in high volume and dedicated centers [Citation16]. Successful CTO revascularization has been associated with a reduction in angina, positive left ventricular (LV) remodeling, and improvement in quality of life [Citation17–Citation18–Citation19–Citation20–Citation21–Citation23]. However, the beneficial prognostic role of a CTO revascularization is still under debate [Citation22,Citation24]. There is a paucity of randomized studies which have focused on a prognostic benefit in comparison to medical management [Citation16,Citation25–Citation27]. Currently, factors that are taken into account when selecting patients for a CTO revascularization, include symptomatic angina, extent of ischemia, and degree of myocardial viability [Citation28]. Considering the increased vulnerability for VA in patients with CTO, more refined risk stratification is necessary to identify potential candidates for an implantable cardioverter-defibrillator (ICD). The current ICD recommendations for primary prevention are largely based on LV ejection fraction () [Citation29–Citation31].
Figure 1. Current recommendations for an ICD in patients with a CTO. Footnote. Based on the recommendations from the European Society of Cardiology (ESC), American College of Cardiology (ACC), American Heart Association (AHA) and Heart Rhythm Society (HRS) [Citation29–Citation31]. Abbreviations: EP, electrophysiology study; ICD, implantable cardioverter-defibrillator; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; SCA, sudden cardiac arrest; VF, ventricular fibrillation; VT, ventricular tachycardia.
![Figure 1. Current recommendations for an ICD in patients with a CTO. Footnote. Based on the recommendations from the European Society of Cardiology (ESC), American College of Cardiology (ACC), American Heart Association (AHA) and Heart Rhythm Society (HRS) [Citation29–Citation31]. Abbreviations: EP, electrophysiology study; ICD, implantable cardioverter-defibrillator; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; SCA, sudden cardiac arrest; VF, ventricular fibrillation; VT, ventricular tachycardia.](/cms/asset/a7fee5c4-6749-48b5-855c-c0fb7f20e3d5/ierk_a_1793671_f0001_oc.jpg)
The aims of this review are: 1) to provide an overview of the clinical evidence demonstrating a relationship between CTO and VA; 2) to expand on the potential mechanisms for the increased VA susceptibility; 3) to evaluate the potential beneficial role of CTO revascularization on electrophysiological parameters; and 4) to discuss potential future research areas with regard to risk stratification.
2. CTO and risk for ventricular arrhythmias
2.1. CTO in ICD recipients
The last decade, several studies have evaluated the association between CTO and the occurrence of VA in ICD recipients with coronary artery disease [Citation5–Citation8,Citation32] (). One of the first studies was published in 2012 by Nombela-Franco et al (VACTO Primary Study) [Citation6]. They evaluated in a single-center cohort the impact of unrevascularized CTO on the occurrence of VA in 162 patients with ischemic cardiomyopathy and a primary prevention ICD. The presence of an unrevascularized CTO was an independent predictor of appropriate ICD therapy. In contrast, Raja et al. found that the presence of a CTO (revascularized or not) was not associated with a higher mortality or VA [Citation32]. In this single-center study, 307 patients with ischemic cardiomyopathy and an ICD for primary prevention were included. The study population consisted of 114 patients with unrevascularized CTO, 99 patients with revascularized CTO, and 94 patients without CTO. During a median follow-up of 4.1 years, 17% died and 32% had at least one episode of appropriate ICD therapy. Presence of a CTO (irrespective of revascularization) was not associated with the risk of appropriate ICD therapy or mortality.
Table 1. CTO and appropriate ICD therapy/mortality in ICD recipients with coronary artery disease.
It is important to realize that patients who receive an ICD for primary prevention have severe LV dysfunction (LVEF <35%). Thus, abovementioned ICD studies evaluating the role of CTO and the occurrence of VA do not involve patients with preserved or midrange ejection fraction. Further insight is provided by studies evaluating the association between CTO and the occurrence of VA in patients who received an ICD for secondary prevention. Two studies have specifically evaluated the effect of a CTO on VA recurrence in survivors of SCD or sustained VA [Citation5,Citation7]. In a retrospective single-center study, Yap et al. evaluated 217 patients with aborted SCD who received an ICD [Citation7]. The proportion of patients with a LVEF <35% was 52%. Appropriate ICD therapy was higher in patients with CTO (37% versus 25% at 5 years). The presence of an unrevascularized CTO and LVEF <35% were independent predictors of appropriate ICD therapy. The results were confirmed by the large multicenter VACTO Secondary Study, which included 425 patients from 8 centers who received an ICD for secondary prevention [Citation5]. The proportion of patients with a LVEF ≤40% was 63%. Appropriate ICD therapy was higher in patients with CTO (51% versus 36% at 4 years). Also, in this study the only independent predictors of appropriate ICD therapy were the presence of a CTO and LVEF. Among patients with CTO, renal dysfunction and LVEF <40% were independent factors associated with appropriate ICD therapy. Van Dongen et al. evaluated 722 patients with ischemic cardiomyopathy who received an ICD for primary and secondary prevention [Citation33]. A CTO was an independent predictor of appropriate ICD therapy. Interestingly, primary prevention patients without a CTO experienced the lowest appropriate ICD therapy rate compared with primary prevention patients with a CTO and secondary prevention patients.
A meta-analysis by Wang et al. in 2018, included 6 studies evaluating the prognostic role of CTO with regard to appropriate ICD therapy [Citation34]. The authors conclude that CTO was a predictor of VA in patients with ICD for secondary prevention (HR 1.96, 95% CI 1.55–2.48), but not for patients with an ICD for primary prevention (HR 1.84, 95% CI 0.63–5.33). This may suggest that in patients with already severe LV dysfunction, the presence of CTO does not give an incremental risk of VA. However, it is important to highlight that in patients with ICD for primary prevention only 1 of 3 studies did not demonstrate a relationship between CTO and VA [Citation32].
2.2. CTO in an infarct-related artery
Some authors have suggested that a CTO in an infarct-related artery (IRA-CTO) may be a better predictor of VA than a CTO without a myocardial infarction in its territory (non-IRA-CTO) [Citation9,Citation11] (). The lower arrhythmic risk of a non-IRA-CTO may be related the absence of a postinfarction scar. An IRA-CTO is defined as a CTO associated with a previous myocardial infarction in the territory of the coronary artery. Evidence for a previous myocardial infarction is demonstrated by the presence of Q-waves in the electrogram and/or evidence of scar at imaging, such as clear wall motion abnormalities at echocardiogram or late gadolinium enhancement at cardiac MRI [Citation9]. Using this definition approximately two thirds of patients with a CTO have an IRA-CTO [Citation9,Citation10]. Di Marco et al. performed a two-center study (n = 342) including ischemic cardiomyopathy patients who received an ICD for primary or secondary prevention [Citation10]. At least 1 CTO was present in 182 patients (53%) and at least one IRA-CTO was found in 161 patients (47%). An IRA-CTO was significantly more prevalent in patients with secondary prevention ICD as compared to primary prevention (62% versus 32%, P < 0.001). Patients with IRA-CTO had higher proportions of appropriate ICD therapies (57% versus 26%, P < 0.001) and appropriate ICD shocks (40% versus 17%, P < 0.001). Patients with a non-IRA-CTO had a similar risk of VA compared to those without CTO (HR 1.0, 95% CI 0.4–2.3, P = 0.97). However, it is good to realize that the number of patients with a non-IRA-CTO was small (n = 21), precluding strong statements that an IRA-CTO is more vulnerable to VA than a non-IRA-CTO.
In 2018, Chi et al. performed a systematic review and meta-analysis examining the relationship between CTO and arrhythmic and mortality outcome [Citation8]. Compared to patients without IRA-CTO (i.e., patients without CTO and patients with non-IRA-CTO), patients with IRA-CTO have a higher risk of VA or appropriate ICD therapy (HR, 2.47, 95% CI, 1.76–3.46), cardiac mortality (HR, 2.73, 95% CI, 1.02–7.30), and all-cause mortality (HR, 1.69, 95% CI, 1.19–2.40).
3. Proarrhythmogenic substrate in CTO territory
Based on abovementioned clinical studies, there seems to be an increased risk of VA in patients with a CTO (). In patients with previous myocardial infarction, the mechanism of VA usually depends on a scar-related reentry circuit [Citation35]. One study showed that the properties of spontaneous VA between patients with and without CTO is similar with respect to cycle length and type of onset. However, the coupling interval of the initiating beat is shorter in patients with CTO [Citation5]. This suggest that the trigger or substrate is different in patients with a CTO.
Figure 2. Schematic overview of pro-arrhythmic effects of IRA-CTO and potential beneficial effects of CTO PCI.
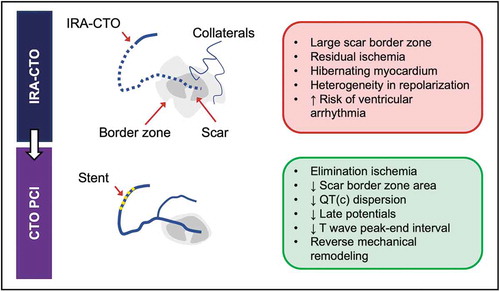
The scar border zone seems critical for the occurrence of VA. This zone consists of surviving cardiomyocytes interposed between fibrotic tissue and is characterized by areas of fractionation and activation delay [Citation36,Citation37]. Several studies using contrast-enhanced MRI have demonstrated that a larger scar border zone, as defined by infarct gray zone or peri-infarct zone, was associated with an increased risk of spontaneous and inducible VA in ICD recipients [Citation38–Citation39–Citation40]. Electrophysiological mapping studies have shown that a CTO in an infarct-related artery was associated with a larger scar border zone (defined by bipolar voltage of 0.5–1.5 mV) in comparison to patients with no CTO in an infarct-related artery [Citation11]. This large border zone may be associated with heterogenous ventricular repolarization. After CTO revascularization there is immediate decrease in scar border zone area. Furthermore, there is improvement of markers of heterogeneity of repolarization as reflected by QT dispersion [Citation41,Citation42]. It is known that heterogenous ventricular repolarization is associated with an increased risk for ischemia-related VA after myocardial infarction [Citation43]. Thus, a larger scar border zone may explain the increased vulnerability for VA in patients with IRA-CTO.
The proarrhythmogenic milieu is further enhanced by the presence of chronic residual ischemia in the CTO territory despite the presence of collaterals. It is well-known that ischemia is an important trigger for VA. Collateral connections are observed in almost all CTOs and they have the capability to prevent myocardial necrosis [Citation44]. Rentrop grade ≤1 collateral flow (poor collateral flow) varies between 10% to 28% depending on patient population [Citation5,Citation10]. Collateral function can develop to a similar functional level in patients after myocardial infarction with large areas of akinesia as it does in patients with normal preserved regional function [Citation44,Citation45]. During exercise, collaterals have limited functional reserve and most patients will experience ischemia due to coronary steal despite angiographically well-developed collaterals [Citation45]. Using fraction flow reserve (FFR), Sachdeva et al. demonstrated that the majority of CTO patients have an ischemic FFR (<0.80), even those with severe regional dysfunction or well-developed collaterals [Citation46]. Furthermore, resting ischemia was present in 78% of CTO patients despite a negative noninvasive stress test. These data suggest that the myocardium supplied by a CTO is chronically repetitive ischemic in a majority of patients. Sympathetic stimulation, for example during exercise, increases the difference in refractoriness in ischemic areas, potentially leading to regional conduction block and the propensity to reentrant arrhythmias [Citation47].
There is inconsistency with regard to the role of better collateral flow and the occurrence of VA. Some studies in patients with ICDs for primary prevention have shown a nonsignificant trend toward less VA in those with better collateral flow [Citation6,Citation9,Citation10]. In contrast, the VACTO Secondary study demonstrated that good collateral flow (Rentrop grade 3) was associated with a higher incidence of VA in comparison to patients with less collateral flow (Rentrop grade ≤2 flow) (HR 1.54, 95% CI 1.04–2.26) [Citation5]. The increased susceptibility to VA in patients with good collateral flow can be explained by a greater amount of hibernating myocardium, especially in a peri-infarct area [Citation46]. Contrast-enhanced MRI studies have demonstrated less segmental transmurality and higher chance of improvement of dysfunctional segments after percutaneous coronary intervention (PCI) of a CTO with improving Rentrop grade [Citation48,Citation49]. Although this implies that a good collateral circulation is more likely to supply viable myocardium, this chronic hibernating myocardium is a potential pathophysiologic substrate for VA [Citation50]. Hibernating myocardium displays abnormal and heterogeneous electrical properties and contributes to the formation of a substrate for reentry.
Chronic ischemia results in regional cellular remodeling including myocyte hypertrophy, interstitial fibrosis, and inhomogeneity of sympathetic innervation [Citation47,Citation51–Citation52–Citation53]. Furthermore, electrical remodeling occurs due to abnormal intracellular ionic homeostasis secondary to metabolic derangement and increased oxidative stress [Citation54,Citation55]. These changes may contribute to cardiac arrhythmogenesis. This remodeling process may take time and may explain the observation that there is a delay in the occurrence of VA after a myocardial infarction in CTO patients [Citation10]. Thus, regional adaptations that promote myocyte survival in the setting of CTO may result in a substrate with enhanced susceptibility to VA.
4. CTO revascularization lowers arrhythmic risk
The rationale of CTO revascularization is that it relieves ischemia in the CTO territory by restoring coronary flow. Successful CTO PCI has been associated with reverse LV remodeling and improved quality of life [Citation25,Citation26,Citation49]. Evidence that CTO PCI improves survival is largely based on observational cohort studies comparing patients with a successful and failed CTO PCI. A meta-analysis in 2015, including 25 observational studies, demonstrated a twofold higher risk of all-cause mortality in patients with a failed CTO PCI (odds ratio [OR] 1.92, 95% CI 1.59–2.33) in comparison to a successful CTO PCI [Citation22]. However, a recent meta-analysis of 3 randomized controlled trials (i.e., EUROCTO, DECISION-CTO, REVASC) (N = 1416) demonstrated that CTO PCI was not associated with a lower all-cause mortality (OR 0.71, 95% 0.38–1.30) or cardiac mortality (OR 0.64, 95% CI 0.28–1.43) in comparison to medical therapy [Citation56]. Thus, to verify the hypothesis that CTO PCI enhances survival a randomized controlled trial with appropriate sample size will be necessary.
Data on the effect of CTO PCI on the incidence of VA is very limited. Recently, a multicenter observational study by Godino et al. (N = 1162) demonstrated that a failed CTO PCI was associated with an almost threefold increased risk of SCD and/or sustained VA (HR 2.71, 95% CI 1.48–4.99), even after correcting for confounders such as LVEF [Citation57]. The baseline LVEF in the study population was relatively high (53 ± 10%). During a median follow-up of 6 years, the rate of arrhythmic events was 7.5% and 2.5% (P < 0.001) in the failed and successful CTO PCI, respectively. Interestingly, a subgroup analysis demonstrated that a failed CTO PCI in IRA-CTO was associated with a higher risk of SCD/sustained VA (HR 2.79, 95% CI 1.43–5.45, P = 0.003). This was not the case for a failed CTO PCI in a non-IRA-CTO. Approximately half (55%) of all CTO PCI procedures were performed in an IRA-CTO.
The lower arrhythmic risk in patients with successful CTO PCI of an IRA-CTO may be related to elimination of myocardial ischemia in chronic hibernating myocardium, regional cellular remodeling, decrease in electrical heterogeneity, improvement of autonomic nervous activity, and/or lower dependency of collateral flow [Citation47,Citation57–Citation58–Citation59] (). Van Dongen et al. performed the first systematic review and meta-analysis focusing on the effect of CTO PCI on electrocardiographic parameters [Citation41]. They included 8 studies incorporating 467 patients. After CTO PCI there was an improvement of several surrogate arrhythmic markers that reflect heterogeneity in depolarization and repolarization. Successful CTO PCI was associated with a decrease in QT dispersion (17 ms, 95% CI 11–24 ms) and QTc dispersion (19 ms, 95% CI 12–26 ms) within one day after PCI [Citation60]. These effects remain stable at 6 months. Furthermore, there was a reduction of T-wave peak-to-end (Tp-e) interval in lead V2 and V5 within 48 after CTO PCI, also after correction for QTc interval (Tp-e/QTc) [Citation61]. Finally, there was a decrease in the presence of late potentials after CTO PCI [Citation60].
Interestingly, recent 3D electro-anatomically mapping studies demonstrated reduction of the area of the scar border zone after successful CTO PCI [Citation42,Citation62]. Yamashita et al. performed high-density endocardial mapping during sinus rhythm before and 8 months after CTO PCI in 16 patients [Citation42]. Thirteen patients had a successful CTO PCI and no restenosis; 3 patients had a failed CTO PCI or in-stent occlusion during follow-up. There was a significant reduction of border zone area (0.5–1.5 mV) (−10.5 cm2 [−16.8 to −4.1], P = 0.004) without a change in dense scar area (<0.5 mV) in patients with a successful CTO PCI. Furthermore, a small study of 3 patients demonstrated that the reduction in border zone area occurs immediately after IRA-CTO PCI [Citation62].
Thus, based on abovementioned observations most electrophysiological parameters seem to improve immediately after CTO PCI. This suggests that electrophysiological properties in the CTO region can improve immediately after CTO PCI in contrast to mechanical properties (i.e., reverse remodeling). The combination of homogenization of repolarization, disappearance of late potentials, and reduction of scar border zone area may potentially reduce the vulnerability for VA in patients who have undergone successful CTO PCI. There is a lack of randomized trials which has focused on the incidence of VA or SCD.
5. Conclusion
A CTO seems to be associated with a higher risk of VA and mortality in ICD recipients. This increased arrhythmic risk may be related to the presence of a chronically hibernating myocardium in the CTO territory despite the presence of a well-developed collateral system. The limited available data on the effect of CTO PCI on electrophysiological parameters suggest that revascularization may provide immediate electrical stability. This is reflected by a decrease in heterogeneity in repolarization, reduction of late potentials, and decrease in scar border zone area. Further studies are necessary to evaluate whether CTO PCI renders patients less vulnerable to VA.
6. Expert opinion
SCD is a devastating consequence of a myocardial infarction. Previous studies have shown that systolic LV function is the most important predictor for future arrhythmic events. Patients with ischemic cardiomyopathy and a LVEF <30-35% are candidates for a prophylactic ICD [Citation29–Citation31] (). However, in clinical practice we also encounter patients with CTO after an out-of-hospital cardiac arrest without severe LV dysfunction. Studies in patients who received an ICD for secondary prevention demonstrated that CTO and LVEF were independent predictors of appropriate ICD therapy [Citation5,Citation7]. The myocardial territory of a CTO is a proarrhythmogenic milieu characterized by scar tissue, large scar border zone, hibernating myocardium, residual ischemia despite collaterals, areas of slow conduction, and heterogeneity in repolarization. Restoring coronary flow by CTO PCI is associated with electrical homogenization. This is reflected by a decrease in QT dispersion, decrease in QTc dispersion, decrease in T wave peak-to-end interval, reduction of late potentials, and decrease in scar border zone area.
These observations mandate further evaluation focused on several research areas. First, although a large observational study demonstrated that a successful CTO PCI was associated with a lower arrhythmic risk [Citation57], an appropriately designed prospective study should explore whether revascularization of CTO reduces the risk of VA/SCD. In this respect, also surrogate markers of increased arrhythmic risk can be used as detailed in the systematic review of Van Dongen et al [Citation41]. A randomized controlled trial will be difficult to conduct considering the low event rate of VA/SCD, thus, requiring a large sample size.
Second, a CTO seems to identify patients who may benefit from a prophylactic ICD implant. Future studies should focus on refinement of risk stratification in this group, especially in those without severe LV dysfunction. Prospective studies evaluating the value of electrophysiological studies, contrast enhanced MRI, body surface mapping and long-term rhythm monitoring using implantable cardiac monitor (ICM) may be interesting [Citation63]. Previously, the MUSTT study has shown that electrophysiologic testing in patients with coronary artery disease (90% with previous myocardial infarction), nonsustained VA and LVEF ≤40%, may identify patients who are at higher risk of arrhythmic death [Citation64] (). The likelihood of a randomized controlled ICD trial in this specific patient population seems low. The first step could be to study the VA inducibility before and after CTO PCI thereby using VA inducibility as a surrogate marker for future arrhythmic risk.
The use of high-resolution body surface mapping to study heterogenous ventricular repolarization has been evaluated as a risk marker for arrhythmic events in post-myocardial infarction patients [Citation65–Citation66–Citation67]. However, body surface mapping has not been studied in patients with a CTO.
We expect that the decision to implant a prophylactic ICD in this population in the future will be based on a multiparametric analysis of different risk factors (e.g., unexplained syncope, repetitive nonsustained VA, extensive late gadolinium enhancement, inducible VA, markers of heterogenous ventricular repolarization). Such a strategy is also employed in patients with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and Brugada syndrome [Citation30].
Considering the current knowledge on the increased risk of VA in patients with CTO, we expect more clinical studies confirming this relationship. Van Dongen et al. performed an interesting systematic review to identify electrophysiological parameters associated with increased VA risk which could be used for future research. Suggested parameters are fragmented QRS, late potentials, QT interval, QT dispersion, T-wave peak to end interval and T-wave alternans [Citation41]. Future studies will identify which parameter will be useful and which not. In this respect, post-hoc analysis of randomized CTO PCI trials focusing on the effect of revascularization on electrocardiographic parameters may be interesting and feasible. Besides electrophysiologic parameters, another research area is to focus on VA burden in CTO patients by evaluating ectopy burden and incidence of nonsustained VA. For example, a Holter study before and after CTO PCI can establish whether revascularization reduces ectopy burden [Citation41]. Furthermore, we are currently conducting a multicenter pilot study evaluating the incidence of VA using ICM in patients with CTO without severe LV dysfunction (Incidence of Ventricular Arrhythmias in patients with Chronic Total Occlusion Recanalization, VACTOR study, NCT03475888). The study population consists of patients with successful CTO PCI, failed CTO PCI and no CTO PCI attempt. This study will provide as more insight in the burden of (non)sustained VA in the different CTO populations. The results are to be expected in 2023.
Article highlights
A CTO is associated with an increased risk of ventricular arrhythmias.
The territory supplied by a CTO is a proarrhythmogenic milieu characterized by scar tissue, large scar border zone, hibernating myocardium, residual ischemia despite collaterals, areas of slow conduction, and heterogeneity in repolarization.
Restoring coronary flow by CTO PCI is associated with electrical homogenization as reflected by a decrease in QT(c) dispersion, decrease in T wave peak-to-end interval, reduction of late potentials, and decrease in scar border zone area.
Future studies should focus on the effect of CTO PCI and better risk stratification of patients with a CTO for a prophylactic ICD.
Declaration of interest
SC Yap has received a research grant from Medtronic for the VACTOR study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Azzalini L, Jolicoeur EM, Pighi M, et al. Epidemiology, management strategies, and outcomes of patients with chronic total coronary occlusion. Am J Cardiol. 2016;118(8):1128–1135.
- Redfors B, Ramunddal T, Angeras O, et al. Angiographic findings and survival in patients undergoing coronary angiography due to sudden cardiac arrest in western Sweden. Resuscitation. 2015;90:13–20.
- Nishiyama K, Shizuta S, Doi T, et al. Sudden cardiac death after PCI and CABG in the bare-metal stent era: incidence, prevalence, and predictors. Int J Cardiol. 2010;144(2):263–266.
- Arslan U, Balcioglu AS, Timurkaynak T, et al. The clinical outcomes of percutaneous coronary intervention in chronic total coronary occlusion. Int Heart J. 2006;47(6):811–819.
- Nombela-Franco L, Iannaccone M, Anguera I, et al., Impact of chronic total coronary occlusion on recurrence of ventricular arrhythmias in ischemic secondary prevention implantable cardioverter-defibrillator recipients (VACTO secondary study): insights from coronary angiogram and electrogram analysis. JACC Cardiovasc Interventions. 10(9): 879–888. 2017.
- Nombela-Franco L, Mitroi CD, Fernandez-Lozano I, et al., Ventricular arrhythmias among implantable cardioverter-defibrillator recipients for primary prevention: impact of chronic total coronary occlusion (VACTO Primary Study). Circ Arrhythm Electrophysiol. 5(1): 147–154. 2012.
- Yap SC, Sakhi R, Theuns D, et al., Increased risk of ventricular arrhythmias in survivors of out-of-hospital cardiac arrest with chronic total coronary occlusion. Heart Rhythm. 15(1): 124–129. 2018.
- Chi WK, Gong M, Bazoukis G, et al., Impact of coronary artery chronic total occlusion on arrhythmic and mortality outcomes: a systematic review and meta-analysis. JACC Clin Electrophysiol. 4(9): 1214–1223. 2018.
- Di Marco A, Anguera I, Teruel L, et al., Chronic total occlusion of an infarct-related artery: a new predictor of ventricular arrhythmias in primary prevention implantable cardioverter defibrillator patients. Europace. 19(2): 267–274. 2017.
- Di Marco A, Anguera I, Teruel L, et al. Chronic total occlusion in an infarct-related coronary artery and the risk of appropriate ICD therapies. J Cardiovasc Electrophysiol. 2017;28(10):1169–1178.
- Di Marco A, Paglino G, Oloriz T, et al. Impact of a chronic total occlusion in an infarct-related artery on the long-term outcome of ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2015;26(5):532–539.
- Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions: the Canadian multicenter chronic total occlusions registry. J Am Coll Cardiol. 2012;59(11):991–997.
- Ramunddal T, Hoebers LP, Henriques JP, et al. Chronic total occlusions in Sweden–a report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). PLoS One. 2014;9(8):e103850.
- Tomasello SD, Boukhris M, Giubilato S, et al. Management strategies in patients affected by chronic total occlusions: results from the Italian Registry of Chronic Total Occlusions. Eur Heart J. 2015;36(45):3189–3198.
- Jeroudi OM, Alomar ME, Michael TT, et al. Prevalence and management of coronary chronic total occlusions in a tertiary Veterans Affairs hospital. Catheter Cardiovasc Interv. 2014;84(4):637–643.
- Lee SW, Lee PH, Ahn JM, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation. 2019;139(14):1674–1683.
- Safley DM, Grantham JA, Hatch J, et al. Quality of life benefits of percutaneous coronary intervention for chronic occlusions. Catheter Cardiovasc Interv. 2014;84(4):629–634.
- Safley DM, Koshy S, Grantham JA, et al. Changes in myocardial ischemic burden following percutaneous coronary intervention of chronic total occlusions. Catheter Cardiovasc Interv. 2011;78(3):337–343.
- Rossello X, Pujadas S, Serra A, et al. Assessment of inducible myocardial ischemia, quality of life, and functional status after successful percutaneous revascularization in patients with chronic total coronary occlusion. Am J Cardiol. 2016;117(5):720–726.
- Azzalini L, Vo M, Dens J, et al. Myths to debunk to improve management, referral, and outcomes in patients with chronic total occlusion of an epicardial coronary artery. Am J Cardiol. 2015;116(11):1774–1780.
- Jang WJ, Yang JH, Choi SH, et al. Long-term survival benefit of revascularization compared with medical therapy in patients with coronary chronic total occlusion and well-developed collateral circulation. JACC Cardiovasc Interv. 2015;8(2):271–279.
- Christakopoulos GE, Christopoulos G, Carlino M, et al. Meta-analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am J Cardiol. 2015;115(10):1367–1375.
- Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and meta-analysis. Am Heart J. 2010;160(1):179–187.
- Lee NH, Cho YH, Seo HS. Successful recanalization of in-stent coronary chronic total occlusion by subintimal tracking. J Invasive Cardiol. 2008;20(4):E129–32.
- Henriques JP, Hoebers LP, Ramunddal T, et al. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol. 2016;68(15):1622–1632.
- Werner GS, Martin-Yuste V, Hildick-Smith D, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39(26):2484–2493.
- Obedinskiy AA, Kretov EI, Boukhris M, et al. The IMPACTOR-CTO Trial. JACC Cardiovasc Interv. 2018;11(13):1309–1311.
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Heart Rhythm. 2018;15(10):e73–e189.
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–2867.
- Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200.
- Raja V, Wiegn P, Obel O, et al. Impact of chronic total occlusions and coronary revascularization on all-cause mortality and the incidence of ventricular arrhythmias in patients with ischemic cardiomyopathy. Am J Cardiol. 2015;116(9):1358–1362.
- van Dongen IM, Yilmaz D, Elias J, et al. Evaluation of the impact of a chronic total coronary occlusion on ventricular arrhythmias and long-term mortality in patients with ischemic cardiomyopathy and an implantable cardioverter-defibrillator (the eCTOpy-in-ICD Study). J Am Heart Assoc. 2018;7(10). doi:10.1161/JAHA.118.008609.
- Wang ZQ, Qiang H, Luo X, et al. Meta-analysis of risk of ventricular arrhythmias and all-cause mortality in patients with chronic total occlusion of a coronary artery and/or implantable cardioverter-defibrillator. Am J Cardiol. 2018;121(10):1149–1154.
- Horowitz LN, Josephson ME, Harken AH. Epicardial and endocardial activation during sustained ventricular tachycardia in man. Circulation. 1980;61(6):1227–1238.
- de Bakker JM, Coronel R, Tasseron S, et al. Ventricular tachycardia in the infarcted, Langendorff-perfused human heart: role of the arrangement of surviving cardiac fibers. J Am Coll Cardiol. 1990;15(7):1594–1607.
- de Bakker JM, van Capelle FJ, Janse MJ, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77(3):589–606.
- Roes SD, Borleffs CJ, van der Geest RJ, et al. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging. 2009;2(3):183–190.
- Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115(15):2006–2014.
- de Haan S, Meijers TA, Knaapen P, et al. Scar size and characteristics assessed by CMR predict ventricular arrhythmias in ischaemic cardiomyopathy: comparison of previously validated models. Heart. 2011;97(23):1951–1956.
- van Dongen IM, Elias J, Meijborg VMF, et al., Electrocardiographic changes after successful recanalization of a chronic total coronary occlusion. A systematic review and meta-analysis. Cardiovasc Revasc Med. 19(2): 221–228. 2018.
- Yamashita K, Igawa W, Ono M, et al., Impact of recanalization of chronic total occlusion on left ventricular electrical remodeling. Pacing Clin Electrophysiol. 42(6): 712–721. 2019.
- Swann MH, Nakagawa H, Vanoli E, et al. Heterogeneous regional endocardial repolarization is associated with increased risk for ischemia-dependent ventricular fibrillation after myocardial infarction. J Cardiovasc Electrophysiol. 2003;14(8):873–879.
- Werner GS. The role of coronary collaterals in chronic total occlusions. Curr Cardiol Rev. 2014;10(1):57–64.
- Werner GS, Surber R, Ferrari M, et al. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur Heart J. 2006;27(20):2406–2412.
- Sachdeva R, Agrawal M, Flynn SE, et al., The myocardium supplied by a chronic total occlusion is a persistently ischemic zone. Catheter Cardiovasc Interv. 83(1): 9–16. 2014.
- Opthof T, Coronel R, Vermeulen JT, et al. Dispersion of refractoriness in normal and ischaemic canine ventricle: effects of sympathetic stimulation. Cardiovasc Res. 1993;27(11):1954–1960.
- Ripley DP, Gosling OE, Bhatia L, et al. The relationship between the contralateral collateral supply and myocardial viability on cardiovascular magnetic resonance: can the angiogram predict functional recovery? Int J Cardiol. 2014;177(2):362–367.
- Elias J, van Dongen IM, Hoebers LP, et al. Improved recovery of regional left ventricular function after PCI of chronic total occlusion in STEMI patients: a cardiovascular magnetic resonance study of the randomized controlled EXPLORE trial. J Cardiovasc Magn Reson. 2017;19(1):53.
- Canty JM Jr., Suzuki G, Banas MD, et al. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res. 2004;94(8):1142–1149.
- Lim H, Fallavollita JA, Hard R, et al. Profound apoptosis-mediated regional myocyte loss and compensatory hypertrophy in pigs with hibernating myocardium. Circulation. 1999;100(23):2380–2386.
- Fallavollita JA, Perry BJ, Canty JM Jr. 18F-2-deoxyglucose deposition and regional flow in pigs with chronically dysfunctional myocardium. Evidence for transmural variations in chronic hibernating myocardium. Circulation. 1997;95(7):1900–1909.
- Luisi AJ Jr., Fallavollita JA, Suzuki G, et al. Spatial inhomogeneity of sympathetic nerve function in hibernating myocardium. Circulation. 2002;106(7):779–781.
- Kouvas N, Kontogiannis C, Georgiopoulos G, et al. The complex crosstalk between inflammatory cytokines and ventricular arrhythmias. Cytokine. 2018;111:171–177.
- Yang KC, Kyle JW, Makielski JC, et al. Mechanisms of sudden cardiac death: oxidants and metabolism. Circ Res. 2015;116(12):1937–1955.
- Li KHC, Wong KHG, Gong M, et al. Percutaneous coronary intervention versus medical therapy for chronic total occlusion of coronary arteries: a systematic review and meta-analysis. Curr Atheroscler Rep. 2019;21(10):42.
- Godino C, Giannattasio A, Scotti A, et al., Risk of cardiac and sudden death with and without revascularisation of a coronary chronic total occlusion. Heart. 105(14): 1096–1102. 2019.
- Hoebers LP, Claessen BE, Elias J, et al. Meta-analysis on the impact of percutaneous coronary intervention of chronic total occlusions on left ventricular function and clinical outcome. Int J Cardiol. 2015;187:90–96.
- van Dongen IM, Kolk MZH, Elias J, et al. The effect of revascularization of a chronic total coronary occlusion on electrocardiographic variables. A sub-study of the EXPLORE trial. J Electrocardiol. 2018;51(5):906–912.
- Pristipino C, Granatelli A, Capasso M, et al. Effects of reperfusion obtained two to six months after acute myocardial infarction on myocardial electrical stabilization in patients with an occluded infarct-related coronary artery. Am J Cardiol. 2005;96(6):769–772.
- Cetin M, Zencir C, Cakici M, et al. Effect of a successful percutaneous coronary intervention for chronic total occlusion on parameters of ventricular repolarization. Coron Artery Dis. 2014;25(8):705–712.
- Myat A, Patel M, Silberbauer J, et al. Chronic total coronary occlusion revascularisation positively modifies infarct-related myocardial scar responsible for recurrent ventricular tachycardia. EuroIntervention. 2019. Published online 2019 Jul 2.
- Baumert M. Measurement of T wave variability in body surface ECG. J Electrocardiol. 2016;49(6):883–886.
- Buxton AE, Lee KL, DiCarlo L, et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. Multicenter unsustained tachycardia trial investigators. N Engl J Med. 2000;342(26):1937–1945.
- Korhonen P, Husa T, Konttila T, et al. Complex T-wave morphology in body surface potential mapping in prediction of arrhythmic events in patients with acute myocardial infarction and cardiac dysfunction. Europace. 2009;11(4):514–520.
- Fereniec M, Stix G, Kania M, et al. Risk assessment of ventricular arrhythmia using new parameters based on high resolution body surface potential mapping. Med Sci Monit. 2011;17(3):MT26–33.
- Fereniec M, Stix G, Kania M, et al. An analysis of the U-wave and its relation to the T-wave in body surface potential maps for healthy subjects and MI patients. Ann Noninvasive Electrocardiol. 2014;19(2):145–156.
